鄰炔基苯基環丁酮類化合物的高效合成及其開環-環化反應
蔡潤達, 高繼強, 劉永盛, 郭梓騰, 王宇超, 劉 宇
(長春工業大學 化學與生命科學院, 長春 130012)
0 引 言
環丁酮作為一類重要的小環化合物, 其合成及轉化具有重要意義[1-7]. 近年來已經得到了許多有關不飽和化合物參與的環丁酮開環-環化反應[8-9], 其中炔基取代的環丁酮類化合物具有獨特的反應活性, 可以制備多種環狀化合物. 如Deng等[10]通過“cut and sew”變換[11]實現了環丁酮與炔烴的分子內反應形成稠環-環己烯酮, 該反應中的炔基環丁酮類原料由鄰炔基苯甲醇制備, 鄰炔基苯甲醇由經典的Sonogashira反應[12]制備. Matsuda等[13]用炔烴取代環丁酮的擴環反應合成了苯并七元環酮類化合物, 該反應中的α-(2-乙炔基苯基)環丁酮類化合物可用鄰溴苯基環丁酮與末端炔烴經Sonogashira偶聯反應高效制備. 由于β-(2-乙炔基苯基)環丁酮類化合物難以通過類似方法合成, 因此限制了該類反應的研究進程. 錢艷艷等[14]將無銅參與的鈀催化Sonogashira偶聯反應用于上述化合物合成, 并進一步實現了Lewis酸催化的β-(2-乙炔基苯基)環丁酮類化合物的開環-復分解反應, 用一步法制備了一系列雙芳基酮類化合物(圖1(A))[15]. 在此基礎上, 本文對該Sonogashira偶聯反應體系進行優化, 得到更佳的反應條件, 反應產率更高, 底物普適性更好(圖1(B)), 利用銅類催化劑可實現該反應產物的開環-復分解反應, 制備出一系列萘酮類化合物.
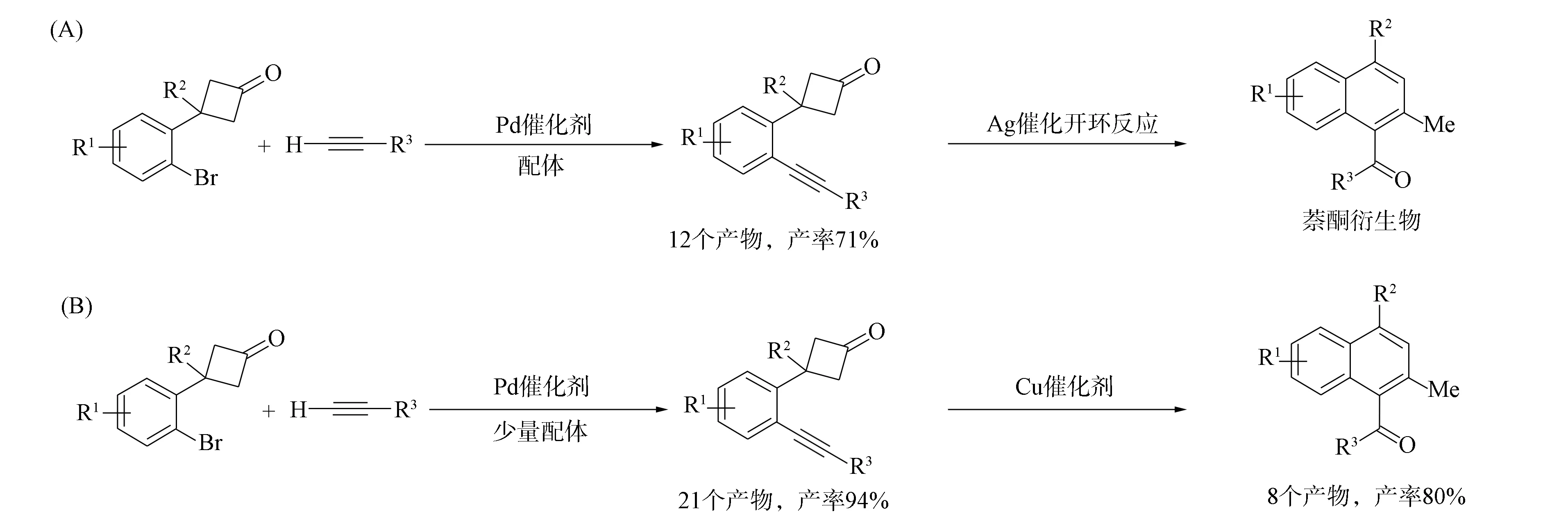
圖1 β-(2-乙炔基苯基)環丁酮類化合物的制備及其開環-復分解反應Fig.1 Preparation and ring-opening-metathesis reactions of β-(2-alkynylphenyl)cyclobutanone compounds
1 實 驗
1.1 試劑和儀器
4-甲氧基苯乙炔、4-甲基苯乙炔、苯乙炔、1-己炔、4-硝基苯乙炔等購自阿拉丁試劑(上海)有限公司; 碳酸鉀、三氟乙酸鈀(Pd(TFA)2)、三氟甲磺酸銅(Cu(OTf)2)等購自薩恩化學技術(上海)有限公司; 鄰溴芳基環丁酮等底物根據文獻[16-17]制備, 所用溶劑為超干溶劑或經除水處理.
