食源性抗生素耐藥菌的污染現狀、傳播擴散及健康風險研究進展
曹弘揚 ?汪慶?趙佳麗?梁海崟?郭紹月?萬春云?駱慧曉

摘要:抗生素的過度使用加劇了環境耐藥菌的產生,給人類健康造成潛在風險。在食品鏈多個環節過度使用或濫用抗生素,造成肉制品、水產品和水果蔬菜等多種食品中細菌耐藥性逐年增強。攜帶耐藥質粒的耐藥菌通過“農場到餐桌”轉移、定植在人體腸道,引起人體腸道菌群變化和免疫功能改變。食品的全球化貿易進一步加劇了細菌耐藥性的全球性傳播。本文綜述了食源性耐藥菌污染現狀、傳播途徑和人體健康風險。同時,結合國內外研究現狀,對食品環境中抗生素耐藥菌的研究重點進行展望。
關鍵詞:抗生素耐藥菌;食源性;傳播途徑;健康風險
中圖分類號:R978文獻標志碼:A
Progress on the contamination status, dissemination, and health risks of foodborne antibiotic resistant bacteria
Cao Hong-yang1, Wang Qing1, Zhao Jia-li2, Liang Hai-yin1, Guo Shao-yue1, Wan Chun-yun1, and Luo Hui-xiao1
(1 College of Energy and Environmental Engineering, Hebei Key Laboratory of Air Pollution Cause and Impact, Hebei University of Engineering, Handan 056038; 2 Medical College, Hebei University of Engineering, Handan 056038)
Abstract The abuse of antibiotics has aggravated the pollution of antibiotic resistant bacteria, posing a potential hazard to human health. Antibiotics have been widely used in many parts of the food chain, which resulted in the increase of antibiotic resistance in meat foods, aquatic foods, fruits, and vegetables. These antibiotic resistant bacteria with resistant plasmid are transferred and colonized in the human gut through 'farm-to-fork', causing changes in the human gut flora and immune functions. The global trade of food has further intensified the antibiotic resistance bacteria transfer and dissemination among the microorganisms. This paper introduced the current status of food-borne drug-resistant bacteria contamination, the transmission routes of antibiotic resistance, and human health risks. In addition, some countermeasures and prospects for the research on antibiotic resistant bacteria in food are reviewed.
Key words Antibiotic resistance bacteria; Food-borne; Transmission routes; Health risk
