復合益生菌對超早期斷奶杜藏乳仔豬腸道微生物群落結構的影響
劉韶娜 郭飛 張斌 相德才 趙智勇 趙素梅 趙彥光
劉韶娜(1983-),副研究員,畢業于浙江大學動物營養與飼料科學專業,主要從事動物營養與腸道微生物學研究工作。先后主持或作為主要成員參與云南省重點研發項目“云南省地方豬選育開發利用及糞便資源化利用示范”、 國家自然科學基金地區科學基金項目“冷凍豬GV期卵母細胞體外成熟后蛋白質組差異及調控策略研究”、云南省畜牧獸醫科學院基礎研究項目“芽孢桿菌和益生菌對迪慶藏豬腸道菌群結構與功能的影響機制研究”及云南省畜禽種質創新及標準化養殖技術推廣專項等科研項目10余項;制定地方標準2項,參編專著2本,獲授權國家發明專利1項;在《Theriogenology》《動物營養學報》《微生物學通報》《南方農業學報》等學術期刊上發表科技論文20余篇。
摘要:【目的】探究復合益生菌對超早期斷奶(7 d)杜藏乳仔豬腸道微生物多樣性及物種豐度的影響,以減輕乳仔豬斷奶應激,為微生物飼料添加劑的研發提供科學依據。【方法】選取7日齡杜藏乳仔豬30頭,隨機分為3組,每組10頭。對照組(ZM組)隨母豬哺乳,試驗I組(ZD組)哺喂代乳粉,試驗II組(ZY組)哺喂代乳粉+復合益生菌,試驗周期21 d。試驗結束當天(28日齡)收集乳仔豬糞便,采用Illumina高通量測序分析糞便樣品的菌群結構組成。【結果】Illumina高通量測序獲得ZM組、ZY組和ZD組杜藏乳仔豬糞便樣品共有OTU為327個,ZM組的特有OTU為247個,ZD組的特有OTU為84個,ZY組的特有OTU為96個。在門分類水平上,ZM組杜藏乳仔豬糞便樣品中相對豐度最高的菌門為厚壁菌門,ZY組和ZD組為擬桿菌門;ZY組和ZD組的厚壁菌門/擬桿菌門比值分別為0.77和0.92,較ZM組(1.76)分別下降56.25%和47.73%。在屬分類水平上,ZD組和ZY組杜藏乳仔豬糞便樣品中的優勢菌屬為普氏菌屬_9,ZM組則為乳桿菌屬;ZD組杜藏乳仔豬糞便中布勞特氏菌屬、腸球菌屬和吉氏副擬桿菌屬的相對豐度較其他2個處理組顯著上升(P<0.05,下同);而ZY組杜藏乳仔豬糞便中普雷沃氏菌科_NK3B31群、普雷沃氏菌科_UCG-003、厭氧弧菌屬、罕見小球菌屬、瘤胃菌科_NK4A214群、瘤胃菌科_UCG-005、未明確普雷沃氏菌科及Family_XIII_AD3011_group的相對豐度顯著高于ZD組。【結論】在杜藏超早期斷奶仔豬代乳粉中添加復合益生菌能有效提高其腸道菌群結構多樣性,同時提高與碳水化合物代謝和產短鏈脂肪酸相關菌群的相對豐度,即復合益生菌具有潛在促進營養物質代謝和抗炎癥的功能,可縮短消化道微生物區系由哺乳型向飼料型的轉變歷程。
關鍵詞: 杜藏乳仔豬;超早期斷奶;代乳粉;復合益生菌;腸道微生物;多樣性
中圖分類號: S816.73? ? ? ? ? ? ? ? ? ? ? ? ? ? ? 文獻標志碼: A 文章編號:2095-1191(2021)03-0547-12
Effects of the probiotic combinations on intestinal microbiota in ultra-early weaned piglets of Duroc×Diqing Tibetan pig
LIU Shao-na1, GUO Fei2, ZHANG Bin1, XIANG De-cai1, ZHAO Zhi-yong1,
ZHAO Su-mei2*, ZHAO Yan-guang1*
(1Yunnan Academy of Animal Husbandry and Veterinary Sciences, Kunming? 650224, China; 2Key Laboratory
of Animal Nutrition and Feed, Yunnan Agricultural University, Kunming? 650201, China)
Abstract:【Objective】The study aimed to explore the effects of the probiotic combinations on the intestinal microbiota diversity and species abundance of ultra-early weaned(7 d) piglets of Duroc×Diqing Tibetan pig. Then released the stress of weaned piglets, and provided scientific basis for the development of microbial feed additives. 【Method】A total of 30 piglets of Duroc×Diqing Tibetan pig, aged 7-day-old were divided into 3 groups with 10 piglets in each group. The piglets of control group(group ZM) were fed with breast milk,the piglets of experimental group I (group ZD) were fed with milk replacer and the piglets of experimental group II (group ZY) were fed with milk replacer and probiotic combinations. The experimental period was 21 d. Collected the faeces samples of the piglets in the last day of the experiment(28-day-old),then analyzed the samples fecal microbiota in structure with IIIumina high throughput sequencing technology. 【Result】The IIIumina high throughput sequencing data showed that there were 327 common OTUs in the three groups, while there were 247 unique OTUs in the group ZM, 84 unique OTUs in the group ZD and 96 unique OTUs in the group ZY, respectively. At the phylum level,the relative abundance of Firmicutes in group ZM was the highest one,while that was Bacteroidetes in group ZD and group ZY. The Firmicutes/Bacteroidetes ratios were 0.77 and 0.92 of the group ZY and ZD,respectively. Compared with group ZM(1.76), they were decreased by 56.25% and 47.73%, respectively. At the genus level,the dominant genus of group ZD and ZY was Prevotella_9 in the faeces samples of the Duroc×Diqing Tibetan Pig,while that of group ZM was Lactobacillus. The relative abundance of Blautia, Enterococcus and Subdoligranulum in group ZD was significantly higher than the other two groups(P<0.05, the same below). The relative abundance of Prevotellaceae_NK3B31_group, Prevotellaceae_UCG-003, Anaerovibrio, Subdoligranulum, Ruminococcaceae_NK4A214_ group, Ruminococcaceae_UCG-005, norank_f_Prevotellaceae and Family_XIII_AD3011_group in group ZY were signi-ficantly higher than the group ZD. 【Conclusion】In conclusion,after feeding the milk replacer with adding the probiotic combinations, the diversity of structure of intestinal flora are increased,? and the relative abundance of the microflora which is related to metabolism of carbohydrate and producing short chain fatty acids in ultra-early weaned piglets of Duroc×Diqing Tibetan pig were increased. The potential functions of the probiotic combinations which can promote nutrient metabolism and anti-inflammation can shorten the transformation process of digestive tract microflora from lactation type to feed type.