RCTBS025型磁力攪拌器(德國IKA公司); 核磁共振波譜儀(AV-400型, 瑞士Bruker公司); 高分辨質譜儀(HRMS, 瑞士Bruker公司).
1.2 β-(2-乙炔基苯基)環丁酮類化合物的合成
先在干燥的封管中加入Pd(TFA)2(0.005 mmol), DPEphos(雙(2-二苯基膦)苯醚, 0.01 mmol)和K2CO3(0.1 mmol), 再對體系進行氬氣保護. 將鄰溴芳基環丁酮1(0.2 mmol)和末端炔烴2(0.3 mmol)溶于2 mL 1,4-二氧六環中, 用注射器將其加入封管中并將封管密封. 將封管置于90 ℃油浴鍋中攪拌12 h, 通過薄層色譜分析(TLC)檢測反應, 待原料反應完全后將其冷卻至室溫, 加入5 mL 2 mol/L的稀鹽酸, 攪拌3 min后加入5 mL乙酸乙酯萃取, 有機相用5 mL飽和氯化鈉溶液洗滌, 無水硫酸鈉干燥. 減壓濃縮后用層析色譜柱分離(200~300目硅膠,V(石油醚)∶V(乙酸乙酯)=20∶1~2∶1), 得到鄰炔基芳基環丁酮產物3, 反應路線如圖2所示.

圖2 β-(2-乙炔基苯基)環丁酮的合成Fig.2 Synthesis of β-(2-alkynylphenyl)cyclobutanones
1.3 炔基化茚滿酮(4gk)的合成
用1.2的合成方法將Pd(TFA)2(0.005 mmol), DPEphos(0.01 mmol)和K2CO3(0.1 mmol), 鄰溴芳基環丁酮1g(0.2 mmol)和末端炔烴2k(0.3 mmol)反應后, 用層析色譜柱分離(200~300目硅膠,V(石油醚)∶V(乙酸乙酯)=2∶1), 得到鄰炔基芳基環丁酮產物3gk和炔基化茚滿酮4gk, 反應路線如圖3所示.

圖3 炔基茚滿酮(4gk)的合成Fig.3 Synthesis of alkynyl indanone (4gk)
1.4 三氟甲磺酸銅催化萘酮類化合物的合成
在干燥的封管中加入Cu(OTf)2(0.03 mmol), 對體系進行氬氣保護. 將鄰炔基芳基環丁酮3(0.15 mmol)溶于2 mL三氯甲烷中, 用注射器將其加入封管中并將封管密封. 將封管置于在65 ℃油浴鍋中攪拌18 h, 通過TLC檢測反應, 待原料反應完全后, 將反應液直接減壓濃縮后用層析色譜柱分離(200~300目硅膠,V(石油醚)∶V(乙酸乙酯)=20∶1), 得到萘酮產物5, 反應路線如圖4所示.

圖4 萘酮類化合物的合成Fig.4 Synthesis of naphthone compounds
1.5 產物的表征
產物的1H NMR和13C NMR核磁數據由Bruker 400 MHz核磁共振儀檢測, CDCl3為溶劑, 四甲基硅烷(TMS)為內標.
3-(2-(苯乙炔基)苯基)環丁-1-酮(3aa).1H NMR(400 MHz, CDCl3)δ: 7.56(d,J=7.5 Hz, 1H), 7.53~7.48(m, 2H), 7.38~7.31(m, 5H), 7.27~7.22(m, 1H), 4.09(p,J=8.4 Hz, 1H), 3.59~3.48(m, 2H), 3.43~3.33(m, 2H);13C NMR(101 MHz, CDCl3)δ: 206.7,144.6,132.9,131.4,128.7,128.51,128.46,126.6,125.5,123.1,122.7,94.5,87.8,53.6,27.7. HRMS(C18H14O, [M]+, 理論值): 246.103 8(246.103 9).
3-(2-(4-甲基苯乙炔基)苯基)環丁-1-酮(3ab).1H NMR(400 MHz, CDCl3)δ: 7.55(d,J=7.5 Hz, 1H), 7.40(d,J=8.0 Hz, 2H), 7.33(d,J=3.9 Hz, 2H), 7.27~7.22(m, 1H), 7.16(d,J=7.9 Hz, 2H), 4.08(p,J=8.4 Hz, 1H), 3.60~3.48(m, 2H), 3.45~3.34(m, 2H), 2.37(s, 3H);13C NMR(101 MHz, CDCl3)δ: 206.7,144.5,138.7,132.8,131.3,129.2,128.4,126.6,125.5,122.9,120.0,94.7,87.2,53.6,27.8,21.5. HRMS(C19H16O, [M]+, 理論值): 260.119 3(260.119 6).
3-(2-(4-甲氧基苯乙炔基)苯基)環丁-1-酮(3ac).1H NMR(400 MHz, CDCl3)δ: 7.55(d,J=7.5 Hz, 1H), 7.45(d,J=8.7 Hz, 2H), 7.33(d,J=4.0 Hz, 2H), 7.27~7.21(m, 1H), 6.89(d,J=8.7 Hz, 2H), 4.13~4.01(m, 1H), 3.82(s, 3H), 3.59~3.48(m, 2H), 3.46~3.34(m, 2H);13C NMR(101 MHz, CDCl3)δ: 207.0,159.8,144.3,132.8,132.7,128.3,126.6,125.5,122.9,115.1,114.1,94.5,86.5,55.3,53.6,27.7. HRMS(C19H16O2, [M]+, 理論值): 276.114 9(276.114 5).