食品中抗生素耐藥性污染問題十分嚴重,已成為全球廣泛關注的熱點話題[1]。隨著畜牧業、水產養殖和農業等迅速發展,人們在預防和治療動植物疾病等方面過度使用抗生素,導致環境中細菌的耐藥性逐年增強[1-3]。中國是抗生素生產和使用量最大的國家,僅在2013年抗生素使用量已到達1.62×105噸,占據全世界抗生素總使用量的23%[4]。并且抗生素的使用量依然會逐年增加,預估在2030年抗生素的使用量將是2010年的1.67倍[5]。證據表明,抗生素使用不當會加快抗生素耐藥菌在大氣[6]、海洋[7]和土壤[8]中的傳播。美國疾病控制與預防中心表明,每年僅美國因感染抗生素耐藥菌患病人數超200萬人,死亡人數約2.3萬人,如果不及時采取有效措施,抗生素耐藥菌的污染將進一步擴大[9]。
目前,我國食源性耐藥菌引發的疾病和耐藥率呈現快速上升趨勢,給人體健康造成潛在健康風險[10]。在雞肉、豬肉、海產品、水果和蔬菜等多種食品中都已檢測到抗生素耐藥菌的存在,同時,至少已發現沙門菌、大腸埃希菌、空腸彎曲菌、單增李斯特菌和副溶血弧菌等致病菌表現出較強的耐藥性[11-18]。因此,加強食品中耐藥細菌的監測和管控迫在眉睫。本文綜述了多種食品中抗生素耐藥菌的研究現狀,介紹了耐藥菌在食物鏈中的來源、傳播途徑和人體健康風險,并對未來研究重點進行展望。
1 食源性耐藥菌的污染現狀
抗生素耐藥菌廣泛存在于多種食品,并且具有污染范圍廣和污染率高等特點[11]。近年來,不同國家和地區食品中檢測出抗生素耐藥菌種類和數量逐年上升[19]。在我國,食源性耐藥菌的污染與傳播同樣不容忽視,其中肉類和水產食品被耐藥菌污染最為嚴重,最高可達到59%[20]。
1.1 肉類食品
過度使用抗生素導致肉類食品中存在大量的抗生素耐藥菌。研究人員從零售店的肉類食品中分離出了耐藥菌[21-22],且種類多、豐度大[23-24]。根據大量數據調研表明(表1),發展中國家禽類食品中檢測的沙門菌陽性菌株比例較高,南美地區陽性樣本數量為13%~39%[25],非洲地區檢測到陽性樣品比例約為35%[26-27],亞洲地區檢測到陽性樣品比例為35%~60%[28-30],而在美國、英國等發達國家食品中檢測到的抗生素耐藥菌明顯較低[22]。
肉類食品中分離出的耐藥菌主要包括產志賀毒素大腸埃希菌[31-33]、金黃色葡萄球菌[33]、單核細胞增生李斯特菌[34-35]和鼠寒沙門菌[36]。由于耐藥性具有可轉移的特性,耐藥菌中的抗性基因能夠通過水平轉移分子機制轉移到腸道菌群內[37]。但根據最新的研究,停止給動物喂含有抗生素的飼料3~4周后,動物腸道、血液和肌肉中抗生素含量下降,其體內耐藥菌豐度減少[38]。因此,可以在屠宰前停止喂含抗生素的飼料,以降低動物體內耐藥菌的占比。
1.2 水產食品
水產養殖中大量使用的抗生素最終會進入到養殖水環境和水產食品中,導致產生越來越多耐藥菌[39]。水產食品中耐藥菌多數具有耐低溫和耐高滲透壓的特性,可以在極端環境中生存。人類在通過手拿、生吃等方式感染耐藥菌,極易對人體健康造成潛在危害[40]。Tan等[41]對馬來西亞的水產食品檢測發現,副溶血弧菌污染了85.7%的水產食品,且對青霉素、氨芐西林和頭孢唑林的耐藥性較高,其中青霉素的耐藥性為100%。Tran等[42]對越南水產食品中分離的菌株進行檢測后同樣發現,副溶血弧菌感染了86.2%的樣品,且大多數菌株對氨芐西林、磺胺異惡唑和鏈霉素具有耐藥性。Jiang等[43]通過檢測發現,黃海和渤海水產食品中的副溶血弧菌污染較重,并對阿米卡星和頭孢唑林等有多重耐藥性。Ellis-Iversen等[44]收集丹麥零售店的水產食品進行耐藥性檢測,有89.7%的水產食品中檢測到大腸埃希菌,其中45.6%的大腸埃希菌對至少一種抗生素具有耐藥性。因此,水產食品中耐藥菌污染已十分嚴重。
我國沿海沿河地區擁有豐富的水產食品,當地居民多以此為食。未被人體和動物利用的抗生素會隨糞便或尿液進入環境,總量多達5.58×104噸,其中46%排放到水體環境[4],致使水產食品中抗生素耐藥菌普遍增加[45],長期攝入這些耐藥菌是否會對人體健康造成潛在風險值得深入探究。
1.3 乳類食品
生乳及乳制品中檢出耐藥菌對食品安全再次敲響了警鐘[46]。生乳制品的微生物污染狀況主要取決于動物的健康狀況、養殖環境、擠奶環境和擠奶器的衛生狀況[47]。生奶中由于水分含量高,pH中性,并且有豐富的營養物質,適合微生物繁殖。因此,乳品加工廠為了保證鮮奶的品質和延長鮮奶儲存,不得已會在生乳的加工過程中添加微量的抗生素。
