A comparison of artificial urinary sphincter outcomes after primary implantation and first revision surgery
Kevin J.Heert ,Brin J.Linder ,Griffin T.Morrisson ,Lureno Rngel Ltuche ,Dniel S.Elliott ,*
a Department of Urology,Mayo Clinic,Rochester,MN,USA
b Department of Health Sciences Research,Mayo Clinic,Rochester,MN,USA
KEYWORDS Artificial urinary sphincter;Outcomes;Revision;Urinary incontinence;Urethra;Post prostatectomy incontinence
Abstract Objective: The artificial urinary sphincter (AUS) is the gold standard for severe male stress urinary incontinence,though evaluations of specific predictors for device outcomes are sparse.We sought to compare outcomes between primary and revision AUS surgery for non-infectious failures.Methods: We identified 2045 consecutive AUS surgeries at Mayo Clinic (Rochester,MN,USA)from 1983 to 2013.Of these,1079 were primary AUS implantations and 281 were initial revision surgeries,which comprised our study group.Device survival rates,including overall and specific rates for device infection/erosion,urethral atrophy and mechanical failure,were compared between primary AUS placements versus revision surgeries.Patient follow-up was obtained through office examination,written correspondence,or telephone correspondence.Results: During the study period,1079 (79.3%) patients had a primary AUS placement and 281(20.7%)patients underwent a first revision surgery for mechanical failure or urethral atrophy.Patients undergoing revision surgery were found to have adverse 1-and 5-year AUS device survival on Kaplan—Meier analysis,90%vs.85%and 74%vs.61%,respectively(p<0.001).Specifically,revision surgery was associated with a significantly increased cumulative incidence of explantation for device infection/urethral erosion (4.2% vs. 7.5% at 1 year; p=0.02),with similar rates of repeat surgery for mechanical failure (p=0.43) and urethral atrophy (p=0.77).Conclusions: Our findings suggest a significantly higher rate of overall device failure following revision AUS surgery,which is likely secondary to an increased rate of infection/urethral erosion events.
1.Introduction
Since its introduction in 1972,the artificial urinary sphincter has been considered the most effective treatment of male stress urinary incontinence [1,2].Long-term device reoperation rates have been studied in multiple series with 5-year device survival rates of 74%—79% [3—6].However,there are limited data comparing device outcomes following primary artificial urinary sphincter (AUS)implantations and revision surgeries [5,7,8].
Device survival following revision surgery can be affected by additional periurethral dissections leading to progressive tissue devascularization.However,in the few series available in the current literature,revision AUS surgery has not been associated with an increased risk of device failure when compared to primary implantations[5,7,8].These studies are limited to relatively small series,with limited follow-up.Alternatively,device reimplantation after a previous explantation for infection/erosion has been associated with an increased risk of repeat infection/erosion [8,9].Understanding the anticipated outcomes of revision surgery,in comparison to primary device implantation,is an important consideration for preoperative counseling and the informed consent process since even with adequate counseling patients often have difficulty recalling the preoperative surgical risks discussed by their physicians [10].
Herein,we sought to compare outcomes of primary AUS implantations and first revision AUS surgeries (for noninfectious complications) in a large cohort with long-term follow-up.
2.Patients and methods
We identified 2045 consecutive AUS surgeries at Mayo Clinic(Rochester,MN,USA) from 1983 to 2013.The study period ranged from 1983 to 2015 to allow for adequate follow-up.Of these,1079 were primary AUS implantations and 281 were initial revision surgeries secondary to AUS mechanical failure or urethral atrophy,which comprised the study cohorts.Patients were excluded from the analysis if they underwent AUS placement secondary to neurogenic bladder,were younger than 18 years,had a non-urethral cuff,or declined research consent.The remaining 685 cases(2045 [total]—1079 [Primary AUS implantation]—281 [initial revision surgery]) included patients having device explantation,multiple surgical revisions(i.e.