3-(2-(4-硝基苯乙炔基)苯基)環丁烷-1-酮(3af).1H NMR(400 MHz, CDCl3)δ: 8.24(d,J=8.7 Hz, 2H), 7.63(dd,J=19.2, 8.1 Hz, 3H), 7.47~7.37(m, 2H), 7.34~7.25(m, 1H), 4.19~4.03(m, 1H), 3.63~3.52(m, 2H), 3.49~3.37(m, 2H);13C NMR(101 MHz, CDCl3)δ: 206.6,166.4,144.8,133.1,131.3,129.6,129.2,127.6,126.7,125.6,122.1,93.6,90.7,53.7,27.7. HRMS(C18H13NO3, [M]+, 理論值): 291.088 8(291.089 0).
3-(2-(環丙基乙炔基)苯基)環丁-1-酮(3ag).1H NMR(400 MHz, CDCl3)δ: 7.41(d,J=7.5 Hz, 1H), 7.27(d,J=4.1 Hz, 2H), 7.21~7.14(m, 1H), 3.96(p,J=8.4 Hz, 1H), 3.53~3.41(m, 2H), 3.39~3.27(m, 2H), 1.47(ddd,J=13.1,8.4,5.1 Hz, 1H), 0.93~0.86(m, 2H), 0.83~0.76(m, 2H);13C NMR(101 MHz, CDCl3)δ: 207.1,144.4,132.8,127.7,126.4,125.4,123.2,98.7,74.3,53.5,27.6,8.6,0.3. HRMS(C15H14O, [M]+, 理論值): 210.103 6(210.103 9).
3-(2-(己炔基)苯基)環丁-1-酮(3ah).1H NMR(400 MHz, CDCl3)δ: 7.43(d,J=7.5 Hz, 1H), 7.28(d,J=3.6 Hz, 2H), 7.22~7.15(m, 1H), 4.06~3.93(m, 1H), 3.54~3.41(m, 2H), 3.40~3.28(m, 2H), 2.44(t,J=7.0 Hz, 2H), 1.65~1.56(m, 2H), 1.53~1.43(m, 2H), 0.95(t,J=7.3 Hz, 3H);13C NMR(101 MHz, CDCl3)δ: 207.1,144.3,132.9,127.8,126.5,125.4,123.5,95.8,79.1,53.6,30.8,27.6,22.1,19.3,13.6. HRMS(C16H18O, [M]+, 理論值): 226.135 4(226.135 2).
3-(2-(壬炔基)苯基)環丁-1-酮(3ai).1H NMR(400 MHz, CDCl3)δ: 7.43(d,J=7.4 Hz, 1H), 7.34~7.24(m, 2H), 7.23~7.16(m, 1H), 4.09~3.93(m, 1H), 3.57~3.41(m, 2H), 3.39~3.29(m, 2H), 2.43(t,J=7.1 Hz, 2H), 1.68~1.55(m, 2H), 1.5~1.4(m, 2H), 1.38~1.26(m, 6H), 0.92~0.86(m, 3H);13C NMR(101 MHz, CDCl3)δ: 207.1,144.3,132.8,127.8,126.4,125.3,123.5,95.8,79.1,53.6,31.7,29.0,28.8,28.7,27.6,22.6,19.6,14.0. HRMS(C19H24O, [M]+, 理論值): 268.182 3(268.182 2).
3-(2-(三甲基硅基乙炔基)苯基)環丁-1-酮(3aj).1H NMR(400 MHz, CDCl3)δ: 7.50(d,J=7.6 Hz, 1H), 7.36~7.25(m, 2H), 7.23~7.18(m, 1H), 4.00(p,J=8.4 Hz, 1H), 3.55~3.44(m, 2H), 3.40~3.31(m, 2H), 0.26(s, 9H);13C NMR(101 MHz, CDCl3)δ: 206.8,144.9,133.2,128.8,126.5,125.4,122.5,103.5,99.8,53.4,27.6,-0.2. HRMS(C15H18OSi, [M]+, 理論值): 242.112 4(242.112 1).
3-(2-(3-噻吩基乙炔基)苯基)環丁-1-酮(3al).1H NMR(400 MHz, CDCl3)δ: 7.58~7.48(m, 2H), 7.39~7.29(m, 3H), 7.25(t,J=7.9 Hz, 1H), 7.18(d,J=4.8 Hz, 1H), 4.06(p,J=8.4 Hz, 1H), 3.61~3.47(m, 2H), 3.47~3.33(m, 2H);13C NMR(101 MHz, CDCl3)δ: 207.1,144.6,132.9,129.7,128.8,128.7,126.7,125.8,125.7,122.6,122.1,89.8,87.4,53.8,27.9. HRMS(C16H12OS, [M]+, 理論值): 252.060 3(252.060 8).