生乳[48]、巴氏滅菌奶[49]和奶酪[46]中均分離出了耐藥菌。Sharma等[50]檢測牛奶樣品發現,生乳中有19.8%的樣品被金黃色葡萄球菌污染,其具有凝固酶活性的金黃色葡萄球菌中,有90%的菌株對至少3種抗生素具有抗性。Ameen等[51]從患有乳腺炎的奶牛身上采集生乳樣品,發現被金黃色葡萄球菌污染的樣品有30%,且多數金黃色葡萄球菌對青霉素具有抗性。Aksomaitiene等[52]對來自牛奶的空腸彎曲桿菌菌株進行測試,結果表明分離的菌株均對至少一種抗生素具有抗性,其中對頭孢曲松、環丙沙星和四環素耐藥率分別為100%、90.2%和85.4%。Wang等[53]對乳制品中分離的乳酸菌進行檢測,發現88.9%的分離株對至少一種抗生素具有耐藥性。因此,不應僅檢測乳制品中致病菌的耐藥性,乳酸菌等益生菌的耐藥性同樣也需要進行監控。
1.4 水果蔬菜類食品
直食性的水果蔬菜中耐藥菌可能對人體具有更大的潛在危害。Wang等[20]對超過一千種食品進行統計研究,發現超過六成直食性的水果蔬菜中檢測出金黃色葡萄球菌,且與肉類食品相比,直食性的水果蔬菜中耐藥菌比例更高,耐藥性更強。Chaj?cka-Wierzchowska等[54]對270份植物源性食品進行鑒定發現,污染最嚴重的為腸球菌,其中對鏈霉素耐藥性最高(55.6%),其次是利福平(51.4%)。與肉類食品相比,水果蔬菜中部分耐藥菌耐藥性更強,且這些耐藥菌可直接進入人體腸道,加快了耐藥菌在腸道中傳播擴散,對人體健康有更大的潛在威脅[55]。
1.5 發酵、腌菜類食品
與其他食品相比,發酵、腌菜類食品中耐藥菌豐度明顯較高[56]。雖然發酵、腌制過程產生的酸、高鹽和厭氧環境對耐藥菌有較好的滅活作用,但是這些環境選擇壓力會進一步促進抗生素耐藥菌的傳播擴散[57-58]。蔡婷等[59]通過研究四川泡菜中乳酸菌的耐藥性,發現經過發酵后耐藥性普遍增強,食竇魏斯菌的抗生素耐藥性甚至提高了3倍。許女等[56]對傳統發酵食品中乳酸菌進行耐藥性分析發現,泡菜、醋醅和發酵乳制品中分離出耐藥表型≥6的多重耐藥菌株明顯增加。
腌肉和臘腸等傳統肉類發酵食品中抗生素耐藥菌污染同樣嚴重[60]。Pisacane等[61]在對意大利傳統香腸進行細菌培養,發現較高數量的葡萄球菌和腸球菌,且多數位于腸衣上。Chajecka-Wierzchowska等[3]對腌肉、臘腸和奶酪的研究顯示,從146份樣品中共分離出58株葡萄球菌,有41.3%的葡萄球菌對頭孢西丁具有耐藥性。Zeng等[62]調查中國在2011—2016年肉制品中的克羅諾桿菌,發現19.1%的臘腸中鑒定出克羅諾桿菌,在所有肉制品中污染最為嚴重。Wang等[63]通過對比中國臘腸和意大利臘腸,發現中國臘腸多為自發發酵,個別衛生條件較差,在細菌豐度和耐藥性方面均超過意大利臘腸。發酵食品中存在的耐藥菌種類較少、總量較低,但耐藥性較強,直接食用含有抗生素耐藥菌的發酵食品,對人體腸道健康的影響值得廣泛關注。
2 食源性耐藥菌在食物鏈的傳播
食物鏈被認為是耐藥菌從動植物傳播到人體的重要途徑[64]。農業和畜牧業的快速發展,使得產品的生產、加工、儲存和分配方式產生了巨大變化,增加了抗生素耐藥菌的傳播風險[20,24,33]。
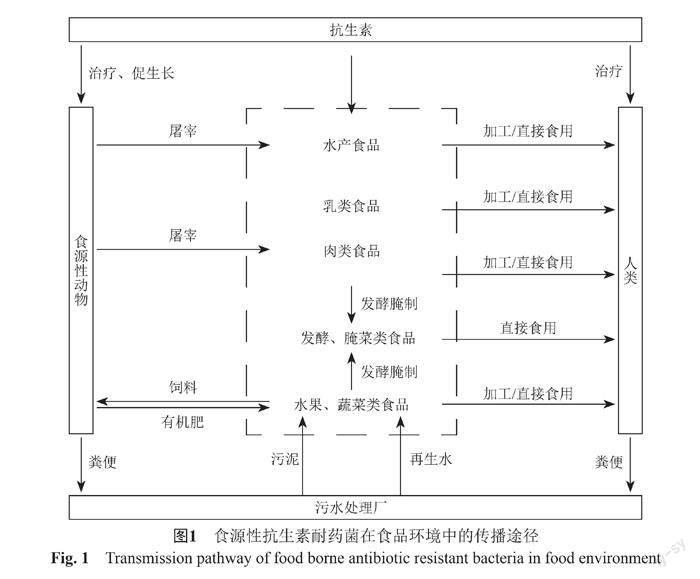
植物源性食品中耐藥菌主要來源于農場環境[65]。進入動物體內的抗生素僅少量參與新陳代謝被生物利用,60%~90%的抗生素在動物體內誘導出抗生素耐藥菌后,同耐藥菌一起隨糞便排出體外[66]。這些糞便直接或堆肥后施用于農田,造成土壤環境中耐藥菌豐度顯著增加。同時,糞便中殘留的抗生素會對土壤中微生物產生選擇壓力,再次誘導產生抗生素耐藥菌[67-68],且灌溉時使用再生水也可對農作物造成污染,這些耐藥菌和抗生素通過食物鏈可以進入人體(圖1)。Zhao等[69]研究農田土壤中施加動物糞便對農作物中細菌的影響,與未施肥相比,施加糞肥的土壤中細菌的耐藥率明顯增加,甚至高出5倍。Xiang等[70]對比有機肥種植與自然生長的蔬菜,發現有機肥種植的蔬菜不僅會增加耐藥菌的豐度,也會增加耐藥菌的多樣性。常旭卉等[71]對施用糞肥的土壤進行檢測,發現土壤中tetG、sulⅠ、qnrA、aadA2、aadD和intⅠ的絕對豐度明顯增加。植物源性食品中耐藥菌在腸道中的傳播尤其值得關注。Zhu等[72]對灌溉再生水的農田進行研究,發現經過污水處理工藝和消毒工藝的再生水,僅含有少量的抗生素耐藥菌,但對農田進行長期灌溉再生水后,依舊可導致農田中抗生素耐藥菌的種類和豐度顯著增加。Ma等[73]通過研究人體腸道和植物源性食品中的乳酸桿菌,發現人體腸道微生物可以從食物中獲得抗生素耐藥性。Losasso等[74]研究不同飲食習慣對腸道菌群耐藥性的影響,發現素食主義者腸道內blaTEM耐藥基因明顯多于雜食者。植物源性食品中抗生素耐藥菌轉移到人體腸道仍是一個亟需解決的問題。
