Histopathology and immunophenotyping of late onset cutaneous manifestations of COVID-19 in elderly patients: Three case reports
Maria Mazzitelli, Stefano Dastoli, Chiara Mignogna, Luigi Bennardo, Elena Lio, Maria Chiara Pelle, Enrico Maria Trecarichi, Branca Isabel Pereira, Steven Paul Nisticò, Carlo Torti
Maria Mazzitelli, Elena Lio, Enrico Maria Trecarichi, Carlo Torti, Department of Medical and Surgical Sciences, Infectious and Tropical Disease Unit, Magna Graecia University, Catanzaro 88100, Italy
Stefano Dastoli, Steven Paul Nisticò, Department of Health Sciences, Magna Graecia Università of Catanzaro, Catanzaro 88100, Italy
Chiara Mignogna, Interdipartimental Service Center, Pathology Unit, Pugliese Ciaccio Hospital,Catanzaro 88100, Italy
Luigi Bennardo, Department of Health Sciences, Magna Graecia University, Catanzaro 88100,Italy
Maria Chiara Pelle, Department of Medical and Surgical Sciences, Magna Graecia Università of Catanzaro, Catanzaro 88100, Italy
Branca Isabel Pereira, HIV/GUM Directorate, Chelsea and Westminster Hospital Foundation Trust, London SW109NH, United Kingdom
Abstract BACKGROUND Several cutaneous manifestations such as urticarial rash, erythematous patches and chilblain-like lesions have been described in young adults with coronavirus disease 2019 (COVID-19) and are present in up to 20% patients, but few reports exist describing histopathological and immunophenotypic characteristics of dermatological lesions in older patients. Our aim was to characterize skin lesions in elderly patients during late stages of COVID-19 from clinical, histological and immunophenotypic perspectives.CASE SUMMARY Three patients, admitted for COVID-19, and who developed cutaneous manifestations underwent skin biopsies. Immunophenotypic analysis for CD20, CD3, CD4 and CD8 was performed on skin biopsies to assess immune cell infiltrates. CD1a was used as a marker of Langerhans cells, and CD31 as a marker of endothelial cells. In the three study patients, cutaneous manifestations were evident in the late-stage of COVID-19 (mean time from the first positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) swab to rash onset was 35 d). Skin biopsies showed a similar pattern of T lymphocyte infiltration in all patients.Indeed, a chronic dermatitis with perivascular lymphocytic infiltrate was observed with predominance of CD3+ T-cell (CD3+).CONCLUSION Our study confirms previous reports. Histological and immunophenotypic patterns in our patients confirm results described in the two previous reported experiences. This pattern is similar to what is found in some lympho-proliferative disorders. Therefore, since these findings are non-specific, SARS-CoV-2 infection should be suspected.
Key Words: COVID-19; SARS-CoV-2; Rash; T-helper infiltrates; Cutaneous manifestation; Case report
INTRODUCTION
Several cutaneous manifestations have been described in the context of coronavirus disease 2019 (COVID-19)[1-5]. An Italian study of 88 hospitalized patients with COVID-19 first reported the occurrence of skin lesions in up to 20% patients with COVID-19 in the form of erythematous rash, widespread urticaria and chickenpox-like vesicles[3]. A larger study in Spain described five major clinical patterns of cutaneous manifestations of COVID-19 with the following prevalence: Erythema with vesicles or pustules (pseudo-chilblain) (19%), other vesicular eruptions (9%), urticarial lesions(19%), maculopapular eruptions (47%) and livedo or necrosis (6%)[6]. Since then,several other studies reported skin lesions in the context of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with prevalence ranging from 0.19%to 20.45%, most of them present at diagnosis of COVID-19[7]. The pathogenesis of such skin manifestations in the context of SARS-CoV-2 infection remains unclear. As described for other diseases, urticarial rashes are related with alteration of local T-cell homeostasis[8,9]. However, only a few studies characterized the histopathology of skin lesions associated with COVID-19[10-13]. Skin lesions were characterized by a superficial perivascular lymphocytic infiltrate. To our knowledge, this is one of the first case series describing both the histopathology and immunophenotyping of COVID-19-related skin lesions with late onset.