3-(2-(4-氯苯乙炔基)-4-甲基苯基)環丁-1-酮(3bd).1H NMR(400 MHz, CDCl3)δ: 7.46~7.37(m, 3H), 7.33(d,J=8.4 Hz, 2H), 7.26~7.21(m, 1H), 7.17(d,J=7.8 Hz, 1H), 4.10~3.95(m, 1H), 3.60~3.46(m, 2H), 3.43~3.28(m, 2H), 2.34(s, 3H);13C NMR(101 MHz, CDCl3)δ: 206.9,141.7,136.4,134.5,133.4,132.6,129.7,128.8,125.6,122.0,121.6,92.9,89.0,53.8,27.4,20.7. HRMS(C19H15ClO, [M]+, 理論值):294.080 5(294.080 6).
3-(4-甲基-2-(三甲基硅基乙炔基)苯基)環丁-1-酮(3bj).1H NMR(400 MHz, CDCl3)δ: 7.25(s, 1H), 7.07(dd,J=19.1,7.9 Hz, 2H), 3.88(p,J=8.4 Hz, 1H), 3.45~3.33(m, 2H), 3.30~3.19(m, 2H), 2.23(s, 3H), 0.17(s, 9H);13C NMR(101 MHz, CDCl3)δ: 207.1,142.0,136.1,133.7,129.6,125.4,122.2,103.7,99.2,53.4,27.3,20.6,-0.2. HRMS(C16H20OSi, [M]+, 理論值): 256.127 4(256.127 8).
3-(4-甲氧基-2-(苯乙炔基)苯基)環丁-1-酮(3ca).1H NMR(400 MHz, CDCl3)δ: 7.51(dd,J=6.5,3.0 Hz, 2H), 7.35(dd,J=4.9,1.7 Hz, 3H), 7.22(d,J=8.6 Hz, 1H), 7.10(d,J=2.6 Hz, 1H), 6.89(dd,J=8.6,2.6 Hz, 1H), 4.07~3.96(m, 1H), 3.81(s, 3H), 3.56~3.45(m, 2H), 3.41~3.27(m, 2H);13C NMR(101 MHz, CDCl3)δ: 206.8,158.0,136.9,131.4,128.5,128.4,126.6,123.4,122.9,117.5,115.1,94.2,87.8,55.4,53.8,27.0. HRMS(C19H16O2, [M]+, 理論值): 276.114 8(276.114 5).
3-(4-甲氧基-2-(4-甲苯乙炔基)苯基)環丁-1-酮(3cb).1H NMR(400 MHz, CDCl3)δ: 7.40(d,J=7.9 Hz, 2H), 7.22(d,J=8.6 Hz, 1H), 7.17(d,J=7.8 Hz, 2H), 7.09(d,J=2.6 Hz, 1H), 6.93~6.85(m, 1H), 4.01(p,J=8.4 Hz, 1H), 3.81(s, 3H), 3.56~3.46(m, 2H), 3.40~3.29(m, 2H), 2.37(s, 3H);13C NMR(101 MHz, CDCl3)δ: 207.2,158.0,138.8,136.8,131.3,129.2,126.6,123.6,119.8,117.3,114.9,94.4,87.1,55.4,53.7,27.0,21.5. HRMS(C20H18O2, [M]+, 理論值): 290.130 5(290.130 1).
3-(2-(4-氟苯乙炔基)-4-甲氧基苯基)環丁-1-酮(3ce).1H NMR(400 MHz, CDCl3)δ: 7.49(dd,J=8.6,5.4 Hz, 2H), 7.27~7.22(m, 1H), 7.11~7.03(m, 3H), 6.91(dd,J=8.6,2.6 Hz, 1H), 4.05~3.95(m, 1H), 3.83(s, 3H), 3.57~3.46(m, 2H), 3.41~3.31(m, 2H);13C NMR(101 MHz, CDCl3)δ: 207.1,162.7(d,J=250.4 Hz), 158.0,136.9,133.3(d,J=8.4 Hz), 126.8,123.2,119.0(d,J=3.6 Hz), 117.5,115.9,115.7,115.1,93.1,87.5(d,J=1.2 Hz), 55.4,53.8,27.1;19F NMR(376 MHz, CDCl3)δ: -110.28. HRMS(C19H15FO2, [M]+, 理論值): 294.105 0(294.105 1).
3-(5-甲基-2-(苯乙炔基)苯基)環丁-1-酮(3da).1H NMR(400 MHz, CDCl3)δ: 7.54~7.44(m, 3H), 7.39~7.32(m,J=5.6 Hz, 3H), 7.15(s, 1H), 7.07(d,J=7.7 Hz, 1H), 4.06(p,J=8.3 Hz, 1H), 3.60~3.48(m, 2H), 3.44~3.35(m, 2H), 2.39(s, 3H);13C NMR(101 MHz, CDCl3)δ: 207.1,144.4,138.8,132.8,131.3,128.4,128.3,127.4,126.4,123.2,119.6,93.7,88.0,53.6,27.6,21.6. HRMS(C19H16O, [M]+, 理論值): 260.119 8(260.119 6).