Key words: Duro×Diqing Tibetan pig; ultra-early weaned piglets; milk replacer; probiotic combinations; intestinal microbe; diversity
Foundation item:Key Research and Development Project of Yunnan(2018BB003);Yunnan Provincial Financial Breeding and Technology Promotion Project(20151105560055); Basic Research Project of Yunnan Academy of Animal Husbandry and Veterinary Sciences(2019RW014)
0 引言
【研究意義】抗生素濫用已造成細菌耐藥性增強及藥物殘留超標(李威等,2020;李昕等,2020),且擾亂動物腸道屏障功能和改變腸道菌群結構,進而導致動物機體對某些病原菌更具感染性(Pamer,2016;Derosa et al.,2020;Hou et al.,2020),同時誘導腸道菌群代謝通路發生改變(色氨酸代謝和脂代謝失調),或影響某些基因正常表達(Sun et al.,2020;Teichman et al.,2020),嚴重制約了畜禽養殖業的持續健康發展。因此,尋找或研發抗生素替代型飼料添加劑是當前畜禽養殖生產中亟待解決的首要問題,也是目前畜牧學科中的研究熱點之一。【前人研究進展】益生菌(Probiotic)是一類能改變宿主菌群組成且對宿主有益的活性微生物,在促進生長(李雪莉等,2017)、營養消化(夏耀耀等,2017;詹明曄等,2019)、降低發病率(謝全喜等,2017;Singer et al.,2019)及促進神經功能(Patil et al.,2017)和腸道器官發育(馮程程等,2019;吳敏等,2019)等方面發揮重要作用。在實際生產中,添加益生菌可通過直接(王曉丹等,2019)或間接(田時祎等,2018;Tian et al.,2018;Hu et al.,2019)促進腸道微生物代謝作用,從而發揮益生作用,促使腸道常駐菌與宿主的微空間結構間形成一個相互依賴又相互作用的微生態系統,在腸道內構成一個對抗病原體的重要保護屏障,以抵抗外來菌微生物的定殖。目前,常用的益生菌有芽孢桿菌、酵母菌、乳酸菌及梭菌等(楊虹等,2019;關嘉琦等,2020)。其中,枯草芽孢桿菌能提高斷奶仔豬腸道菌群多樣性,提高厚壁菌門相對豐度及短鏈脂肪酸、脫氧膽酸和石膽酸濃度(He et al.,2017);乳酸菌能顯著提高老年鼠的腸道菌多樣性,抑制炎癥因子表達,減輕腸漏現象(Ahmadi et al.,2020);乳桿菌具有提高有益菌并降低有害菌相對豐度,增強菌群磷酸鹽代謝、氨基酸轉運系統和異亮氨酸生物合成,以及降低脂多糖生物合成的功能(Toscano et al.,2017;Hou et al.,2020)。【本研究切入點】至今,有關益生菌在斷奶仔豬和育肥豬上的應用已有較多研究報道,但主要是單一或少數幾種菌株復合使用(Latham et al.,2018;He et al.,2019;Zhang et al.,2020;Zheng et al.,2020),而針對復合益生菌的研究較少。【擬解決的關鍵問題】杜藏仔豬是以杜洛克為父本、迪慶藏豬為母本的雜交品種,具有耐高寒、耐低氧、耐粗飼和強抗病性的特點,通過選育及營養調控等措施已將其產仔數提高至9~12頭,但母豬乳頭數量少,不能滿足其哺乳需求。故選擇杜藏仔豬為試驗動物,給予外源性復合益生菌,探究復合益生菌對超早期斷奶(7 d)杜藏乳仔豬腸道微生物多樣性及物種豐度的影響,旨在減輕乳仔豬斷奶應激,同時為微生物飼料添加劑的研發提供科學依據。
1 材料與方法
1. 1 試驗材料
從10窩杜藏仔豬中隨機挑選初生體重和日齡接近的乳仔豬,每窩挑選3頭,共計30頭。按照血緣和體重隨機分為3個處理組,每組2個重復,每個重復5頭,每個重復同圈飼養。從初生7日齡開始試驗,對照組(ZM組)杜藏乳仔豬隨母豬哺乳至28日齡,2個試驗組的杜藏乳仔豬則在7日齡斷奶,試驗周期21 d[預飼期7 d,正試期(飼喂對應的飼糧)14 d]。試驗 I組(ZD組)哺喂代乳粉,試驗II組(ZY組)哺喂代乳粉+復合益生菌。代乳粉是利用VF123參照國標NY/T 65—2004《豬飼養標準》的肉脂型地方豬3~8 kg計算配制的粉狀配合飼料,其組成和營養成分見表1。代乳粉需用50~65 ℃溫開水按1∶6比例沖調,冷卻至38 ℃左右再進行飼喂。復合益生菌制劑(表2)包含類腸膜魏斯氏菌、植物乳桿菌、戊糖片球菌、貝萊斯芽孢桿菌、枯草芽孢桿菌和賴氨酸芽孢桿菌,均由云南省畜牧獸醫科學院從迪慶藏豬腸道內容物及其糞便中分離獲得(劉韶娜,2019a,2019b),添加量參考Kim和Isaacson(2015)、謝全喜等(2017)、Piuske等(2018)的研究結果。
1. 2 飼養管理