Our objective was to describe the histology and immunophenotyping of late-onset cutaneous manifestations in patients diagnosed with COVID-19.
CASE PRESENTATION
Chief complaints
We report herein three cases of late onset COVID-19-related skin lesions, who were admitted to Infectious and Tropical Disease Unit for COVID-19.
History of present illness
Three elderly patients (Cases 1, 2, and 3) were diagnosed with COVID-19 on March 25,2020, and all received hydroxychloroquine and azithromycin (HCQ/AZI)[14].Skin rashes occurred late in all three patients. Skin biopsies were performed for all patients on May 5, 2020 after patient consent and per clinical indication of a Consultant Dermatologist. Patients gave written consent for publication. Data was retrospectively collected after approval from our Ethics Committee. Immunohistochemical staining of serial sections of formalin-fixed and paraffin embedded skin was performed using a standard automatized protocol with the Autostainer DAKO 48. Antibodies for CD20,CD3, CD4 and CD8 were used to assess immune cell infiltrates. CD1a was used as a marker of Langerhans cells, and CD31 as a marker of endothelial cells. For each patient, follow-up real time polymerase chain reaction (PCR) for SARS-CoV-2 were performed on nasopharyngeal swabs in order to evaluate viral shedding from respiratory samples. A positive PCR result was defined by the detection of one out of three target genes of SARS-CoV-2 and considering number of cycle threshold (CT).
Case 1:Patient 1 was admitted on March 31, 2020 and prescribed HCQ/AZI, methylprednisolone, and enoxaparin at prophylactic dosing (all stopped after 10 d, but enoxaparin, which was continued for 14 d for the abed status). Thirty-eight days after the first positive swab, an erythematous and itchy skin rash appeared in the submammary region (Figure 1A and B), rapidly extending to the trunk and the upper limbs, and spontaneously healing on May 11, 2020.
Case 2:Patient 2 was admitted on April 2, with severe COVID-19 pneumonia and was prescribed oxygen support, HCQ/AZI, piperacillin/tazobactam, methylprednisolone,and enoxaparin (all stopped within 10 d except enoxaparin, which was stopped after 20 d). Twenty-eight days after the first positive swab for SARS-CoV-2, an itchy and urticarial rash appeared on the chest and arms (Figure 1C and D), with mild increase in temperature (37.5 °C) and eosinophil count (from 50/μL to 550/μL). The rash extended to the face, chest, abdomen, wrists, and thighs, and spontaneously healed after 10 d.
Case 3:Patient 3 was admitted on April 2 with severe COVID-19 pneumonia, and prescribed oxygen support, HCQ/AZI, methylprednisolone, and enoxaparin. All drugs, except enoxaparin were stopped within 10 d. Thirty-eight days after the first positive swab for SARS-CoV-2, patient developed an itchy and erythematous rash on both the lower limbs (Figure 1E and F), worsening in the two following days,involving the trunk and the upper limbs, with spontaneous healing on May 16, 2020.
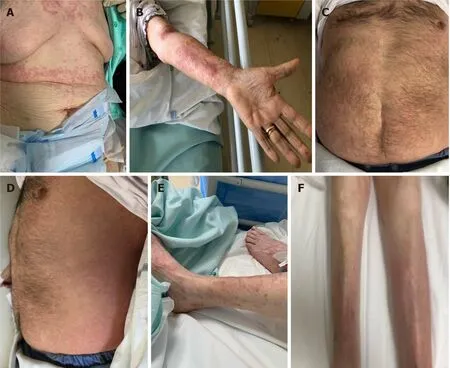
Figure 1 Clinical manifestations in coronavirus disease 2019 patients. A and B: Thirty-eight days after the first positive swab, an erythematous and itchy skin rash appeared in patient 1 the sub-mammary region, rapidly extending to the trunk and the upper limbs; C and D: Twenty-eight days after the first positive swab for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an itchy and urticarial rash appeared in patient 2 on the chest and arms; E and F: Thirtyeight days after the first positive swab for SARS-CoV-2, patient 3 developed an itchy and erythematous rash on both the lower limbs.