3-(5-甲基-2-(2,4,5-三甲氧基苯乙炔基)苯基)環丁烷-1-酮(3dk).1H NMR(400 MHz, CDCl3)δ: 7.46(d,J=7.7 Hz, 1H), 7.14(s, 1H), 7.05(d,J=7.6 Hz, 1H), 6.96(s, 1H), 6.51(s, 1H), 4.16~4.03(m, 1H), 4.00~3.81(m, 9H), 3.63~3.49(m, 2H), 3.49~3.36(m, 2H), 2.38(s, 3H);13C NMR(101 MHz, CDCl3)δ: 207.4,155.4,150.4,144.0,142.9,138.2,132.4,127.2,126.3,120.1,115.5,103.3,97.0,90.7,90.4,56.5,56.0,53.4,27.7,21.6. HRMS(C22H22O4, [M]+, 理論值): 350.151 9(350.151 3).
3-(5-甲氧基-2-(苯乙炔基)苯基)環丁-1-酮(3ea).1H NMR(400 MHz, CDCl3)δ: 7.52~7.46(m, 3H), 7.36~7.30(m, 3H), 6.87(d,J=2.1 Hz, 1H), 6.78(dd,J=8.5, 2.3 Hz, 1H), 4.11~4.00(m, 1H), 3.83(s, 3H), 3.58~3.48(m, 2H), 3.43~3.33(m, 2H);13C NMR(101 MHz, CDCl3)δ: 206.5,160.0,146.4,134.4,131.3,128.4,128.2,123.5,114.9,112.3,111.6,93.0,87.9,55.4,53.6,27.9. HRMS(C19H16O2, [M]+, 理論值):276.114 5(276.114 5).
3-甲基-3-(2-(4-甲基苯乙炔基)苯基)環丁-1-酮(3fb).1H NMR(400 MHz, CDCl3)δ: 7.56(d,J=7.1 Hz, 1H), 7.37(d,J=7.9 Hz, 2H), 7.34~7.21(m, 3H), 7.16(d,J=7.7 Hz, 2H), 3.72~3.57(m, 2H), 3.28~3.17(m, 2H), 2.37(s, 3H), 1.71(s, 3H);13C NMR(101 MHz, CDCl3)δ: 207.3,149.3,138.7,133.6,131.0,129.2,128.3,126.5,126.1,121.5,120.1,94.8,87.9,59.1,34.9,28.8,21.5. HRMS(C20H18O, [M]+, 理論值): 274.135 3(274.135 2).
3-(2-(4-甲氧基苯乙炔基)苯基)-3-苯基環丁-1-酮(3gc).1H NMR(400 MHz, CDCl3)δ: 7.51(dd,J=17.6, 7.6 Hz, 2H), 7.41~7.14(m, 9H), 6.83(d,J=14.9 Hz, 2H), 4.05~3.94(m, 2H), 3.87~3.72(m, 5H);13C NMR(101 MHz, CDCl3)δ: 206.3,159.8,147.6,146.0,133.8,132.5,128.4,128.0,127.4,126.9,126.3,122.9,115.1,114.1,95.5,87.5,60.4,55.3,42.3. HRMS(C25H20O2, [M]+, 理論值): 352.146 2(352.145 8).
3-苯基-3-(2-(2,4,5-三甲氧基苯乙炔基)苯基)環丁-1-酮(3gk).1H NMR(400 MHz, CDCl3)δ: 7.57(d,J=7.5 Hz, 1H), 7.49(d,J=7.7 Hz, 1H), 7.42(d,J=7.6 Hz, 2H), 7.36(t,J=7.5 Hz, 1H), 7.29~7.22(m, 3H), 7.19~7.13(m, 1H), 6.68(s, 1H), 6.50(s, 1H), 4.10~3.99(m, 2H), 3.96~3.78(m, 11H);13C NMR(101 MHz, CDCl3)δ: 206.9,155.4,150.6,147.6,146.3,142.9,134.0,128.3,123.0,127.4,126.7,126.5,126.1,123.0,115.7,103.2,97.1,92.3,91.3,60.6,56.5,56.4,56.0,42.4. HRMS(C27H24O4, [M]+, 理論值): 412.166 8(412.166 9).
3-(1-(苯乙炔基)-2-萘基)環丁-1-酮(3ha).1H NMR(400 MHz, CDCl3)δ: 8.46(d,J=8.4 Hz, 1H), 7.91~7.80(m, 2H), 7.69~7.56(m, 3H), 7.56~7.46(m, 2H), 7.46~7.31(m, 3H), 4.39(p,J=8.4 Hz, 1H), 3.71~3.54(m, 2H), 3.54~3.37(m, 2H);13C NMR(101 MHz, CDCl3)δ: 206.8,143.4,133.7,132.1,131.6,129.1,128.7,128.6,128.2,127.4,126.3,126.2,123.3,123.2,119.5,100.1,86.0,54.4,28.1. HRMS(C22H16O, [M]+, 理論值): 296.119 6(296.119 8).
3-苯基-3-(3-(2,4,5-三甲氧基苯基)丙-2-炔-1-基)-2,3-二氫-1H-茚-1-酮(4gk).1H NMR(400 MHz, CDCl3)δ: 7.81(d,J=7.6 Hz, 1H), 7.63(t,J=7.4 Hz, 1H), 7.50~7.42(m, 2H), 7.35~7.22(m, 5H), 6.56(s, 1H), 6.39(s, 1H), 3.85(s, 3H), 3.79(s, 3H), 3.73(s, 3H), 3.46~3.27(m, 3H), 3.01~2.94(m, 1H);13C NMR(101 MHz, CDCl3)δ: 205.0,159.6,155.2,149.9,145.5,142.8,137.1,134.9,128.5,128.1,126.7,126.6,126.2,123.0,116.0,97.4,88.8,79.8,56.6,56.4,56.0,53.1,49.9,31.9.(C27H24O4, [M]+, 理論值): 412.166 7(412.166 9).