按照正常免疫程序對試驗仔豬進行常規免疫,對豬舍進行清潔和消毒。對照組杜藏乳仔豬隨母豬哺乳;試驗組杜藏乳仔豬哺喂相對應的試驗飼糧,試驗周期內每天哺喂6次,從早晨8:00開始,每隔3 h哺喂1次,自由飲水。于28日齡(試驗結束)當天收集剛排泄的糞便,取中間部分樣品置于液氮中速凍,-80 ℃冰箱保存,用于高通量測序分析。
1. 3 16S rRNA序列PCR擴增
使用DNA提取試劑盒(OMEGA Bio-tek,Norcross,GA,U.S.)對杜藏乳仔豬糞便樣品進行總DNA抽提,利用NanoDrop 2000檢測DNA濃度和純度,并以1.0%瓊脂糖凝膠電泳檢測DNA質量;采用引物對338F(5'-ACTCCTACGGGAGGCAGCAG-3')和806R(5'-GGACTACHVGGGTWTCTAAT-3')擴增16S rRNA序列V3~V4可變區,PCR反應體系20.0 ?L:5×Buffer 4.0 ?L,dNTPs(2.5 mmol/L)2.0 ?L,引物338F和806R(5 ?mol/L)各0.8 ?L,2×FastPfu聚合酶0.4 ?L,BSA 0.2 ?L,DNA模板10 ng,ddH2O補足至20.0 ?L。擴增程序:95 ℃預變性3 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 45 s,進行27個循環;72 ℃延伸10 min。回收PCR擴增產物并進行純化,以Tris-HCl洗脫后進行瓊脂糖凝膠電泳檢測;并利用QuantiFluorTM-ST(Promega,USA)進行定量分析。
1. 4 高通量測序分析
使用Trimmomatic軟件對原始測序結果進行序列質控,以FLASH進行拼接。利用Illumina MiSeq平臺構建PE300的文庫,根據97%相似度對序列進行OTU聚類,并去除單序列和嵌合體,在I-Sanger云平臺對每條序列進行物種分類注釋分析。采用SPSS 17.0進行單因素方差分析(One-way ANOVA)和Duncans多重比較。
2 結果與分析
2. 1 復合益生菌對杜藏乳仔豬腸道菌群結構的影響
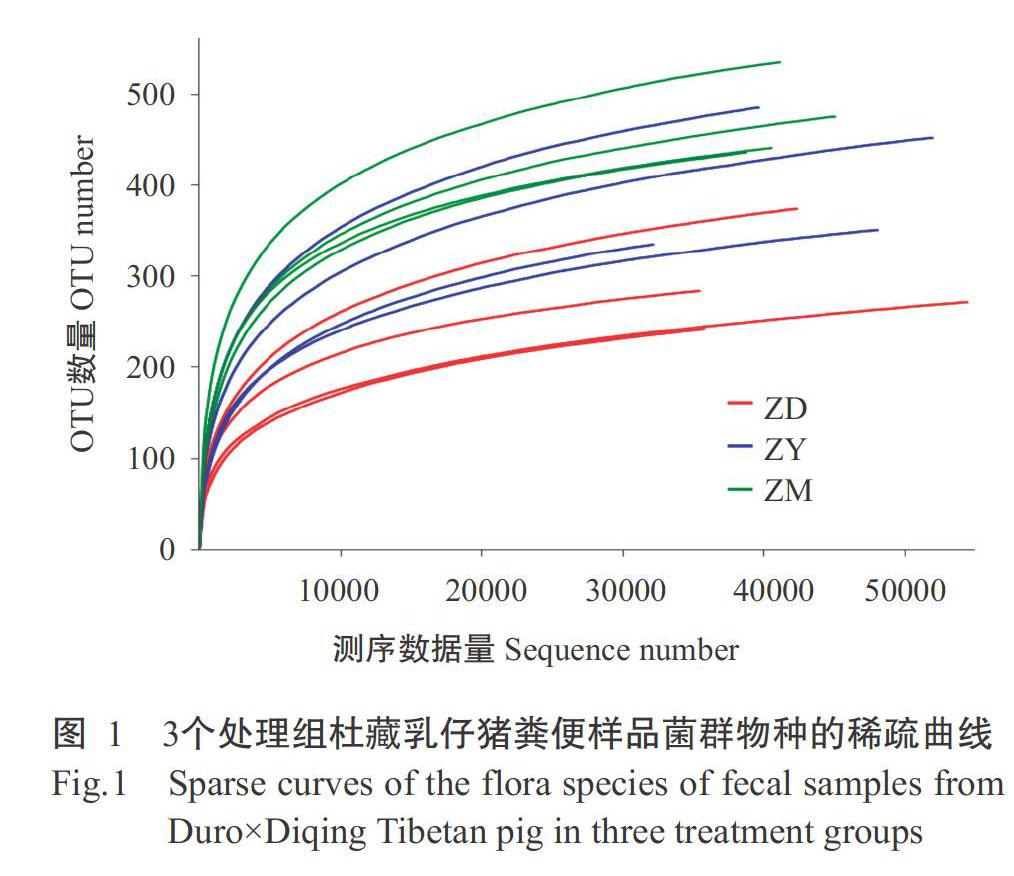
2. 1. 1 OTU聚類分析結果 采用Illumina高通量測序獲得樣本數據后,經數據過濾、拼接及質控,平均每個糞便樣品獲得53810條高質量序列,平均長度為463.62 nt,將相似性達97%的序列聚類成1個OTU,12個糞便樣品共獲得1049個OTUs。杜藏乳仔豬糞便樣品菌群物種的稀疏曲線見圖1,當測序數據量在20000以上時,稀釋曲線趨于平穩,說明測序的深度和數據量足夠,即Illumina高通量測序數據合理,能基本反映樣品中細菌的組成和種類。
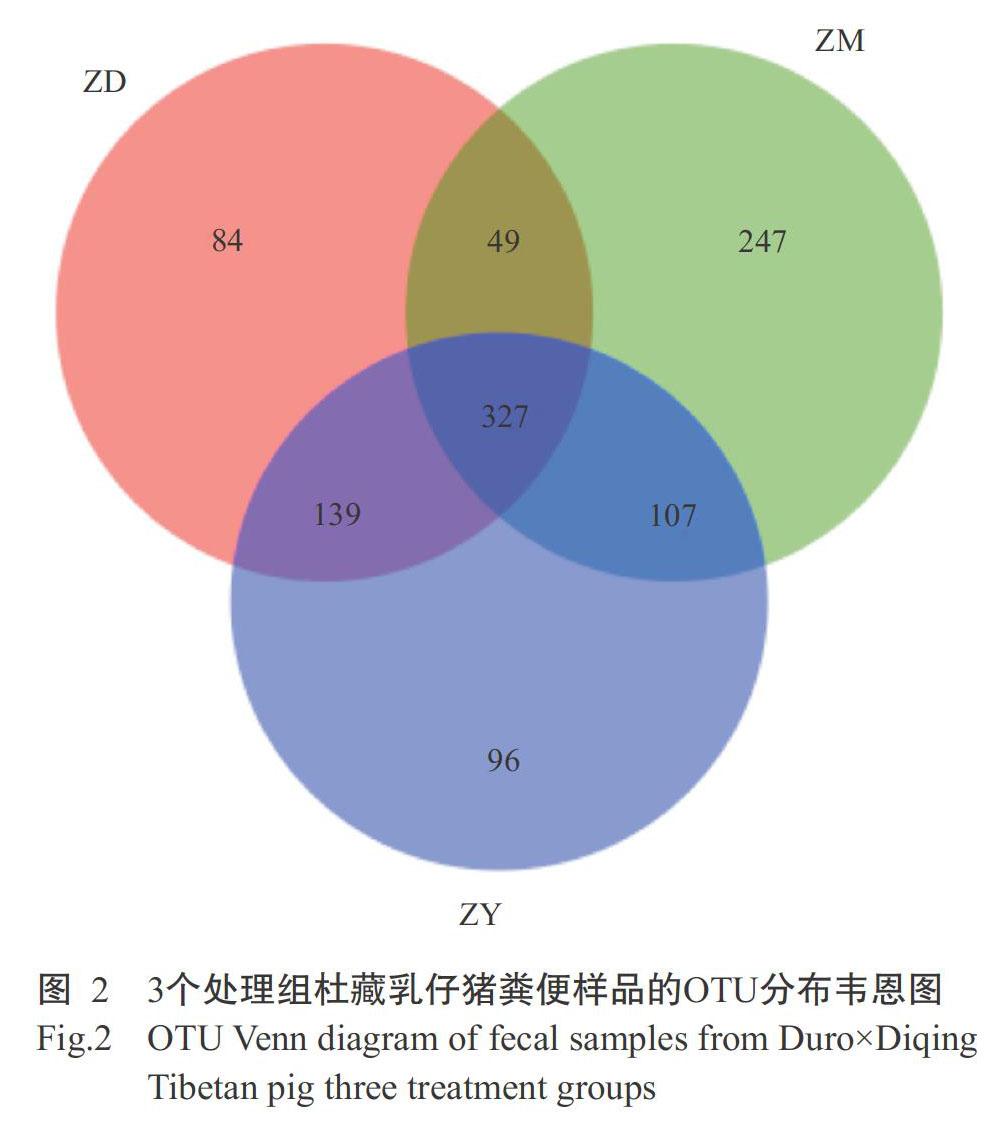
由圖2可知,ZM組、ZY組和ZD組杜藏乳仔豬糞便樣品共有OTU為327個,ZM組的特有OTU為247個,ZD組的特有OTU為84個,ZY組的特有OTU為96個。ZM組的OTU總數為730個,ZD組的OTU總數為599個,兩組的共有OTU為376個,可能是與迪慶藏豬母乳相比,代乳粉成分較單一,杜藏豬乳仔豬采食代乳粉后其腸道細菌物種隨之發生變化,細菌種類下降。ZY組的OTU總數為669個,與ZM組的共有OTU為434個,與ZD組的共有OTU為466個。
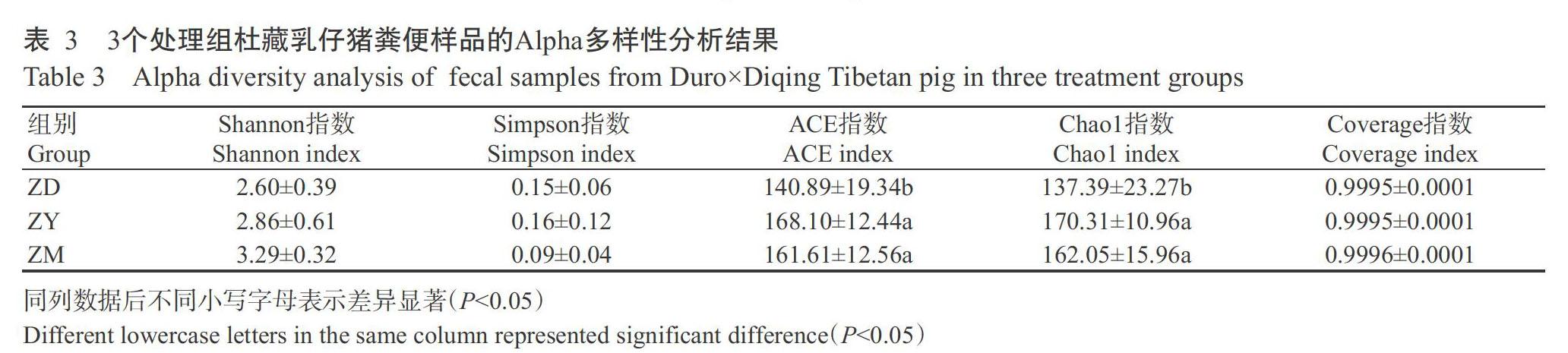
2. 1. 2 Alpha多樣性分析結果 Alpha多樣性指數是反映樣品物種多樣性的指標之一,包括Shannon指數、ACE指數、Chao1指數和Simpson指數等。本研究結果表明,3個處理組杜藏乳仔豬糞便樣品的Coverage指數均在0.9995以上,說明測序的深度和廣度符合試驗要求,試驗數據合理。從表3可看出,ZY 組和ZM組杜藏乳仔豬糞便樣品的Shannon指數、ACE指數和Chao1指數均高于ZD組,ZM組的Simpson指數則低于ZD組,但差異均不顯著(P>0.05,下同)。其中,ZM組和ZY組的ACE指數及Chao1指數均顯著高于ZD組(P?0.05,下同),說明在代乳粉中添加復合益生菌后能顯著提高杜藏乳仔豬腸道菌群Alpha多樣性。
2. 1. 3 Beta多樣性分析結果 Beta多樣性分析是對不同微生物群落間的物種多樣性進行組間比較,以明確不同樣品組間的群落組成相似性或差異性。在基于Weighted Unifrac加權距離的PCoA分析和基于Unweighted Unifrac加權距離的PCoA分析中,橫坐標表示第一主成分(PC1),縱坐標表示第二主成分(PC2)。PCoA分析結果(圖3和圖4)表明,PC1和PC2對樣品差異的貢獻值分別為47.62%和15.54%、38.65%和13.97%。3個處理組12個杜藏乳仔豬糞便樣品明顯分開,但組內樣品聚集在一起。其中,ZY組和ZD組樣品在同一區域,與ZM組樣品明顯分開,說明ZY組和ZD組樣品間差異較小,杜藏乳仔豬腸道菌群組成相似,但與ZM組間差異顯著,即樣品中的菌群組成差異顯著。
2. 2 物種相對豐度影響分析結果