History of past illness
Case 1:An 89-year-old woman (patient 1) with a clinical history of hypertension,osteoporosis, chronic cerebral vasculopathy and on treatment with pantoprazole and allopurinol.
Case 2:A 65-year-old man (patient 2) with cognitive impairment and chronic psychosis on treatment with olanzapine.
Case 3:An 83-year-old female (patient 3) with hypertension, diabetes, osteoporosis,depression and cognitive impairment. She was on regular treatment with lansoprazole, tapentadol, ramipril and sertraline.
Personal and family history
No further details are available aside from those mentioned in the history of past illness section above.
Physical examination
At admission, skin lesions were not detected in all three patients.
Laboratory examinations
Nothing remarkable was found in blood tests for all three patients; however, mild leukopenia, increased C-reactive protein, ferritin and interleukin 6 levels were detected.
Figure 2 depicts the patient clinical course and results of PCR for SARS-CoV-2. For patient 1 the nasopharyngeal swab performed on May 5, 2020 came back as positive only for N gene (33 CT). For patient 2, the nasopharyngeal swab performed 2 d before the rash onset (April 22, 2020) came back as positive only for N gene (36 CT). For patient 3, PCR for SARS-CoV-2 on nasopharyngeal swab performed a week before the rash onset came back as negative, while the one performed a week later (May 16, 2020,the same day of rash healing) was positive only for the N gene (37 CT).
FINAL DIAGNOSIS