人類也可通過接觸或食用動物源性食品而感染耐藥菌。動物源性食品耐藥菌增多主要有兩種方式。首先,過量抗生素會導致動物腸道菌群等耐藥菌增加[75]。Talley等[76]研究養牛場環境中的細菌,發現使用抗生素的養牛場中牛和果蠅均攜帶有耐藥性的大腸埃希菌O157:H7。Liu等[77]研究發現了養殖場環境與產出豬肉中的抗生素耐藥菌之間的關系,使用抗生素后,豬肉中抗生素耐藥菌明顯增加,且與養豬場環境中耐藥菌種類大致相同,甚至和養豬場周圍土壤也呈現出高度的相似性。Chen等[78]研究污水處理廠出水對水產食品的污染,發現污水處理廠出水中抗生素能夠對10 km內的水環境造成不利影響,且可增加區域內水產食品中抗生素耐藥菌豐度。其次,生產加工、運輸儲存和零售消費的各個階段都可造成耐藥菌的交叉污染[39,79-80]。在農場內,動物糞便中帶有大量耐藥菌,這些耐藥菌可以通過糞口傳播和接觸傳播再次污染動物[79,81]。養殖場內不完善的管理措施和較差的衛生條件,促使耐藥菌在養殖動物之間傳播[39]。Molechan等[80]對雞的運輸設備進行檢測,發現運輸設備中同樣存在耐藥菌,且可在運輸的過程中通過接觸污染雞。另外,農貿市場、超市與餐廳中的砧板和刀具等也是傳播耐藥菌的重要途徑[39]。肉類食品中鑒定出的耐藥菌和耐藥基因在人體中被找到,證明了被污染的食品可通過食物鏈感染人類[82]。Lu等[83]分析了中國上海在2006—2016年從腹瀉患者中分離的沙門菌,從患者和當地豬肉中分離的mcr-1陽性菌株,大多來自同一社區,充分表明豬肉是主要傳染源。Otto等[84]發現,在魁北克和安大略地區患者中分離的耐頭孢噻呋腸道沙門菌主要來源于食用雞肉。
人們通常會使用消毒、腌制、加熱和冷凍等處理方式保證食品和食品加工環境的安全[85]。Yu等[86]研究經過消毒劑苯扎氯銨處理過的單核細胞增生李斯特菌,發現單核細胞增生李斯特菌增加了對頭孢噻肟、頭孢菌素和環丙沙星的耐藥性。Govender等[20,33]對冷藏室內分離的金黃色葡萄球菌進行研究,在和國內未經過冷凍處理的金黃色葡萄球菌相比后,發現從冷藏庫中分離到的金黃色葡萄球菌對青霉素有更強耐藥性。
越來越多的證據表明,抗生素的不規范使用和濫用是導致食品中耐藥菌增加的主要原因。因此,應減少抗生素的使用,并制定食源性食品中抗生素的使用指南。尋找合適的方法治療食用動物,禁止抗生素作為生長促進劑,加強抗生素的管理,并采取合適的感染控制措施。
3 食品的全球貿易促進食源性耐藥菌的全球傳播
食品貿易全球化增加了食源性耐藥菌的全球性傳播擴散[87]。Cavaco等[88]在泰國患者、丹麥患者和進口食品中檢測到有相同喹諾酮耐藥基因qnr的科瓦利斯沙門菌,這些耐藥致病菌多數來源于泰國,且最初在丹麥很少發現qnr耐藥基因,隨著丹麥從泰國進口越來越多的食品,耐藥致病菌在丹麥正在快速傳播。現今,在美國、中國、日本和丹麥檢測到含有喹諾酮耐藥基因的致病菌[88-89]。Vounba等[90]在加拿大、塞內加爾和越南的家禽中分離出的大腸埃希菌均攜帶mcr-1耐藥基因。Van等[91]從尼日利亞、突尼斯和阿爾及利亞的家禽食品中分離出帶mcr-1耐藥基因的大腸埃希菌。
雞肉已經成為食源性耐藥菌在全球傳播的主要載體[92]。中國、巴西和泰國等發展中國家已經成為動物源性食品的主要出口國[93],例如巴西生產的雞肉會銷往美國、中國和英國等142個國家[92]。有證據表明,雞肉生產過程中常使用四環素類、磺胺類和氟喹諾酮類等多種抗生素,導致雞肉中致病菌耐藥性增強,食品貿易的全球化進一步把耐藥性致病菌傳播到世界的其他地區[91]。Roth等[92]利用數據庫篩選出美國本土雞肉和進口雞肉的耐藥菌信息,發現進口雞肉中檢測到大腸埃希菌對氟喹諾酮類藥物的耐藥率均高于40%,但在禁止使用氟喹諾酮類藥物的美國本土雞肉中耐藥率則低于5%。Aarestrup等[94]使用數據庫對丹麥食品中多重耐藥鏈球菌進行分析,其中近一半的多重耐藥鏈球菌來自泰國,且從泰國進口的雞肉中分離的鏈球菌對萘啶酸、鏈霉素、慶大霉素、氨芐西林、氯霉素和環丙沙星的耐藥率分別為88.6%、88.6%、63.6%、52.3%、36.4%和5%,均高于丹麥本土生產的雞肉。食源性耐藥菌在美國、加拿大、英國等發達國家也表現出迅速蔓延的趨勢[95]。食品的貿易全球化能夠把耐藥性致病菌傳播到世界各地,局部地區的管控并不能從整體上降低或消除人類感染耐藥菌的風險。