2. 2. 1 門分類水平上的優勢菌群分析結果 在門分類水平上,3個處理組杜藏乳仔豬糞便樣品中均以厚壁菌門(Firmicutes)、擬桿菌門(Bacteroidetes)、螺旋菌門(Spirochaetes)和放線菌門(Actinobacteria)占據絕對優勢(圖5)。其中,厚壁菌門和擬桿菌門的相對豐度最高,其總和占樣品中細菌總數的90%以上。ZM組的優勢菌門為厚壁菌門,ZY組和ZD組的優勢菌門為擬桿菌門。ZY組和ZD組的厚壁菌門/擬桿菌門比值分別為0.77和0.92,較ZM組(1.76)分別下降56.25%和47.73%。可見,采食代乳粉后杜藏乳仔豬糞便中擬桿菌門的相對豐度升高,在代乳粉中添加復合益生菌能進一步提高擬桿菌門的相對豐度,從而降低厚壁菌門/擬桿菌門比值。
2. 2. 2 屬分類水平上的優勢菌群分析結果 在屬分類水平上,3個處理組杜藏乳仔豬糞便菌群主要分布在34個菌屬,其相對豐度排名前10的菌屬(圖6)分別是普氏菌屬_9(Prevotella_9)、norank_f_Muribaculaceae、乳桿菌屬(Lactobacillus)、布勞特氏菌屬(Blautia)、擬桿菌屬(Bacteroides)、考拉桿菌屬(Phascolarctobacterium)、普雷沃氏菌科_NK3B31群(Prevotellaceae_NK3B31_group)、毛螺菌科_NK4A136群(Lachnospiraceae_NK4A136_group)、密螺旋體屬_2(Treponema_2)和毛螺菌科_AC2044群(Lachnospiraceae_AC2044_group)。其中,布勞特氏菌屬、考拉桿菌屬、乳桿菌屬及毛螺菌科(Lachnospiraceae)等屬于厚壁菌門,擬桿菌屬、普氏菌屬(Prevotella)、norank_f_Muribaculaceae及普雷沃氏菌科(Prevotellaceae)屬于擬桿菌門,密螺旋體屬屬于螺旋體門。ZD組和ZY組的優勢菌屬為普氏菌屬_9,ZM組則為乳桿菌屬。此外,ZD組和ZY組的普氏菌屬_9、norank_f_Muribaculaceae、布勞特氏菌屬和考拉桿菌屬均呈上升趨勢,乳桿菌屬、普雷沃氏菌科_NK3B31群、毛螺菌科_AC2044群、毛螺菌科_ NK4A136群和密螺旋體屬_2則呈下降趨勢。
2. 3 物種豐度聚類分析結果
不同處理組杜藏乳仔豬糞便樣品物種豐度聚類分析結果如圖7所示,ZD組和ZY組首先聚類在一起,然后與ZM組聚類在一起,說明試驗組和對照組的杜藏乳仔豬糞便物種豐度明顯分開,也表明2個試驗組的杜藏乳仔豬糞便物種組成及相對豐度更接近。
2. 4 組間差異分析結果
由圖8可知,ZD組和ZY組杜藏乳仔豬糞便中的布勞特氏菌屬、罕見小球菌屬(Subdoligranulum)、腸球菌屬(Enterococcus)、未明確普雷沃氏菌科(norank_ f_Prevotellaceae)及瘤胃菌科(Ruminococcaceae)較ZM組均呈上升趨勢,密螺旋體屬_2、毛螺菌科_ AC2044群、普雷沃氏菌科_NK3B31群、普雷沃氏菌科_UCG-003、瘤胃菌科_NK4A214群、瘤胃菌科_ UCG-005及Family_XIII_AD3011_group則呈下降趨勢。ZD組杜藏仔豬糞便中布勞特氏菌屬、腸球菌屬和吉氏副擬桿菌屬(Parabacteriodes)的相對豐度較其他2個處理組呈顯著上升趨勢;而ZY組杜藏乳仔豬糞便中普雷沃氏菌科_NK3B31群、普雷沃氏菌科_UCG-003、厭氧弧菌屬(Anaerovibrio)、罕見小球菌屬、瘤胃菌科_NK4A214群、瘤胃菌科_UCG-005、未明確普雷沃氏菌科及Family_XIII_AD3011_group的相對豐度顯著高于ZD組,說明在代乳粉中添加復合益生菌能改變杜藏乳仔豬腸道菌群結構,有利于普雷沃氏菌科、瘤胃菌科、厭氧弧菌屬和罕見小球菌屬等菌群的生長。
3 討論
機體腸道內環境復雜,且受年齡、遺傳、營養及生存環境等因素的影響,腸道內的菌群結構復雜且呈多樣性的特點(Zhao et al.,2015;李永洙等,2019;劉晗璐等,2019;詹明曄等,2019)。日糧是影響機體腸道微生物多樣性的主要因素(Claesson et al.,2012;Gudi et al.,2019)。李永洙等(2018)通過研究代乳粉對沂蒙黑山羊瘤胃微生物區系的影響,發現其腸道菌群Alpha多樣性降低,隨著日齡的增加,母乳組和代乳粉組沂蒙黑山羊瘤胃內微生物區系均達到一個動態平衡,且差異越來越小。在本研究中,ZD組杜藏乳仔豬糞便微生物多樣性指標Chao1指數、ACE指數和Shannon指數均低于ZM組,Simpson指數則高于ZM組,說明采食代乳粉后糞便中微生物多樣性降低,與李永洙等(2018)的研究結果一致。此外,ZD組的Chao1指數和ACE指數顯著低于ZY組,說明在代乳粉中添加復合益生菌可有效提高杜藏乳仔豬腸道菌群結構多樣性。