2-甲基-1-萘基苯甲酮(5aa).1H NMR(400 MHz, CDCl3)δ: 7.81(d,J=8.4 Hz, 4H), 7.55~7.45(m, 2H), 7.41~7.30(m, 5H), 2.27(s, 3H);13C NMR(101 MHz, CDCl3)δ: 199.9,137.6,136.0,133.6,132.1,131.6,130.6,129.5,128.8,128.7,128.4,128.0,126.6,125.3,124.8,19.6. HRMS(C18H14O, [M]+, 理論值): 246.103 8(246.103 9).
(2-甲基-1-萘基)4-甲基苯甲酮(5ab).1H NMR(400 MHz, CDCl3)δ: 7.82(d,J=8.4 Hz, 2H), 7.71(d,J=7.8 Hz, 2H), 7.49(d,J=8.3 Hz, 1H), 7.42~7.30(m, 3H), 7.20(d,J=7.9 Hz, 2H), 2.37(s, 3H), 2.30(s, 3H);13C NMR(101 MHz, CDCl3)δ: 199.7,144.7,136.2,135.2,132.0,131.6,130.7,129.8,129.5,128.8,128.4,128.0,126.6,125.3,124.9,21.7,19.6. HRMS(C19H16O, [M]+, 理論值): 260.119 9(260.119 6).
(2-甲基-1-萘基)3-噻吩甲酮(5al).1H NMR(500 MHz, CDCl3)δ: 7.83(dd,J=8.2,3.1 Hz, 2H), 7.71~7.65(m, 1H), 7.59(d,J=4.7 Hz, 1H), 7.55(d,J=8.3 Hz, 1H), 7.44~7.31(m, 4H), 2.35(s, 3H);13C NMR(101 MHz, CDCl3)δ: 193.7,143.5,136.7,135.6,131.9,131.7,130.4,128.9,128.5,128.0,127.1,126.8,126.6,125.4,124.9,19.6. HRMS(C18H12OS, [M]+, 理論值): 252.060 3(252.060 6).
(7-甲氧基-2-甲基-1-萘基)4-甲基苯甲酮(5cb).1H NMR(400 MHz, CDCl3)δ: 7.80~7.69(m, 4H), 7.23(d,J=7.6 Hz, 3H), 7.08(d,J=8.9 Hz, 1H), 6.76(s, 1H), 3.67(s, 3H), 2.40(s, 3H), 2.27(s, 3H);13C NMR(101 MHz, CDCl3)δ: 200.1,158.1,144.7,135.2,135.1,132.7,131.8,129.8,129.54,129.48,128.6,127.2,126.2,118.0,103.5,55.2,21.7,19.8. HRMS(C20H18O2, [M]+, 理論值): 290.130 1(290.130 1).
(2,6-二甲基-1-萘基)苯甲酮(5da).1H NMR(400 MHz, CDCl3)δ: 7.81(d,J=7.5 Hz, 2H), 7.76(d,J=8.4 Hz, 1H), 7.61(s, 1H), 7.57(t,J=7.3 Hz, 1H), 7.46~7.31(m, 4H), 7.19(d,J=8.5 Hz, 1H), 2.47(s, 3H), 2.29(s, 3H);13C NMR(101 MHz, CDCl3)δ: 200.3,137.6,135.8,135.0,133.7,131.9,131.2,129.7,128.9,128.8,128.5,128.3,127.0,124.7,21.5,19.6. HRMS(C19H16O, [M]+, 理論值):260.119 7(260.119 6).
(2,4-二甲基-1-萘基)4-甲苯甲酮(5fb).1H NMR(400 MHz, CDCl3)δ: 8.00(d,J=8.4 Hz, 1H), 7.71(d,J=7.7 Hz, 2H), 7.50(d,J=8.3 Hz, 1H), 7.44(t,J=7.6 Hz, 1H), 7.37~7.31(m, 1H), 7.25~7.17(m, 3H), 2.71(s, 3H), 2.40(s, 3H), 2.26(s, 3H);13C NMR(101 MHz, CDCl3)δ: 200.1,144.6,135.4,135.3,134.6,131.7,130.9,130.8,129.9,129.5,129.2,126.3,125.6,125.2,124.2,21.7,19.6,19.4. HRMS(C20H18O, [M]+, 理論值): 274.135 7(274.135 2).
(2-甲基-4-苯基-1-萘基)4-甲氧基苯甲酮(5gc).1H NMR(400 MHz, CDCl3)δ: 7.93~7.83(m,J=10.9,7.8 Hz, 3H), 7.59~7.42(m, 6H), 7.40~7.29(m, 3H), 6.92(d,J=8.4 Hz, 2H), 3.85(s, 3H), 2.34(s, 3H);13C NMR(101 MHz, CDCl3)δ: 198.7,164.2,141.0,140.3,135.9,132.2,131.4,131.1,130.8,130.0,129.5,128.3,127.4,126.4,126.2,125.4,125.3,114.1,55.5,19.6. HRMS(C25H20O2, [M]+, 理論值):352.145 7(352.145 8).