two or more revision surgeries),and those not meeting inclusion criteria.Three surgeons performed the AUS implantations over the time frame of the study and all implanted AUS devices were American Medical Systems 800 (American Medical Systems,Minnetonka,MN,USA).Work-ups for post-prostatectomy and post-radiotherapy stress urinary incontinence included a detailed history,physical exam(displaying stress urinary incontinence),post void residual,and flexible cystoscopy showing poor coaptation of the external urethral sphincter.Residual prostate volume assessment in patients with a history of primary prostate radiotherapy was not routinely performed if clinical and cystoscopic evidence of stress urinary incontinence was present.For primary AUS placement in males,we use a perineal approach with placement of the urethral cuff around the proximal bulbar urethra.Throughout the timeframe of the study,it has been standard at our institution to preserve the bulbospongiosus muscle during perineal dissection.The cuff is placed around the muscle and not in direct contact with the urethra.Following circumferential dissection of the proximal bulbar urethra between the corpora cavernosum and corpora spongiosum,the appropriate-sized cuff is selected.For revision AUS procedures,management was based on surgeon preference.Our typical approach for evaluating patients with recurrent incontinence after AUS has previously been reported [11].Briefly,for mechanical failure (verified with abdominal Xray given placement of iso-osmotic contrast at the time of AUS placement),the AUS was replaced completely or by components depending on the age of device and intraoperative findings.For cases involving urethral atrophy,the approach was to downsize the cuff,move the cuff to a new location,or place a tandem cuff depending on intraoperative findings.
Individual patient charts were evaluated for pertinent clinical and surgical comorbidities,device details,and device outcomes including etiology of reoperations (i.e.
,explantation for urethral erosion or device infection,revision for device malfunction,urethral atrophy,tubing,or pump complications).Standardized follow-up protocol was not able to be obtained due to the retrospective nature of the study.Typically,patients returned 6 weeks after surgery for device activation and instruction on device usage.Follow-up after activation was performed on an as-needed basis;most follow-up appointments were due to incontinence or other device concerns.Finally,the Mayo Clinic AUS Registry monitors outcomes periodically by correspondence to the patient.Information regarding device survival was acquired from the most recent office visit,operative report,and/or correspondence via mail or telephone.Statistical analysis was performed using the SAS software package (SAS Institute,Cary,NC,USA).Continuous features were summarized with medians and interquartile ranges (IQRs);categorical features were summarized with frequency counts and percentages.Device survival was estimated as time from AUS implantation (or revision) to subsequent repeat surgery (including explantation or device revision for any reason) using the Kaplan-Meier method.For this analysis,patients were censored at the time of last known follow-up if no event occurred.All statistical tests were two-sided,with ap
-value <0.05 considered statistically significant.3.Results
We identified 1360 primary or first revision AUS procedures from 1983 to 2013.Of these,1079 were first time(primary)AUS procedures and 281 were revision procedures secondary to mechanical failure or urethral atrophy.Clinical and demographic features of those undergoing primary implantation or first revision surgery are shown in Table 1,and 18% of patients undergoing primary implantation were included in the revision cohort (i.e.
both surgeries at our institution).Thus statistical analysis of differences in clinical and demographic factors between the cohorts was not possible.Not surprisingly,patients undergoing revision tended to be older (72.5 years oldvs.
70.5 years old) with the most common etiology for incontinence being radical prostatectomy in both groups (81.1%vs.
76.9%).A higher percentage of primary implantation patients had a history of coronary artery disease and prior myocardial infarction.Of the revision cohort,revision surgery was performed for urethral atrophy in 161 cases and for device malfunction in 120 cases.The median follow-up for all patients undergoing AUS surgery was 4.1 (IQR 0.8,7.9) years,during which time there were 281 additional surgeries(195 among those in the primary AUS cohort and 86 among those in the first revision cohort).Follow-up was longer in primary implantations compared to first revision cases (4.4 yearsvs.
2.9 years).Notably,revision surgery was associated with adverse 5-year overall AUS survival compared to primary AUS surgery(61%vs.
74%;p
<0.001)(Fig.1).The median(IQR)time to repeat surgery was not significantly different between primary implantations and revision surgery (4.4 [0.9,8.3]yearsvs.