機體腸道內細菌種類繁多,形成一個錯綜復雜的微生物體系,促進個體發育并參與養分代謝。厚壁菌門細菌參與能量的吸收,促進機體從飼料中吸收能量,從而滿足機體生長需求(Davis,2017;馬燕等,2019)。擬桿菌門細菌參與碳水化合物、膽汁酸和類固醇的代謝,能提高營養物質的利用率,與腸黏膜修復、免疫發育及腸道微生態平衡等密切相關(Turnbaugh and Gordon,2010;馬燕等,2019),擁有與碳水化合物利用降解和轉運有關的多糖利用基因座,可根據碳水化合物種類和濃度的不同,針對性合成利用底物的碳水化合物活性酶(Turnbaugh et al.,2006;金磊等,2019)。本研究中,代乳粉中添加有葡萄糖等碳水化合物,可能是導致杜藏乳仔豬腸道菌群結構改變的原因之一。芽孢桿菌能產生大量脂肽類物質,而抑制病原菌生長及促進營養物質消化吸收(曹護群等,2019;王曉丹等,2019)。在ZY組代乳粉中添加含有芽孢桿菌和乳桿菌的復合益生菌,對杜藏乳仔豬的生長發育具有潛在促進作用,結果顯示其糞便樣品中芽孢桿菌和乳桿菌的相對豐度略有提高,但差異不顯著,說明低劑量的益生菌并不能顯著提高添加益生菌的相對豐度,也進一步證實飼糧才是影響腸道微生物的主要因素。厚壁菌門/擬桿菌門比值與熱量和脂肪的貯存呈正相關(Turnbaugh et al.,2006;金磊等,2019),同時調控脂代謝相關基因表達與脂肪貯存和代謝,如厭氧弧菌屬、普雷沃氏菌屬與肌肉中甘油三酯的含量呈顯著負相關(嚴鴻林,2018)。本研究結果顯示,代乳粉中添加復合益生菌后能降低厚壁菌門/擬桿菌門比值,同時提高擬桿菌門、厭氧弧菌屬和普雷沃氏菌屬的相對豐度,說明添加復合益生菌具有提高碳水化合物、類固醇膽汁酸等營養物質利用率相關菌群相對豐度的趨勢,進而提高與腸道黏膜修復和降低機體脂肪貯存的潛能。厚壁菌門和擬桿菌門是杜藏乳仔豬腸道內最主要的微生物,與王一冰等(2013)、劉晗璐等(2019)的研究結果一致。本研究觀察到ZD組和ZY組杜藏乳仔豬腸道的優勢菌為擬桿菌門和普氏菌屬,ZM組為厚壁菌門和乳桿菌屬,究其原因可能與飼糧的成分不同有關(王一冰等,2013;李永洙等,2019;劉晗璐等,2019;劉萍等,2019),腸道菌群結構的改變有利于仔豬適應斷奶帶來的飼糧成分變化及腸道黏膜損傷等應激反應。普氏菌屬是腸道內的優勢菌,其相對豐度與飼糧中的纖維含量呈正比,與飼糧中蛋白和脂肪的含量呈反比(姜濤等,2019),與碳水化合物的代謝密切相關(Murtaza et al.,2019)。說明分離于成年豬消化道的復合益生菌具有提高杜藏乳仔豬體內與碳水化合物代謝相關菌群的潛在功能,同時菌群結構發生改變,脂肪沉積型菌群的相對豐度降低,而促進斷奶仔豬腸道內的菌群結構由哺乳型向碳水化合物等營養代謝型轉變。說明復合益生菌的添加原則應以提高腸道微生物穩定性、多樣性及環境適應性為前提,而不是以提高添加菌的相對豐度為目標。
短鏈脂肪酸是食物在機體腸道內被微生物發酵而獲得的一種物質,其合成主要與布勞特氏菌、梭菌屬、瘤胃球菌屬、糞桿菌屬、罕見小球菌屬、羅斯氏菌屬、糞球菌屬、毛螺菌科、普氏棲糞菌屬、擬桿菌屬及考拉桿菌屬相關(Zhang et al.,2015;Kang et al.,2018)。短鏈脂肪酸能增強腸黏膜免疫屏障作用,而降低腸道炎癥反應,與動物的健康有密切聯系。布勞特氏菌屬是一種具有潛在保護作用的菌群,與腸道健康相關(Bai et al.,2018),能抵御碳青霉烯耐藥型銅綠假單胞菌等致病菌的入侵(Pettigrew et al.,2019)。本研究結果顯示,添加復合益生菌后,ZY組的毛螺菌科、罕見小球菌屬和布勞特氏菌屬相對豐度顯著提高,表明復合益生菌能有效提高與短鏈脂肪酸產生有關及具有潛在抗菌作用菌群的相對豐度,對動物體抵抗腸道炎癥具有潛在功能,但其作用機理尚需進一步探究。
復合益生菌的作用并非完全依靠其數量的增加,還需通過調節腸道內微生物菌群的種類和數量,才能發揮益生作用。本研究通過對比ZD組和ZY組發現,在代乳粉中添加益生菌后,杜藏乳仔豬糞便中的菌群多樣性升高,尤其是與碳水化合物等代謝和短鏈脂肪酸產生相關的菌群(擬桿菌門、普雷沃氏菌屬、罕見小球菌屬、布勞特氏菌屬、厭氧弧菌屬及毛螺菌科等)相對豐度顯著升高,說明添加分離自同種源成年豬消化道的益生菌具有潛在促進營養物質代謝和抗腸道炎癥的功能,故推測復合益生菌的添加可縮短消化道微生物區系由哺乳型向飼料型的轉變歷程。
4 結論
在杜藏超早期斷奶仔豬代乳粉中添加復合益生菌能有效提高其腸道菌群結構多樣性,同時提高與碳水化合物代謝和產短鏈脂肪酸相關菌群的相對豐度,即復合益生菌具有潛在促進營養物質代謝和抗炎癥的功能,可縮短消化道微生物區系由哺乳型向飼料型的轉變歷程。
參考文獻:
曹護群,賀濛初,舒迎霜,夏曉東,馮士彬,李玉,王希春,吳金節. 2019. 犬源復合益生菌對脾氣虛犬盲腸菌群的影響[J]. 動物營養學報,31(8):3810-3820. doi:10.3969/j.issn. 1006-267x.2019.08.043. [Cao H Q,He M C,Shu Y S,Xia X D,Feng S B,Li Y,Wang X C,Wu J J. 2019. Effects of canine derived compound probiotics on cecal microflora in splenic qi asthenia canines[J]. Chinese Journal of Animal Nutrition,31(8):3810-3820.]