不同的國家和地區,食源性耐藥菌污染程度不同,但食品在世界各地消費量逐年增加,食品供應鏈全球化加劇了耐藥菌的傳播擴散。目前對食源性抗生素耐藥菌在全球傳播的研究多以數據庫為基礎進行,此種研究方法對解析耐藥菌耐藥機制和預防耐藥菌傳播等有不可比擬的優勢,但龐大數據庫的建立需要長時間大量的耐藥菌與耐藥基因信息,前期投入巨大。此外,抗生素耐藥性已經成為一個全球性問題,它不僅限于某些地區或國家,各個國家都需要持續監測耐藥性致病菌的遷移和進出口狀況,采取多種方式來抑制抗生素耐藥性的出現和傳播[96]。
4 健康風險
人類腸道被認為是抗生素耐藥基因的儲存庫[97]。有證據顯示,農業和養殖業中的耐藥菌是人體腸道微生物中主要的耐藥菌,證明了“農場到餐桌”的假設,即食用被細菌污染的食品能明顯改變人體腸道菌群[98]。腸道微生物在消化和代謝中發揮著至關重要的作用,長期不良的飲食習慣可能導致一些微生物豐度下降,另一些微生物豐度增加,易導致肥胖、炎癥和過敏等不良反應。Takewaki等[99]利用宏基因組技術發現,多發性硬化癥患者腸道菌群與健康人之間存在較大差異,并且證明改善飲食習慣可以改善病癥。Haidar等[100]對一位急性膽囊炎患者進行病因分析,發現可能為食品中耐萬古霉素腸球菌引發的疾病。食品中的耐藥菌和耐藥基因可以直接或間接影響腸道菌群結構,進而影響機體的消化系統[101]、免疫系統[102]和中樞神經系統[103]。
肉類食品引發的人體健康風險亟待解決[104]。Schoen等[105]使用定量微生物風險評估模型對豬肉中耐甲氧西林金黃色葡萄球菌進行評估,被感染的風險為3.20×10-3~1.30×10-2之間,存在人體被耐甲氧西林金黃色葡萄球菌污染的可能。Presi等[106]采用半定量模型對雞肉、豬肉和牛肉中抗生素耐藥菌造成的潛在風險進行評估,雞肉、豬肉和牛肉的風險值分別為6.7、4.0和0.4,且凍肉的風險值最高。
人們普遍認為有機種植的水果、蔬菜更安全健康,然而研究發現有機肥種植的水果、蔬菜可能被抗生素耐藥菌污染[107]。OFlaherty等[108]通過定量模型分析不同種植方式生菜中耐藥大腸埃希菌,結果表明,人體對耐藥大腸埃希菌的平均暴露水平為1.00×10-2~1.35×106 CFU/g之間,每食用100g被耐藥致病性大腸埃希菌污染的生菜,患病的平均概率在1.46×10-9到1.88×10-2之間。Njage等[109]對萵苣中耐藥大腸埃希菌進行模擬,3%的南非居民暴露水平在2.51×106到5.01×106 CFU/g之間。與歐盟食品微生物限量1441/2007的標準進行比較,生菜和萵苣中耐藥大腸埃希菌暴露水平均已經超過1×103 CFU/g,因此,使用有機肥種植的水果蔬菜存在超過微生物限量標準的情況。
發酵食品和多數水果蔬菜都是直食性食品,這些食品中的耐藥菌可以直接進入人體,增加病原菌感染人體的幾率[20,80]。Lakhanpal等[110]研究游牧人直食性牛奶和肉類食品,發現食品中有6.7%被金黃色葡萄球菌所污染,其中對萬古霉素耐藥率為43.8%,游牧人患者萬古霉素治療的失敗和較高的死亡率可能與直接食用攜帶耐萬古霉素金黃色葡萄球菌的食品有關。
大量證據表明,抗生素耐藥菌和抗生素耐藥基因會破壞腸道菌群的穩定,改變菌群組成,進而對人體健康造成影響[11]。目前,食源性耐藥菌污染在中國、印度、巴西和南非共和國等發展中國家較為嚴重,這些國家的人民需要承擔更大健康風險,主要是因為缺乏適當的方法預防和控制食源性耐藥菌的傳播[89]。宏基因組測序技術可以獲得耐藥菌的全部遺傳信息,可對環境中微生物菌群的多樣性、功能活性等宏觀特征進行研究。與傳統技術相比,宏基因測序技術在發現新的耐藥基因和耐藥機制有著其他技術無可比擬的優勢,但測序過程較為繁瑣、價格昂貴。為了降低食源性耐藥菌的感染,應盡可能從源頭降低抗生素耐藥菌的產生,減少抗生素的使用或使用抗生素替代物,同時完善監測體系,長期對食源性耐藥菌進行監測。
5 研究展望
目前,國內外對食源性抗生素耐藥菌做了大量研究,但研究結果多局限在食物中發現或檢測到的耐藥菌,而抗生素耐藥菌在食品中的來源、傳播和轉移機制以及控制對策尚不清楚。因此,對未來食源性耐藥菌的研究提供一些建議,主要包括:
(1)研究抗生素耐藥菌在不同食品環境中的傳播擴散機制,為有效遏制食源性耐藥菌的水平轉移提供新的思路。
(2)探究抗生素耐藥菌通過食物鏈進入人體的傳播機制,揭示耐藥菌在人體腸道的污染水平及潛在健康風險,為控制耐藥菌污染提供理論依據。
(3)加強畜牧業和水產養殖業等抗生素使用監管,探究食品生產、加工、儲存和消費過程中耐藥菌的傳播或去除效果,并對過程中耐藥菌的去除機制進行深入研究。
參 考 文 獻