2.9 [0.5,6.6] years).We next assessed the association of type of surgery with specific device outcomes (infection/erosion,mechanical failure,and urethral atrophy).Here,we found that first revision surgery was associated with a significantly increased cumulative incidence of device infection and urethral erosion (p
=0.02) (Fig.2).Notably,there was no significant difference in the incidence of mechanical failure(p
=0.43) and atrophy (p
=0.77) between the cohorts.Given our findings of adverse device survival identified with revision surgery,we performed univariate analysis to evaluate for factors associated with subsequent device revision after either primary implantation or first revision surgery (Table 2).The only statistically significant factor was myocardial infarction (hazard ratio 1.64,p
=0.04)which was only significant for first time failure.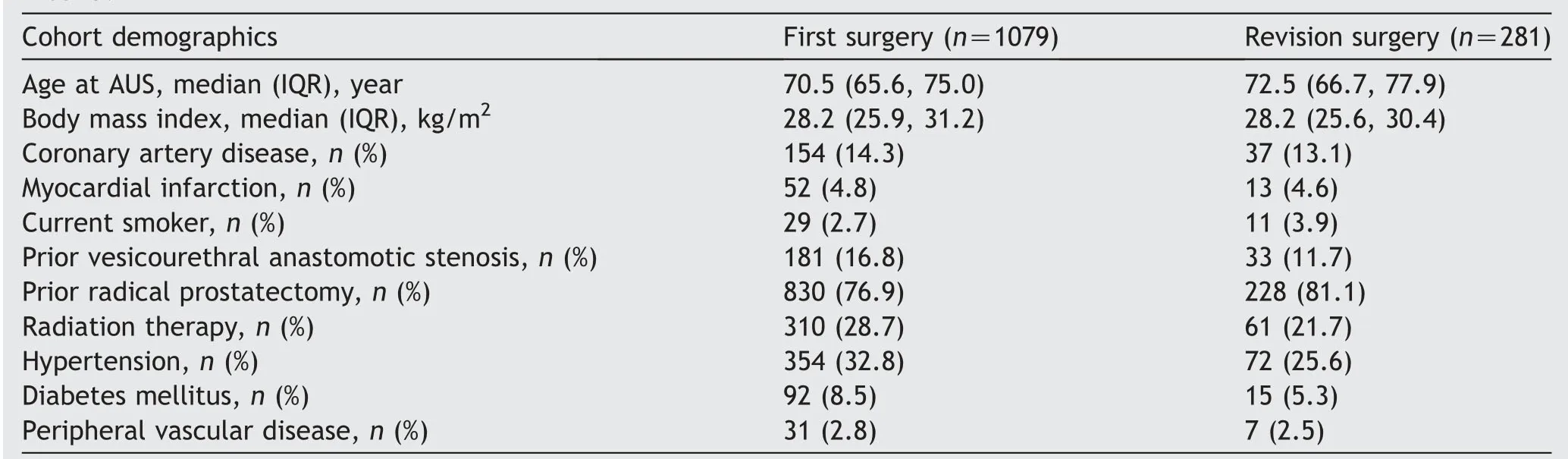