馮程程,白凱文,王安諳,葛曉可,張莉莉,王恬. 2019. 日糧添加二甲基甘氨酸鈉對宮內發育遲緩斷奶仔豬肝臟抗氧化能力及免疫指標的影響[J]. 南京農業大學學報,42(2):336-344. doi:10.7685/jnau.201806011. [Feng C C,Bai K W,Wang A A,Ge X K,Zhang L L,Wang T. 2019. Effect of N,N-dimethylglycine sodium salt supplementation on hepatic antioxidant capacity and immune indices in intrauterine growth retardation weanling piglets[J]. Journal of Nanjing Agricultural University,42(2):336-344.]
關嘉琦,李柏良,焦雯姝,李慧臻,岳瑩雪,李娜,史佳鷺,趙莉,霍貴成. 2020. 益生菌對促進腸道發育作用的研究進展[J]. 食品科學,41(21):278-285. doi:10.7506/spkx1002- 6630-20191015-132. [Guan J Q,Li B L,Jiao W S,Li H Z,Yue Y X,Li N,Shi J L,Zhao L,Huo G C. 2020. Recent advances in understanding the role of probiotics in promoting intestinal development[J]. Food Science,41(21):278-285.]
姜濤,王雯磊,張明和,張軍. 2019. 普雷沃菌和飲食及糖代謝關系的研究進展[J]. 中華糖尿病雜志,11(4):296-298. doi:10.3760/cma.j.issn.1674-5809.2019.04.015. [Jiang T,Wang W L,Zhang M H,Zhang J. 2019. Research pro-gress of the relationship between Prevotella copri,diet and glucose metabolism[J]. Chinese Journal of Diabetes Me-llitus,11(4):296-298.]
金磊,王立志,王之盛,薛白,彭全輝. 2019. 基于高通量測序技術對山羊盲腸細菌多樣性的分析[J]. 微生物學通報,46(6):1423-1433. doi:10.13344/j.microbiol.china.180599. [Jin L,Wang L Z,Wang Z S,Xue B,Peng Q H. 2019. Analysis of cecum bacterial diversity of goat based on Illumina MiSeq sequencing[J]. Microbiology China,46(6):1423-1433.]
李威,李佳熙,李吉平,呂寶玲,張銀龍. 2020. 我國不同環境介質中的抗生素污染特征研究進展[J]. 南京林業大學學報(自然科學版),44(1):205-214. doi:10.3969/j.issn. 1000-2006.201902002. [Li W,Li J X,Li J P,Lü B L,Zhang Y L. 2020. Pollution characteristics of antibiotics in different environment media in China:A review[J]. Journal of Nanjing Forestry University(Natural Science Edition),44(1):205-214.]
李昕,曾潔,王岱,薛云新,趙西林. 2020. 細菌耐藥耐受性機制的最新研究進展[J]. 中國抗生素雜志,45(2):113-121. doi:10.13461/j.cnki.cja.006866. [Li X,Zeng J,Wang D,Xue Y X,Zhao X L. 2020. Recent advances in the mecha-nism of bacterial resistance and tolerance[J]. Chinese Journal of Antibiotics,45(2):113-121.]
李雪莉,王超,虞德夫,丁立人,朱偉云,杭蘇琴. 2017. 微生態制劑對斷奶仔豬生長性能、器官重及其胃腸道發育的影響[J]. 草業學報,26(8):192-199. doi:10.11686/cyxb2016438. [Li X L,Wang C,Yu D F,Ding L R,Zhu W Y,Hang S Q. 2017. Effects of probiotics on the growth performance,organ relative weight,and intestine development of weaned piglets[J]. Acta Prataculturae Sinica,26(8):192-199.]
李永洙,金太花,韓照清,夏春峰,晁洪雨,張乃鋒,王世琴,刁其玉. 2018. 代乳粉對早期斷奶沂蒙黑山羊羔小腸發育、菌群多樣性及葡萄糖轉運載體基因表達的影響[J]. 中國農業科學,51(11):2193-2205. doi:10.3864/j.issn.0578-1752.2018.11.016. [Li Y Z,Jin T H,Han Z Q,Xia C F,Chao H Y,Zhang N F,Wang S Q,Diao Q Y. 2018. Effects of the milk replacer on the development of intestine,the flora diversity and the relative expression of glucose transporter gene of early weaned Yimeng black goat lambs[J]. Scientia Agricultura Sinica,51(11):2193-2205.]
李永洙,韓照清,金太花,夏春峰,晁洪雨,張乃鋒,王世琴,刁其玉. 2019. 代乳粉對沂蒙黑山羊羔羊早期生長性能及其瘤胃微生物區系的影響[J]. 動物營養學報,31(8):3600-3611. doi:10.3969/j.issn.1006-267x.2019.08.021. [Li Y Z,Han Z Q,Jin T H,Xia C F,Chao H Y,Zhang N F,Wang S Q,Diao Q Y. 2019. Effects of milk replacer on growth performance and rumen microflora in early wea-ning Yimeng black lambs[J]. Chinese Journal of Animal Nutrition,31(8):3600-3611.]
劉晗璐,鐘偉,司華哲,李志鵬,李光玉. 2019. 鮮飼料與混合飼糧對咖啡貂(Mustela iutreola)腸道微生物多樣性的影響[J]. 動物營養學報,31(1):226-235. doi:10.3969/j.issn. 1006-267x.2019.01.028. [Liu H L,Zhong W,Si H Z,Li Z P,Li G Y. 2019. Effects of fresh feed and mixed diets on gut microbiota diversity in European mink(Mustela iutreola)[J]. Chinese Journal of Animal Nutrition,31(1):226-235.]
劉萍,趙金標,耿正穎,王軍軍,劉嶺,王春林,郭娉婷,吳怡,張剛,黃冰冰. 2019. 日糧添加褐藻糖膠對斷奶仔豬抗炎能力和腸道微生物多樣性的影響[J]. 微生物學報,59(4):700-710. [Liu P,Zhao J B,Geng Z Y,Wang J J,Liu L,Wang C L,Guo P T,Wu Y,Zhang G,Huang B B. 2019. Influence of dietary fucoidan on inflammatory response and intestinal microbial diversity in weaned pigs[J]. Acta Microbiology Sinica,59(4):700-710.]
劉韶娜,張斌,相德才,趙智勇,趙彥光. 2019a. 芽孢桿菌B13的分離鑒定及其抑菌作用研究[J]. 中國畜牧獸醫,46(2):573-581. doi:10.16431/j.cnki.1671-7236.2019.02.029. [Liu S N,Zhang B,Xiang D C,Zhao Z Y,Zhao Y G. 2019a. Isolatiog,identification and study of a Bacillus sp. B13 with high antibiotic character[J]. China Animal Husbandry & Veterinary Medicine,46(2):573-581.]