A similar histological pattern was found in all patients with a chronic dermatitis with perivascular lymphocytic infiltrate. The immunohistochemical analysis (Figure 3)showed dermatitis with a perivascular lymphocytic infiltrate (Figure 3A and B) in all three cases. There was a predominance of T-cell populations (CD3+) (Figure 3C), while B-cell infiltrates were scarce (Figure 3D), with a prevalence of CD4+ cells (T-helper lymphocytes) and few CD8+ cells (Figure 3E and F). Epidermotropism was visible in all cases (Figure 3G), with CD4+ cells predominance. CD31 staining showed small vessel damage with fibrinoid necrosis of the walls (N) whereas CD1a staining highlighted the presence of Langerhans cells in epidermidis and in perivascular infiltrates (Figure 3S and T).
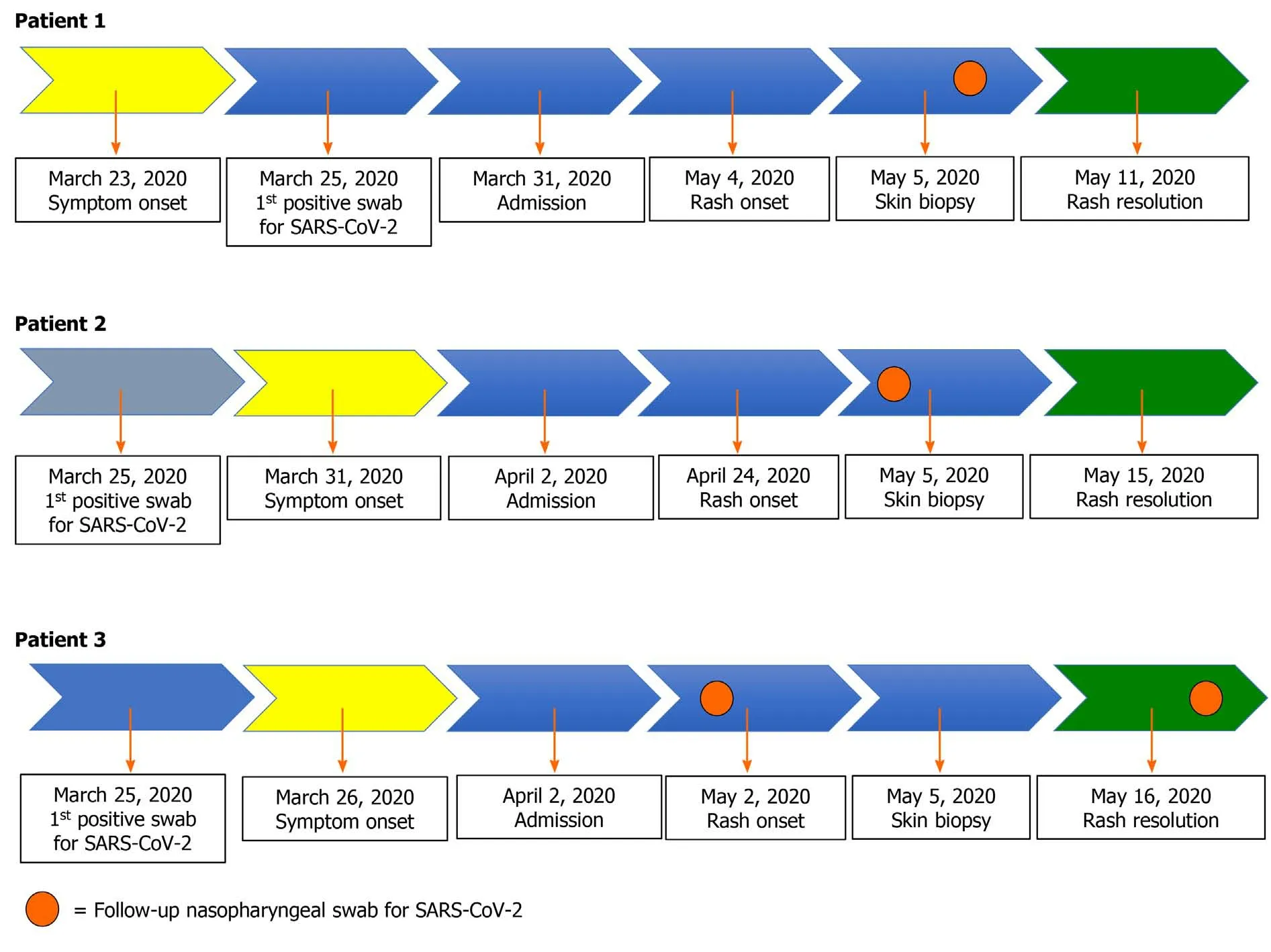
Figure 2 Clinical course for each patient. For patient 1 the polymerase chain reaction (PCR) for severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) on nasopharyngeal swab performed on May 5 came back as positive for the N gene (33 cycle threshold). Similarly, for patient 2, the nasopharyngeal swab performed 2 d before the rash onset (April 22, 2020) also came back as positive for the N gene (36 cycle threshold). For patient 3, PCR for SARS-CoV-2 on nasopharyngeal swab performed a week before the rash onset was negative, while the one performed a week later (May 16, 2020, the same day of rash healing) was positive only for the N gene (37 cycle threshold).
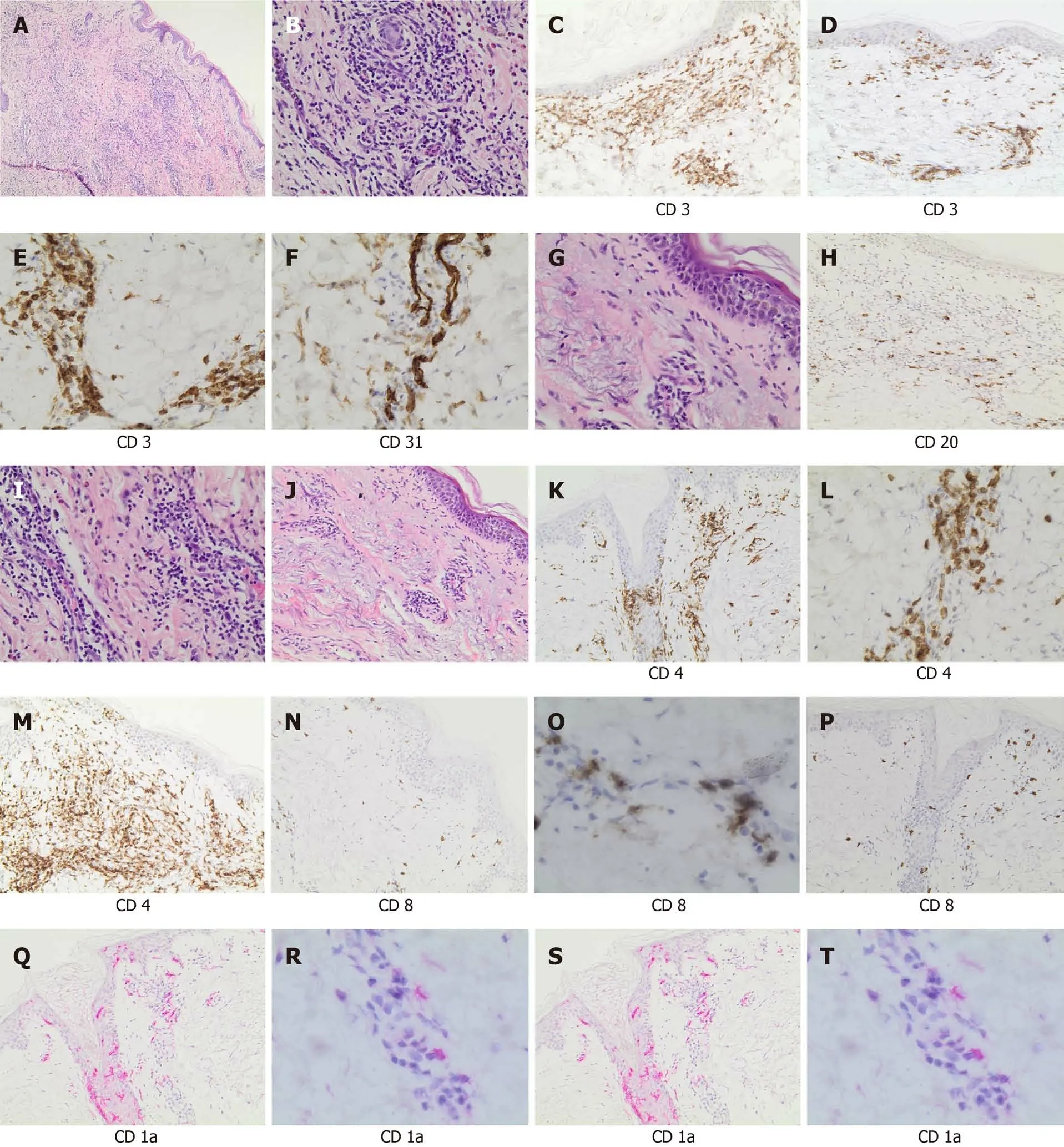