Table 1 Clinical and demographic information for patient undergoing AUS surgery,stratified by primary implantation versus first revision surgery.
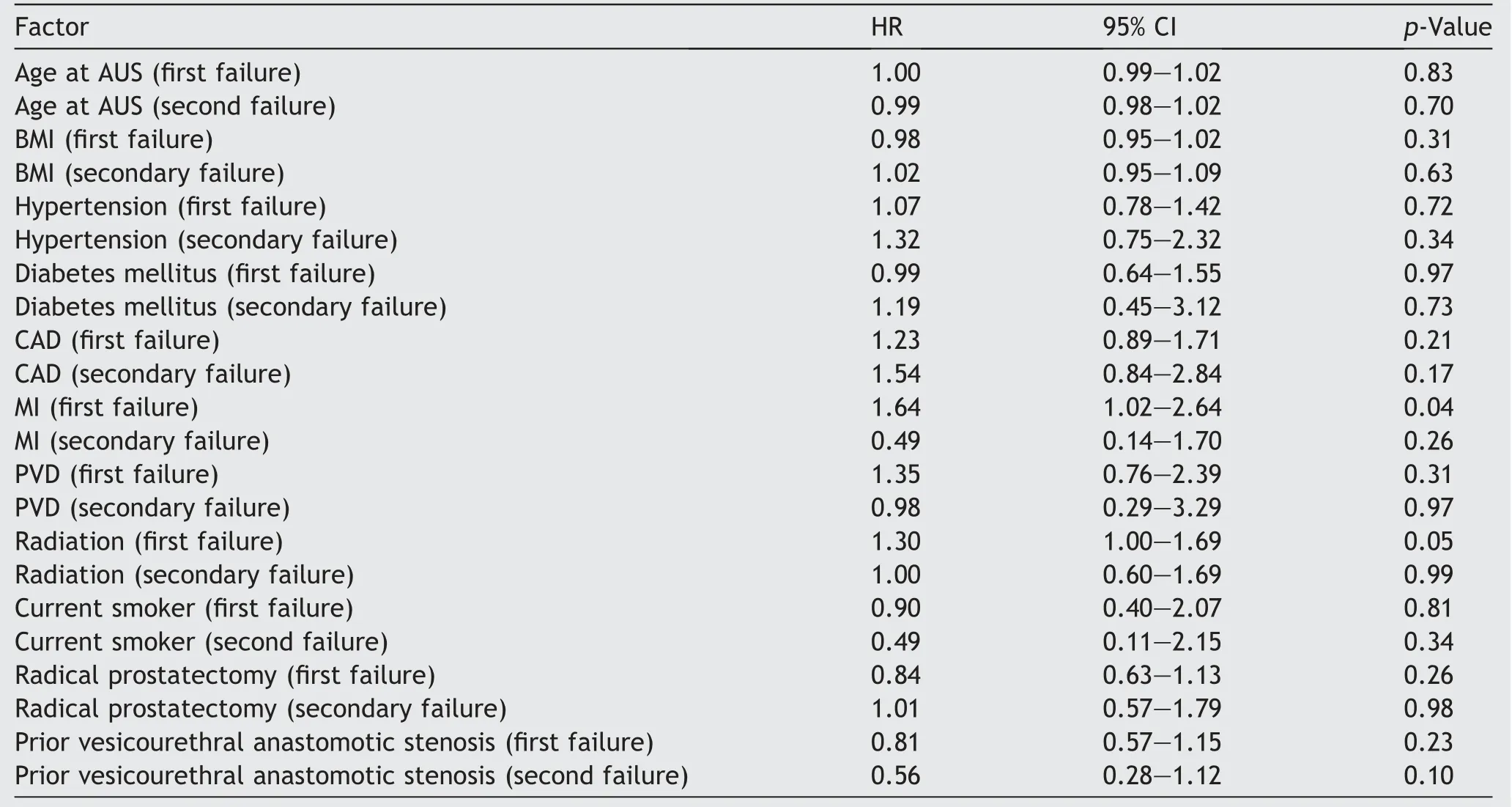
Table 2 Univariate analysis of factors associated with repeat AUS surgery (any cause).
4.Discussion
Our results from a large series of AUS revision surgeries reveals reduced overall device survival and increased incidence of device infection/urethral erosion in patients undergoing first revision surgeries (for device malfunction or urethral atrophy) as compared to primary AUS implantations.However,no increase in mechanical failure or atrophy rates in the revision cohort was identified.This study further augments the available literature,by providing information on outcomes of AUS revision surgery in a large cohort of patients with long-term follow-up and can be used to better inform patients during preoperative counseling.