劉韶娜,張斌,相德才,趙智勇,趙彥光. 2019b. 一株植物乳桿菌的分離、鑒定及其特性研究[J]. 飼料研究,(9):73-77. doi:10.13557/j.cnki.issn.1002-2813.2019.09.018. [Liu S N,Zhang B,Xiang D C,Zhao Z Y,Zhao Y G. 2019b. Isola-ting,identification and characteristic research of a Lactobacillus plantarum[J]. Feed Research,(9):73-77.]
馬燕,馬爽,尚春香,格日力. 2019. 低氧暴露對大鼠腸道微生物群落的影響[J]. 微生物學通報,46(1):120-129. doi:10.13344/j.microbiol.china.180306. [Ma Y,Ma S,Shang C X,Ge R L. 2019. Effects of hypoxic exposure on rats? gut microbiota[J]. Microbiology China,46(1):120-129.]
田時祎,王玨,汪晶,朱偉云. 2018. 早期低聚半乳糖干預對哺乳仔豬回腸形態、功能發育相關基因及回腸菌群的影響[J]. 南京農業大學學報,41(5):917-924. doi:10.7685/jnau.201711031. [Tian S Y,Wang J,Wang J,Zhu W Y. 2018. The effect of early intervention with galacto-oligosaccharides on the morphological structure,functional development,and microbial community in ileum of suckling piglets[J]. Journal of Nanjing Agricultural University,41(5):917-924.]
王曉丹,孔祥峰,趙越,章文明,王占彬. 2019. 枯草芽孢桿菌對斷奶仔豬生長性能和血漿生化參數的影響[J]. 動物營養學報,31(2):605-611. doi:10.3969/j.issn.1006-267x. 2019.02.016. [Wang X D,Kong X F,Zhao Y,Zhang W M,Wang Z B. 2019. Effects of Bacillus subtilis on growth performance and plasma biochemical parameters of weaned piglets[J]. Chinese Journal of Animal Nutrition,31(2):605-611.]
王一冰,張小平,黃怡,李衛芬. 2013. 運用454焦磷酸測序技術對斷奶前后仔豬腸道菌群的分析[J]. 動物營養學報, 25(10):2440-2446. doi:10.3969/j.issn.1006-267x.2013.10. 028. [Wang Y B,Zhang X P,Huang Y,Li W F. 2013. Intestinal microflora analysis of pre-and post-weaning piglets using by 454 pyrosequencing technology[J]. Chinese Journal of Animal Nutrition,25(10):2440-2446.]
吳敏,劉作華,齊仁立. 2019. 腸道微生物調控動物肌肉的生長和發育[J]. 動物營養學報,31(9):3976-3982. doi:10. 3969/j.issn.1006-267x.2019.09.008. [Wu M,Liu Z H,Qi R L. 2019. Growth and development of muscle in animals controlled by intestinal microbes[J]. Chinese Journal of Animal Nutrition,31(9):3976-3982.]
夏耀耀,任文凱,黃瑞林,曾本華,魏泓,印遇龍. 2017. 仔豬腸道微生物研究進展[J]. 中國實驗動物學報,25(6):681-688. doi:10.3969/j.issn.1005-4847.2017.06.018. [Xia Y Y,Ren W K,Huang R L,Zeng B H,Wei H,Yin Y L. 2017. Current understanding of the intestinal microbiota of piglets[J]. Acta Laboratorium Animalis Scientia Sinica,25(6):681-688.]
謝全喜,亓秀曄,陳振,于佳民,徐海燕,谷巍. 2017. 復合微生態制劑對斷奶仔豬生長性能、腹瀉率、免疫性能和腸道菌群的影響[J]. 動物營養學報,29(3):850-858. doi:10. 3969/j.issn.1006-267x.2017.03.015. [Xie Q X,Qi X Y,Chen Z,Yu J M,Xu H Y,Gu W. 2017. Effects of compound probiotics on growth performance,diarrhea rate,immunity performance and intestinal microflora of weaned piglets[J]. Chinese Journal of Animal Nutrition,29(3):850-858.]
嚴鴻林. 2018. 腸道微生物及其與營養互作對豬骨骼肌表型及代謝的調控[D]. 雅安:四川農業大學. [Yan H L. 2018. Regulation of porcine skeletal muscle-phenotyoes and metabolism by gut microbita and its interaction with nutition[D]. Yaan:Sichuan Agriculture University.]
楊虹,謝明寶,于輝,農興新,楊文豪,楊映. 2019. 2種微生態制劑對烏鱧生長性能、腸道形態及免疫功能的影響[J]. 河南農業科學,48(1):134-140. doi:10.15933/j.cnki. 1004-3268.2019.01.022. [Yang H,Xie M B,Yu H,Nong X X,Yang W H,Yang Y. 2019. Effects of microecological preparations on growth performance,intestinal morphology and immune function of Channa argus[J]. Journal of Henan Agricultural Sciences,48(1):134-140.]
詹明曄,付小花,張姝,張鑫,楊海迪,俞錦華,王愛善,王磊. 2019. 不同地區成體大熊貓腸道微生物結構差異性及其與纖維素消化能力的相關性[J]. 應用與環境生物學報,25(3):736-742. doi:10.19675/j.cnki.1006-687x.2018.080-12. [Zhan M Y,Fu X H,Zhang S,Zhang X,Yang H D,Yu J H,Wang A S,Wang L. 2019. Differences of the intestinal microbial structure of adult giant panda in diffe-rent regions and its correlation with the digestibility of cellulose[J]. Chinese Journal of Applied & Environmental Biology,25(3):736-742.]
Ahmadi S,Wang S H,Nagpal R,Wang B,Jain S L,Razazan A,Mishra S P,Zhu X W,Wang Z,Kavanagh K,Yadav H. 2020. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating microbiota/taurine/tight junction axis[J]. JCI Insight,5(9):e132055. doi:10.1172/jci.insight.132055.
Bai J,Zhu Y,Dong Y. 2018. Modulation of gut microbiota and gut-generated metabolites by bitter melon results in improvement in the metabolic status in high fat diet-induced obese rats[J]. Journal of Functional Foods,41:127-134. doi:10.1016/j.jff.2017.12.050.
Claesson M J,Jeffery I B,Conde S,Power S E,O?Connor E M,Cusack S,Harris H M B,Coakley M,Lakshmina-rayanan B,OSullivan O,Fitzgerald G F,Deane J,OConnor M,Harnedy N,OConnor K,OMahony D,van Sinderen D,Wallace M,Brennan L,Stanton C,Marchesi J R,Fitzgerald A P,Shanahan F,Hill C,Ross R P,O?Toole P W. 2012. Gut microbiota composition correlates with diet and health in the elderly[J]. Nature,488(7410):178-184. doi:10.1038/nature11319.
Davis H C. 2017. Can the gastrointestinal microbiota be modu-lated by dietary fibre to treat obesity?[J]. Irish Journal of Medical Science,187(1):393-402. doi:10.1007/s11845-017-1686-9.
Derosa L,Routy B,Fidelle M,Iebba V,Alla L,Pasolli E,Segata N,Desnoyer A,Pietrantonio F,Ferrere G,Fahrner J E,Le Chatellier E,Pons N,Galleron N,Roume H,Duong C P M,Mondragón L,Iribarren K,Bonvalet M,Terrisse S,Rauber C,Goubet A G,Daillère R,Lemaitre F,Reni A,Casu B,Alou M T,Silva C A C,Raoult D,Fizazi K,Escudier B,Kroemer G,Albiges L,Zitvogel L. 2020. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients[J]. European Urology,78(2):195-206. doi:10.1016/j.eururo.04. 044.