Figure 3 Histological section and immunophenotyping. A: Panoramic view of cutaneous lesion showed chronic dermatitis with perivascular lymphocytic infiltrate (hematoxylin and eosin staining, × 40 magnification); B: A detail of perivascular infiltrate (× 200 magnification); C: CD3 staining highlighted the diffuse T-cell infiltrate (× 200 magnification); D: Rare B cell lymphocytes (CD20+) were present (× 200 magnification); E: CD4 staining showed the prevalence of the T-helper lymphocytes (× 200 magnification); F: Only rare CD8 positive lymphocytes were observed (× 200 magnification); G: Epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) (hematoxylin and eosin staining, × 200 magnification); H: CD3 staining highlighted the presence of T cell infiltrate, perivascular and intra-epidermal (× 100 magnification); I: CD4 staining was prevalent and demonstrated the presence of T-helper lymphoid cells in the epidermidis (× 100 magnification); L: CD8 staining evidentiated only rare cytotoxic (× 100 magnification); M: Morphologic evaluation demonstrated vascular proliferation associated to vasculitic phenomena (hematoxylin and eosin staining, × 200 magnification); N: CD31 staining highlighted vascular component (× 200 magnification); O: CD3 staining showed T cell vascular/perivascular infiltrate (× 200 magnification); P: CD4 staining indicated the prevalence of T-helper lymphoid cells in perivascular and vascular component (× 200 magnification); Q: CD8 staining indicated only rare cytotoxic (× 100 magnification); R: Morphologic evaluation showed a chronic dermatitis with perivascular infiltrate (hematoxylin and eosin staining, × 40 magnification); S: CD1a staining highlighted Langerhans cell intra-epidermal component (× 100 magnification); T: CD1a staining showed the presence of Langerhans cell in vascular/perivascular infiltrate (× 200 magnification).