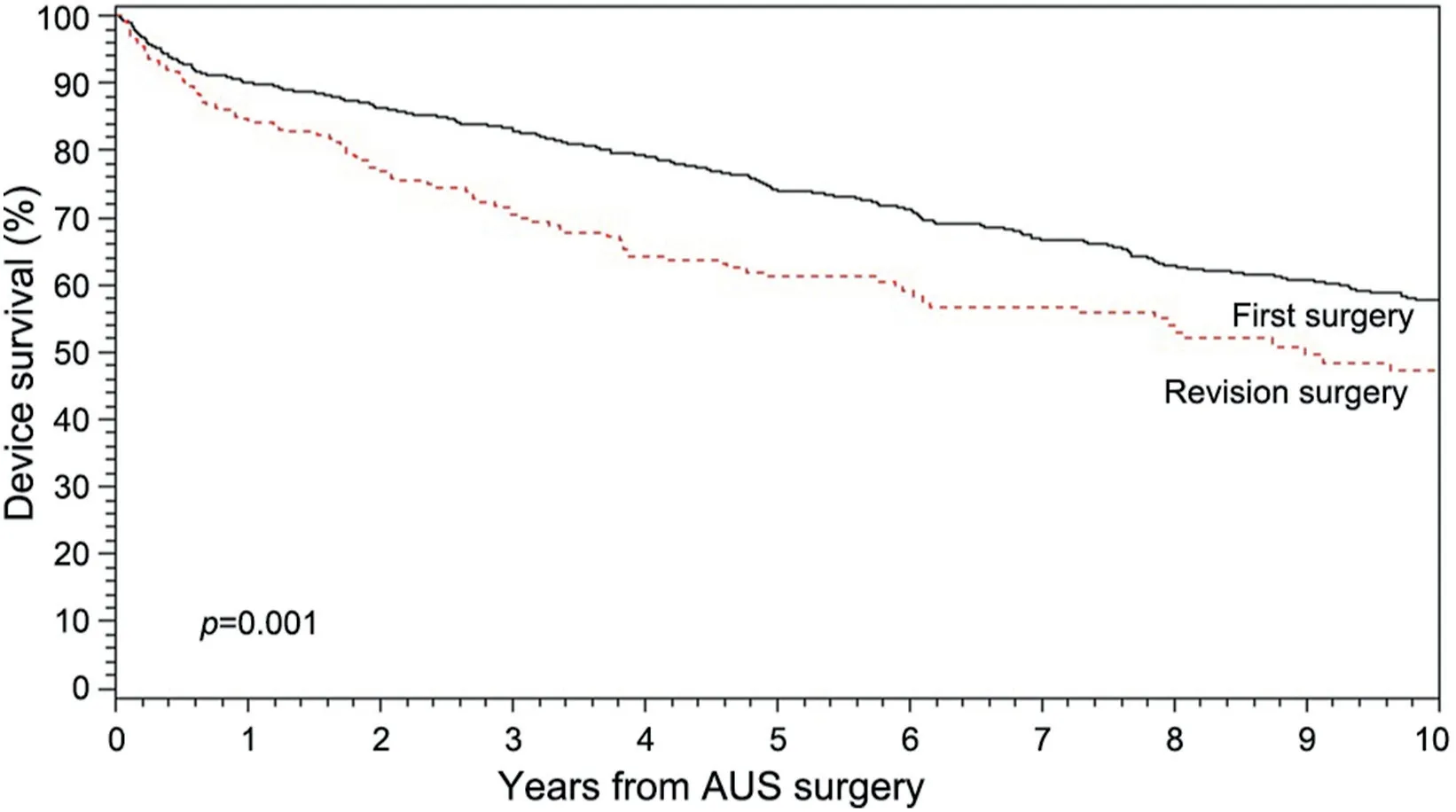
Figure 1 Kaplan-Meier survival analysis stratified by primary vs. first revision AUS surgery.AUS,artificial urinary sphincter.
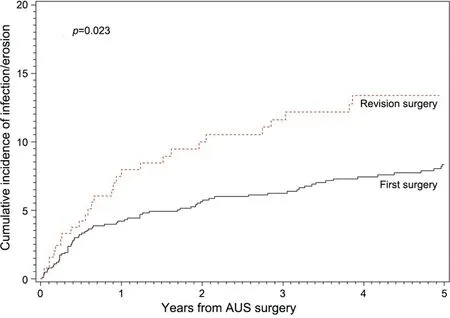
Figure 2 Cumulative incidence curve of infection/erosion compared between primary and first revision AUS surgeries.AUS,artificial urinary sphincter.
Limited available literature regarding device outcomes following revision surgery,as compared to primary implantations,has shown no difference in device outcomes between primary and revision surgery [5,7,8].For instance,Lai and Boone[8]reported their experience with 37 revision AUS procedures and found no significant increase in reoperation rates or urethral erosion rates that when compared to 169 primary cases.With a mean followup of 31.9 months for primary implantations and 16.8 months for revision cases,there was non-statistically significant difference in device malfunction rate (10.8%vs.
3.6%,p
=0.06) [8].Likewise,Raj et al.[5] reported their experience with 435 primary and 98 revision procedures,and reported no difference in device survival in revision compared to primary procedures,and interestingly saw an improved device survival at 5 years compared with primary(88%vs.