Duan M, Gu J, Wang X, et al. Factors that affect the occurrence and distribution of antibiotic resistance genes in soils from livestock and poultry farms[J]. Ecotoxicol Environ Saf, 2019, 180(5): 114-122.
Badi S, Cremonesi P, Abbassi M S, et al. Antibiotic resistance phenotypes and virulence-associated genes in Escherichia coli isolated from animals and animal food products in Tunisia[J]. FEMS Microbiol Lett, 2018. 365(10): 88.
Chajecka-Wierzchowska W, Zadernowska A, Nalepa B, et al. Coagulase-negative Staphylococci (CoNS) isolated from ready-to-eat food of animal origin-phenotypic and genotypic antibiotic resistance[J]. Food Microbiol., 2015, 46(4): 222-226.
Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance[J]. Environ Sci Technol, 2015, 49(11): 6772-6782.
Van Boeckel T P, Brower C, Gilbert M, et al. Global trends in antimicrobial use in food animals[J]. Proc Natl Acad Sci U S A, 2015, 112(18): 5649-5654.
付星宇, 汪慶, 畢聰聰, 等. 大氣環境中抗生素耐藥菌的來源與傳播擴散研究進展[J/OL]. 中國抗生素雜志, https://doi.org/10.13461/j.cnki.cja.007088 1224, 2020.
趙小慧, 蘇潔, 樊景鳳, 等. 海洋環境中細菌耐藥性研究進展[J]. 中國抗生素雜志, 2019, 44(4): 406-412.
Chen C, Pankow C A, Oh M, et al. Effect of antibiotic use and composting on antibiotic resistance gene abundance and resistome risks of soils receiving manure-derived amendments[J]. Environ Int, 2019, 128(4): 233-243.
Saharan V V, Verma P, Singh A P, et al. High prevalence of antimicrobial resistance in Escherichia coli, Salmonella spp. and Staphylococcus aureus isolated from fish samples in India[J]. Aquac Res, 2020, 51(3): 1200-1210.
Wu S, Huang J H, Zhang F, et al. Staphylococcus argenteus isolated from retail foods in China: Incidence, antibiotic resistance, biofilm formation and toxin gene profile[J]. Food Microbiol, 2020, 91(2): 103531.
Chen J, Ying G G, Deng W J. Antibiotic residues in food: Extraction, analysis, and human health concerns[J]. J Agr Food Chem, 2019, 67(27): 7569-7586.
楊承霖, 舒剛, 趙小玲, 等. 2010—2016年四川省食品動物源大腸桿菌的耐藥性研究[J]. 西北農林科技大學學報(自然科學版), 2020, 48(9): 24-30.