3-甲基-4-菲基苯甲酮(5ha).1H NMR(400 MHz, CDCl3)δ: 8.33(d,J=8.6 Hz, 1H), 7.90(d,J=8.1 Hz, 1H), 7.81(d,J=6.9 Hz, 3H), 7.73(dd,J=19.0,8.8 Hz, 2H), 7.50(dd,J=6.9,4.8 Hz, 2H), 7.43(t,J=7.4 Hz, 1H), 7.34(t,J=7.7 Hz, 2H), 7.30~7.23(m, 1H), 2.34(s, 3H);13C NMR(101 MHz, CDCl3)δ: 202.3,137.5,136.5,133.9,133.6,133.4,131.3,129.7,129.5,129.2,129.1,128.8,128.8,128.0,127.3,127.2,127.0,126.4,126.1,20.3. HRMS(C22H16O, [M]+, 理論值): 296.124 5(296.124 0).
2 結果與討論
2.1 反應條件優化
首先, 用3-(2-溴苯基)環丁烷-1-酮1a和苯乙炔2a為模板底物對鈀催化的Sonogashira偶聯反應條件進行系統研究(表1), 其合成路線如圖5所示.

表1 反應條件優化

圖5 化合物3aa的合成Fig.5 Synthesis of compound 3aa
在Pd(TFA)2為催化劑, 1,1′-雙(二苯基膦)二茂鐵(dppf)為配體, K2CO3為堿, 1,4-二氧六環作為溶劑的條件下, 以83%的核磁產率得到了產物3aa(序號 1), 比文獻[14]的產率(71%)有較大提升. 分別將1,4-雙(二苯基膦)丁烷(dppb)、三氟拉嗪(TFP)、2-(二環己基膦基)聯苯(CyJohnphos)、DPEphos作為配體進行實驗, 序號為2~5. 當DPEphos為配體時, 產率可提高到90%且以83%的分離產率得到產物3aa(序號 5). 因此以DPEphos為最優配體進行下一步篩選. 當催化劑的量降為0.005 mmol, 配體的量降為0.01 mmol時, 產物3aa的產率未明顯變化(序號 6). 再對K2CO3的量以及其他堿進行篩選(序號 7~11), 當K2CO3的量降到0.1 mmol時, 能以85.3%的分離產率得到目標產物, 當降至0.05 mmol時, 只有31%的產率. 使用不同堿的反應效果有較明顯的區別: 用Cs2CO3做堿時產率可達89%; 使用堿性較弱的K3PO4時, 反應產率明顯降低; 有機堿三乙烯二胺(DABCO)參與反應時, 產率大幅度下降. 最后, 對鈀催化劑以及溶劑的篩選表明, 其對反應結果的影響較小. 通過對配體、催化劑、堿、溶劑及其量的篩選, 確定了最優反應條件(序號7).
2.2 反應底物擴展
確定最優反應條件后, 對底物官能團的兼容性考察結果如圖6所示. 首先, 考察3-(2′-溴苯基)環丁烷-1-酮1a與各類末端炔烴的反應, 供電子基團甲基和甲氧基取代的苯乙炔化合物均以較高的產率得到目標產物3ab,3ac. 但當苯乙炔底物中引入強吸電子的硝基時, 反應僅以26%的產率得到偶聯產物3af. 此外, 烷基末端炔烴底物也可參與反應, 環狀或直鏈脂肪末端炔烴均以較高的產率得到目標化合物(3ag~3aj), 噻吩基團也可在該體系中以60%的收率獲得相應的產物(3al).