79.4%;nop
-value given).In our larger cohort,with longer follow-up,we found adverse device survival,specifically due to a significantly increased incidence of erosion/infection in first revision surgeries as compared to primary implantations.Differences in our findings may be secondary to differences in sample sizes,disparate patient populations,surgical techniques,and length of follow-up available.One potential explanation for the increased rate of device infection and urethral erosion identified in this study with revisions cases,as compared to primary implantations,is poor urethral tissue quality and impaired vascularity.For instance,with repeat dissection,there may be compromise of blood supply/scarring of the periurethral tissue which may increase the risk of erosion as spongiosum may have been previously attenuated (e.g.
in cases of urethral atrophy).Similar findings have been seen in the inflatable penile prosthesis literature as there is level II evidence that revision surgery increases the risk of infection [12].However,the etiology of device failure in the revision AUS surgery population is likely a “multiplehit” phenomenon related to a combination of prior tissue manipulation in addition to one or more comorbidities which impair small vessel function (diabetes,coronary artery disease,smoking status,and prior radiotherapy).The fact that we found a correlation with myocardial infarction,a small vessel disease,and device failure may complement this theory.However,the absence of an association of AUS failure with diabetes,prior radiotherapy,smoking status,and BMI in our study should not suggest that these comorbidities do not augment the risk of device failure.Several authors have published on AUS survival and its association with coronary artery disease.Raj and colleagues [13] found a history of coronary artery disease was associated with a relative risk of 2.43 (95% CI:1.4—4.25) of erosion.Similarly Rivera and colleges [14]found a hazard ratio of 2.4 in patients with coronary artery disease leading to device erosion and infection,although this was found in a post radiation cohort.This is not surprising since radiation can be thought of as a correlate to small vessel disease.Likewise,Rivera et al.[14] found no relation to device failure in patients receiving radiation (3-year device survival 82%vs.
85%,p
=0.25).We recognize several limitations in our study including that our patient population is a well-selected,single-institution cohort,treated at a tertiary care center by three high-volume AUS surgeons.Thus our findings may not be generalizable to all surgical practices.Additionally,some patients may follow-up with local providers,introducing heterogeneity into patient follow-up.We attempted to remedy this with written patient correspondence regarding their device status.Likewise,we do not have a standardized follow-up or monitoring protocol;thus while we are able to capture length of follow-up,we are not able to capture those lost to follow-up.Given the retrospective nature of our study,with the majority of revision patients having had their primary procedure at a separate institution,direct statistical comparison of the baseline comorbidities in each cohort is not feasible.Lastly,subgroup analyses assessing the effect of prior bladder neck stenosis,history of prostatectomy,and history of radiotherapy on device survival were not performed as the subgroups were significantly underpowered.While prior studies with well powered cohorts have shown no difference in AUS device survival based on incontinence etiology or prior history of radiotherapy [14—16],we recognize that our data cannot support these findings.
5.Conclusions
Our findings suggest that revision surgery is associated with lower,but acceptable,overall AUS device survival compared to primary implantations.This increase is secondary to an increased rate of infection/erosion.
Author contributions
Study concept and design
:Kevin J.Hebert,Brian J.Linder,Griffin T.Morrisson,Laureano Rangel Latuche,Daniel S.ElliottData acquisition
:Laureano Rangel Latuche,Daniel S.ElliottData analysis
:Laureano Rangel LatucheDrafting of manuscript
:Kevin J.Hebert,Brian J.Linder,Griffin T.MorrissonCritical revision of the manuscript
:Kevin J.Hebert,Brian J.Linder,Laureano Rangel Latuche,Daniel S.ElliottConflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2021年3期
Asian Journal of Urology2021年3期
- Asian Journal of Urology的其它文章
- Testing for BRCA1/2 and ataxiatelangiectasia mutated in men with high prostate indices:An approach to reducing prostate cancer mortality in Asia and Africa
- Male genital damage in COVID-19 patients:Are available data relevant?
- Augmented anastomotic urethroplasty with buccal mucosa for post penile fracture urethral injury long segment bulbar urethral stricture review
- The neutrophil-tolymphocyte ratio at the prostate-specific antigen nadir predicts the time to castration-resistant prostate cancer
- Conquering new battlegrounds:Successful management of isolated giant retrovesical hydatid cyst with robotic assistance
- A systematic review of dedicated models of care for emergency urological patients