TREATMENT
No specific treatment was prescribed for skin rashes.
OUTCOME AND FOLLOW-UP
Skin rashes spontaneously healed in all three patients.
DISCUSSION
Our case series describes the histology and immunophenotype of late onset skin rashes in three elderly patients diagnosed with COVID-19. In spite of previously reported data, we observed skin rashes occurring in older patients well after the resolution of lung disease[2]. Our experience is similar to the one reported by Reymundoet al[12]who described clinical and histological characterization of late appearance maculopapular eruptions in 4 patients with COVID-19, but he did not temporally correlate cutaneous manifestations with SARS-CoV-2 shedding.
In two cases in the present study, there was a temporal correlation with viral shedding, demonstrated by the detection of SARS-CoV-2 genome at nasopharyngeal swab after many CT and with the expression of tardive target gene (N). For patient 3,RT PCR for SARS-CoV-2 was found positive 1 wk later (May 16, 2020) on the same day of rash healing[15]. So, it is possible that the appearance of late-onset cutaneous urticarial/erythema multiforme-like rashes are not directly related to the presence of the virus, but it may be due to a delayed immune response to COVID-19, as already hypothesized[12]. We observed a perivascular lymphocytic dermatitis with a cell infiltrate typical of other cutaneous diseases, such as cutaneous lymphomas, pseudolymphomas, or autoimmune condition[16,17]. The finding of a lymphocytic T-helper infiltrate detected when viral clearance is ongoing or has already occurred needs to be confirmed and studied in larger studies. Also, in our cases, a vascular proliferation associated to vasculopathic/vasculitic phenomena was observed in line with other data[11]. SARS-CoV-2 rapidly travels through the vascular system but clinical and physiopathological significance of this finding remains to be elucidated[18,19].
Unfortunately, this study is somewhat limited by the small number of patients.Moreover, we did not perform PCR for SARS-CoV-2 on skin samples.
CONCLUSION
Increased up-taking of skin biopsies with immunohistochemical analysis could improve our understanding of the clinical presentations and pathophysiology of cutaneous manifestations of COVID-19. Whether rashes associated with COVID-19 can simulate those occurring in lympho-proliferative disorders remains to be clarified.
ACKNOWLEDGEMENTS
We first want to thank our patients who accepted to undergo a skin biopsy, and our nurses. We also thank the Infectious Diseases and Tropical Medicine (IDTM) of the University “Magna Graecia” (UMG) COVID-19 Group, which is composed, besides the main authors, by the following: Arrighi E, Barreca GS, Biamonte F, Bertucci B,Bruni A, Busceti MT, Cancelliere A, Costanzo FS, Davoli C, De Francesco A, Fusco P,Gallo L, Garofalo E, Giancotti A, Giudice A, Greco G, La Gamba V, Laganà D,Lamberti A, Liberto MC, Lionello R, Longhini F, Marascio N, Matera G, Petullà M,Perri G, Procopio G, Quirino A, Ricchio M, Scaglione V and Tassone B.
 World Journal of Clinical Cases2021年20期
World Journal of Clinical Cases2021年20期
- World Journal of Clinical Cases的其它文章
- Corrigendum to “Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases”
- Role of gastrointestinal system on transmission and pathogenesis of SARS-CoV-2
- COVID-19: Considerations about immune suppression and biologicals at the time of SARS-CoV-2 pandemic
- Multidisciplinary team therapy for left giant adrenocortical carcinoma: A case report
- Hemorrhagic transformation of ischemic cerebral proliferative angiopathy: A case report
- Splenosis masquerading as gastric stromal tumor: A case report