陳偉冰, 李柏生, 盧向明, 等. 2012—2018年茂名市食品中食源性致病菌污染監測結果分析[J]. 應用預防醫學, 2020, 26(2): 150-152.
鄒志云, 嚴昕宇, 朱惠芳, 等. 2017年無錫地區食品從業人員及食源性疾病患者中分離沙門菌耐藥情況[J]. 江蘇預防醫學, 2020, 31(5): 578-581.
李可維, 劉思潔, 趙薇, 等. 9274份肉及肉制品食源性致病菌監測結果分析[J]. 食品安全質量檢測學報, 2020, 11(23): 9033-9038.
陳偉冰, 李柏生, 李振翠, 等. 廣東省茂名市2017—2018年空腸彎曲菌食品分離株病原特征[J]. 中國熱帶醫學, 2020. 20(5): 452-455.
陶勇, 刁保衛, 王利, 等. 馬鞍山市不同來源副溶血弧菌生物學特征及分子流行病學研究[J]. 公共衛生與預防醫學, 2013. 24(03): 18-22.
馮志寬, 殷文政, 楊榮杰, 等. 乳源性金黃色葡萄球菌的分離及耐藥特性[J]. 食品工業, 2012, 33(6):102-104.
Yang S, Pei X, Wang G, et al. Prevalence of food-borne pathogens in ready-to-eat meat products in seven different Chinese regions[J]. Food Control, 2016, 65(18): 92-98.
Wang Y T, Lin Y T, Wan T W, et al. Distribution of antibiotic resistance genes among Staphylococcus species isolated from ready-to-eat foods[J]. J Food Drug Anal, 2019, 27(4): 841-848.
Osman K, Badr J, Al-Maary K S, et al. Prevalence of the antibiotic resistance genes in coagulase-Positive-and negative-Staphylococcus in chicken meat retailed to consumers[J]. Front Microbiol, 2016, 7(5): 1846.
de Jong A, Simjee S, Garch F E, et al. Antimicrobial susceptibility of enterococci recovered from healthy cattle, pigs and chickens in nine EU countries (EASSA Study) to critically important antibiotics[J]. Vet Microbiol, 2018, 216(2): 168-175.
Ta Y T, Nguyen T T, To P B, et al. Quantification, serovars, and antibiotic resistance of Salmonella isolated from retail raw chicken meat in Vietnam[J]. J Food Prot, 2014, 77(1): 57-66.
Chajecka-Wierzchowska W, Zadernowska A, Laniewska-Trokenheim L. Diversity of antibiotic resistance genes in Enterococcus strains isolated from ready-to-eat meat products[J]. J Food Sci, 2016, 81(11): 2799-2807.
Donado-Godoy P, Byrne B A, Leon M, et al. Prevalence, resistance patterns, and risk factors for antimicrobial resistance in bacteria from retail chicken meat in Colombia[J]. J Food Prot, 2015, 78(4): 751-759.
Adeyanju G T, Ishola O. Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo State, Nigeria[J]. Springerplus, 2014, 3: 139.
Abd-Elghany S M, Sallam K I, Abd-Elkhalek A, et al. Occurrence, genetic characterization and antimicrobial resistance of Salmonella isolated from chicken meat and giblets[J]. Epidemiol Infect, 2015. 143(5): 997-1003.
Ta Y T, Nguyen T T, Phuong B T, et al. Quantification, serovars, and antibiotic resistance of Salmonella isolated from retail raw chicken meat in Vietnam[J]. J Food Protect, 2014, 77(1): 57-66.
Yang B, Cui Y, Shi C, et al. Counts, serotypes, and antimicrobial resistance of Salmonella isolates on retail raw poultry in the Peoples Republic of China[J]. J Food Protect, 2014, 77(6): 894-902.
Yoon R H, Cha S Y, Wei B, et al. Prevalence of Salmonella isolates and antimicrobial resistance in poultry meat from South Korea[J]. J Food Protect, 2014, 77(9): 1579-1582.
Grafskaia E N, Nadezhdin K D, Talyzina I A, et al. Medicinal leech antimicrobial peptides lacking toxicity represent a promising alternative strategy to combat antibiotic-resistant pathogens[J]. Eur J Med Chem, 2019, 180(6): 143-153.
Aguilar-Santelises M, Castillo-Vera J, Gonzalez-Molina R, et al. Clinical isolates of Escherichia coli are resistant both to antibiotics and organotin compounds[J]. Folia Microbiol, 2020, 65(1):87-94.
Govender V, Madoroba E, Magwedere K, et al. Prevalence and risk factors contributing to antibiotic-resistant Staphylococcus aureus isolates from poultry meat products in South Africa, 2015-2016[J]. J S Afr Vet Assoc, 2019, 90(2): 1-8.
Wu S, Wu Q, Zhang J, et al. Listeria monocytogenes prevalence and characteristics in retail raw foods in China[J]. PLoS One, 2015, 10(8): e0136682.