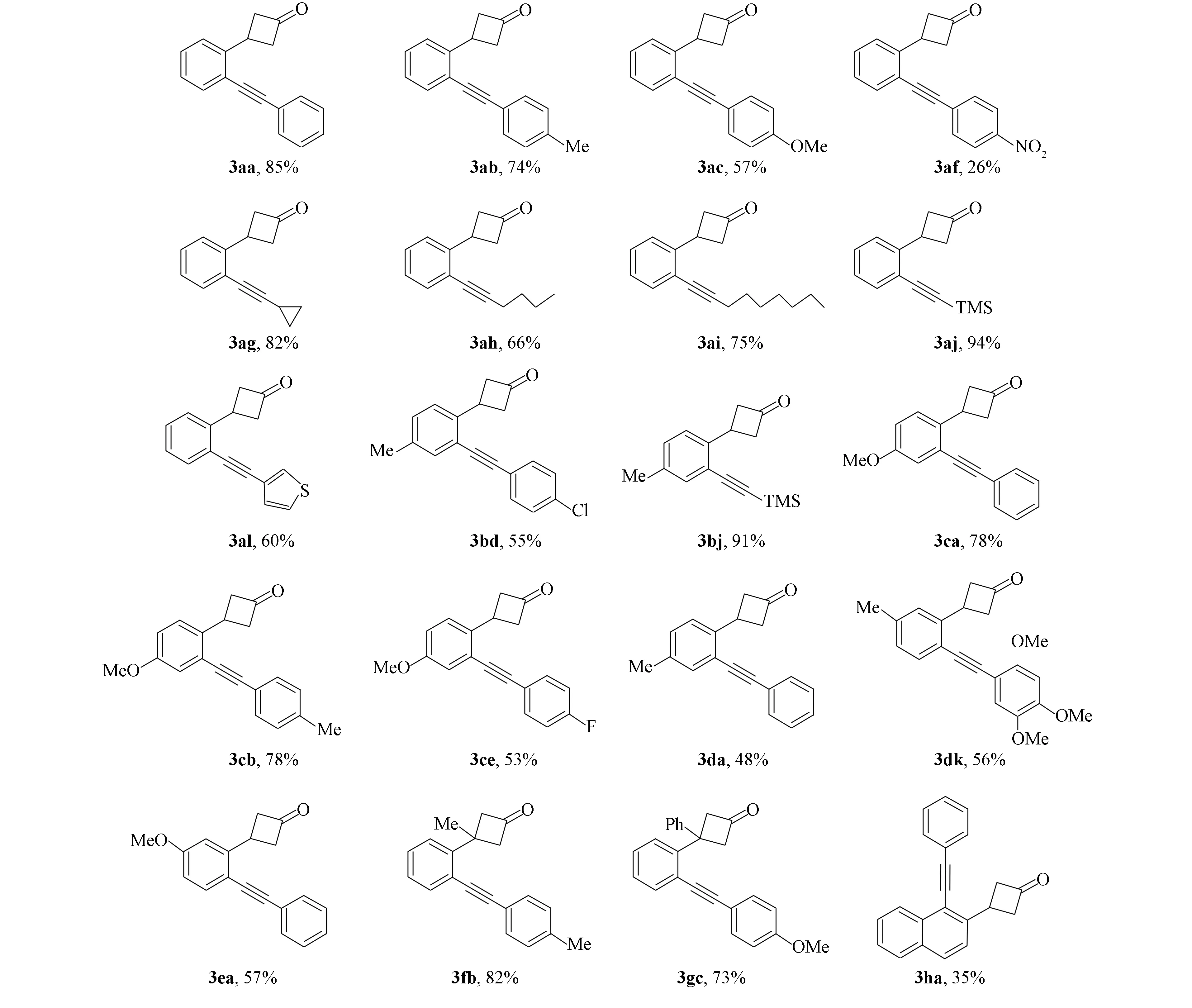
圖6 反應底物擴展及產率Fig.6 Substrate expansion and yield of reaction
再將含有不同取代基的鄰溴芳基環丁酮與末端炔烴用于單鈀催化的Sonogashira偶聯反應, 當在溴原子的間位引入甲基或甲氧基時, 以較高的產率得到產物3bj,3ca,3cb, 但苯乙炔的對位被鹵素Cl或F取代時, 反應僅得到中等的產率(3bd,3ce). 溴的對位被甲基或甲氧基取代時, 產物3da,3dk,3ea的產率較低. 當環丁酮的β位被Me或Ph取代時, 底物也可完成反應, 以較高的產率得到相應的偶聯產物(3fb,3gc). 此外, 含萘環結構底物也適應該反應, 但產率偏低(3ha, 35%).
環丁酮1g與2,4,5-三甲氧基苯乙炔2k在標準條件下反應時, 除生成Sonogashira偶聯產物3gk外, 同時得到串聯C—C鍵活化/Sonogashira型的交叉偶聯產物炔基化茚滿酮4gk[18], 如圖3所示.
2.3 炔基環丁酮類化合物的開環-復分解反應
文獻[15]研究了銀催化的β-(2-乙炔基苯基)環丁酮類化合物的開環-復分解過程, 并提出了相應機理. 本文以此為基礎, 進一步探索廉價金屬催化劑在上述反應中的催化性能. 將偶聯產物3aa作為原料, 以廉價Lewis酸Cu(OTf)2為催化劑, 三氯甲烷為溶劑, 水為氫源, 以63%的產率得到了萘酮產物5aa. 然后對其他底物進行嘗試, 當甲基和甲氧基取代的原料參與反應時,也能以中等到較高的產率(55%~80%)得到相應的萘酮類化合物5ab,5cb. 噻吩基底物3al也適應于該反應體系. 進一步嘗試在環丁酮的β位引入甲基或苯基的鄰炔基芳基環丁酮, 分別能以73%和63%得到目標產物5fb,5gc. 最后, 進行稠碳環類化合物的合成, 雖然底物3ha的反應活性較低, 但仍以23%的產率得到稠碳環產物5ha. 其底物擴展及產率如圖7所示.

圖7 制備萘酮化合物的底物擴展及產率Fig.7 Substrate expansion and yield of preparation of naphthone compounds
綜上所述, 本文優化了鈀催化的無銅Sonogashira反應體系, 通過對配體、催化劑、堿、溶劑及其量的篩選, 以更高的產率和更廣泛的底物普適性制備了一系列不同取代基的鄰炔基苯基環丁酮類化合物, 良好的官能團耐受性為復雜目標分子的構建提供了潛在的轉化途徑. 此外, 實現了銅催化下該類炔基環丁酮類化合物的開環-復分解反應, 以較高的產率制備了一系列萘酮類化合物.