Gudi R,Perez N,Johnson B M,Sofi M H,Brown R,Quan S H,Karumuthil-Melethil S,Vasu C. 2019. Complex die-tary polysaccharide modulates gut immune function and microbiota,and promotes protection from autoimmune dia-betes[J]. Immunology,157(1):70-85. doi:10.1111/imm. 13048.
He B K,Hoang T K,Tian X J,Taylor C M,Blanchard E,Luo M,Bhattacharjee M B,Freeborn J,Park S,Couturier J,Lindsey J W,Tran D Q,Rhoads J M,Liu Y Y. 2019. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota[J]. Frontiers in Immunology,10:385. doi:10. 3389/fimmu.2019.00385.
He Y Y,Mao C X,Wen H,Chen Z Y,Lai T,Li L Y,Lu W,Wu H D. 2017. Influence of ad libitum feeding of piglets with Bacillus subtilis fermented Liquid Feed on gut flora,luminal contents and health[J]. Scientific Reports,7:44553. doi:10.1038/srep44553.
Hou Q C,Zhao F Y,Liu W J,Lü R R,Khine W W T,Han J,Sun Z H,Lee Y K,Zhang H P. 2020. Probiotic-directed modulation of gut microbiota is basal microbiome dependent[J]. Gut Microbes,12(1):1736974. doi:10.1080/19490976.2020.1736974.
Hu P,Zhao F Z,Zhu W Y,Wang J. 2019. Effects of early-life lactoferrin intervention on growth performance,small intestinal function and gut microbiota in suckling piglets[J]. Food & Function,10(9):5361-5373. doi:10.1039/c9fo00676a.
Kang Y F,Guan G,Zhang S M,Ross C F,Zhu M J. 2018. Goji berry modulates gut microbiota and alleviates colitis in IL-10-deficient mice[J]. Molecular Nutrition & Food Research,62(22):e1800535. doi:10.1002/mnfr.201800 535.
Kim H B,Isaacson R E. 2015. The pig gut microbial diversity:Understanding the pig gut microbial ecology through the next generation high throughput sequencing[J]. Vete-rinary Microbiology,177(3-4):242-251. doi:10.1016/j.vetmic.2015.03.014.
Latham E A,Pinchak W E,Trachsel J,Allen H K,Callaway T R,Nisbet D J,Anderson R C. 2018. Isolation,characte-rization and strain selection of a Paenibacillus species for use as a probiotic to aid in ruminal methane mitigation,nitrate/nitrite detoxification and food safety[J]. Bioresource Technology,263:358-364. doi:10.1016/j.biortech. 2018.04.116.
Murtaza N,Burke L M,Vlahovich N,Charlesson B,O'Neill H,Ross M L,Campbell K L,Krause L,Morrison M. 2019. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers[J]. Nutrients,11(2):261. doi:10.3390/nu11020261.
Pamer E G. 2016. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens[J]. Sience,352(6285):535-538. doi:10.1126/science.aad9382.
Patil Y,Gooneratne R,Ju X H. 2019. Interactions between host and gut microbiota in domestic pigs:A review[J]. Gut Microbes,11(3):310-334. doi:10.1080/19490976. 2019.1690363.
Pettigrew M M,Gent J F,Kong Y,Halpin A L,Pineles L,Harris A D,Johnson J K. 2019. Gastrointestinal microbiota disruption and risk of colonization with carbapenem-resistant Pseudomonas aeruginosa in intensive care unit patients[J]. Clinical Infectious Diseases,69(4):604-613. doi:10.1093/cid/ciy936.
Piuske J R,Turpin D L,Kim J C. 2018. Gastrointestinal tract (gut) health in the young pig[J]. Animal Nutrition,4(2):187-196. doi:10.1016/j.aninu.2017.12.004.
Singer J R,Blosser E G,Zindl C L,Silberger D J,Conlan S,Laufer V A,DiToro D,Deming C,Kumar R,Morrow C D,Segre J A,Gray M J,Randolph D A,Weaver C T. 2019. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis[J]. Nature medicine,25(11):1772-1782. doi:10.1038/s41591-019-0640-y.
Sun J,Liao X P,D'Souza A W,Boolchandani M,Li S H,Cheng K,Martínez J L,Li L,Feng Y J,Fang L X,Huang T,Xia J,Yu Y,Zhou Y F,Sun Y X,Deng X B,Zeng Z L,Jiang H X,Fang B H,Tang Y Z,Lian X L,Zhang R M,Fang Z W,Yan Q L,Dantas G,Liu Y H. 2020. Environmental remodeling of human gut microbiota and antibiotic resistome in livestock farms[J]. Nature Communications,11(1):1427. doi:10.1038/s41467-020-15222-y.
Teichman E M,O'Riordan K J,Gahan C G M,Dinan T G,Cryan J F. 2020. When rhythms meet the blues:Circa-dian interactions with the microbiota-gut-brain axis[J]. Cell Metabolism,31(3):448-471. doi:10.1016/j.cmet.2020. 02.008.
Tian S Y,Wang J,Yu H,Wang J,Zhu W Y. 2018. Effects of galacto-oligosaccharides on growth and gut function of newborn suckling piglets[J]. Journal of Animal Science and Biotechnology,9:75. doi:10.1186/s40104-018-0290-9.
Toscano M,Grandi R D,Stronati L,de Vecchi E,Drago L. 2017. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level:A preliminary study[J]. World Journal of Gastroenterology,23(15):2696-2704. doi:10.3748/wjg.v23.i15.2696.
Turnbaugh P J,Gordon J I. 2010. The core gut microbiome,energy balance and obesity[J]. The Journal of Physiology,587(17):4153-4158. doi:10.1113/jphysiol.2009.174136.
Turnbaugh P J,Ley R E,Mahowald M A,Magrini V,Mardis E R,Gordon J I. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest[J]. Nature,444(7122):1027-1031. doi:10.1038/nature05414.
Zhang J C,Guo Z,Xue Z S,Sun Z H,Zhang M H,Wang L F,Wang G Y,Wang F,Xu J,Cao H F,Xu H Y,Lü Q,Zhong Z,Chen Y F,Qimuge S,Menghe B,Zheng Y,Zhao L P,Chen W,Zhang H P. 2015. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles,geography and ethnicities[J]. The ISME Journal,9(9):1979-1990. doi:10.1038/ismej.2015.11.
Zhang M L,Li M,Sheng Y,Tan F,Chen L Q,Cann I,Du Z Y,Rawls J F. 2020. Citrobacter species increase energy harvest by modulating intestinal microbiota in fish:Nondominant species play important functions[J]. mSystems,5(3):e00303. doi:10.1128/mSystems.00303-20.
Zhao W J,Wang Y P,Liu S Y,Huang J J,Zhai Z X,He C,Ding J M,Wang J,Wang H J,Fan W B,Zhao J G,Meng H. 2015. The dynamic distribution of porcine microbiotaacross different ages and gastrointestinal tract segments[J]. PLoS One,10(2):e0117441. doi:10.1371/journal.pone.0117441.
Zheng D W,Pan P,Chen K W,Fan J X,Li C X,Cheng H,Zhang X Z. 2020. An orally delivered microbial cocktail for the removal of nitrogenous metabolic waste in animal models of kidney failure[J]. Nature Biomedical Enginee-ring,4:852-862. doi:10.1038/s41551-020-0582-1.
(責任編輯 蘭宗寶)