Oliveira T S, Varjao L M, da Silva L N N, et al. Listeria monocytogenes at chicken slaughterhouse: Occurrence, genetic relationship among isolates and evaluation of antimicrobial susceptibility[J]. Food Control, 2018, 88(6): 131-138.
Johnson T A, Stedtfeld R D, Wang Q, et al. Clusters of antibiotic resistance genes enriched together stay together in Swine agriculture[J]. Mbio, 2016, 7(2): e02214-15.
Bacanli M, Basaran N. Importance of antibiotic residues in animal food[J]. Food Chem Toxicol, 2019, 125(1): 462-466.
Wang Y, Hu Y, Cao J, et al. Antibiotic resistance gene reservoir in live poultry markets[J]. J Infect, 2019, 78(6): 445-453.
Monte D F, Lincopan N, Fedorka-Cray P J, et al. Current insights on high priority antibiotic-resistant Salmonella enterica in food and foodstuffs: A review[J]. Curr Opin Food Sci, 2019, 26(8): 35-46.
Jeong H W, Kim J A, Jeon S J, et al. Prevalence, antibiotic-resistance, and virulence characteristics of Vibrio parahaemolyticus in restaurant fish tanks in Seoul, South Korea[J]. Foodborne Pathog Dis, 2020, 17(3): 209-214.
Tan C W, Rukayadi Y, Hasan H, et al. Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia[J]. Saudi J Biol Sci, 2020, 27(6): 1602-1608.
Tran T H T, Yanagawa H, Nguyen K T, et al. Prevalence of Vibrio parahaemolyticus in seafood and water environment in the Mekong Delta, Vietnam[J]. J Vet Med Sci, 2018, 80(11): 1737-1742.
Jiang Y, Chu Y, Xie G, et al. Antimicrobial resistance, virulence and genetic relationship of Vibrio parahaemolyticus in seafood from coasts of Bohai Sea and Yellow Sea, China[J]. Int J Food Microbiol, 2019, 290(19): 116-124.
Ellis-Iversen J, Seyfarth A M, Korsgaard H, et al. Antimicrobial resistant E. coli and Enterococci in pangasius fillets and prawns in Danish retail imported from Asia[J]. Food Control, 2020, 114(5): 106958.
Wu J J, Su Y L, Deng Y Q, et al. Prevalence and distribution of antibiotic resistance in marine fish farming areas in Hainan, China[J]. Sci Total Environ, 2019, 653(4): 605-611.
Tabaran A, Mihaiu M, Tabaran F, et al. First study on characterization of virulence and antibiotic resistance genes in verotoxigenic and enterotoxigenic E. coli isolated from raw milk and unpasteurized traditional cheeses in Romania[J]. Folia Microbiol, 2017, 62(2): 145-150.
Guo H, Pan L, Li L, et al. Characterization of antibiotic resistance genes from Lactobacillus isolated from traditional dairy products[J]. J Food Sci, 2017, 82(3): 724-730.
Pizauro L J L, de Almeida C C, Soltes G A, et al. Short communication: Detection of antibiotic resistance, mecA, and virulence genes in coagulase-negative Staphylococcus spp. from buffalo milk and the milking environment[J]. J Dairy Sci, 2019, 102(12): 11459-11464.
Yehia H M, Al-Masoud A H, Alarjani K M, et al. Prevalence of methicillin-resistant (mecA gene) and heat-resistant Staphylococcus aureus strains in pasteurized camel milk[J]. J Dairy Sci, 2020, 103(7): 5947-5963.
Sharma V, Sharma S, Dahiya D K, et al. Coagulase gene polymorphism, enterotoxigenecity, biofilm production, and antibiotic resistance in Staphylococcus aureus isolated from bovine raw milk in North West India[J]. Ann Clin Microbiol Antimicrob, 2017. 16(1): 65.
Ameen F, Reda S A, El-Shatoury S A, et al. Prevalence of antibiotic resistant mastitis pathogens in dairy cows in Egypt and potential biological control agents produced from plant endophytic actinobacteria[J]. Saudi J Biol Sci, 2019, 26(7): 1492-1498.
Aksomaitiene J, Ramonaite S, Tamuleviciene E, et al. Overlap of antibiotic resistant Campylobacter jejuni MLST genotypes isolated from humans, broiler products, dairy cattle and wild birds in Lithuania[J]. Front Microbiol, 2019. 10: 1377.
Wang K, Zhang H, Feng J, et al. Antibiotic resistance of lactic acid bacteria isolated from dairy products in Tianjin, China[J]. J Agr Food Res, 2019, 1(72): 100006.
Chaj?cka-Wierzchowska W, Zarzecka U, Zadernowska A. Enterococci isolated from plant-derived food-analysis of antibiotic resistance and the occurrence of resistance genes[J]. Lwt-food Sci Technol, 2020, 139(3): 110549.
Li Y, Cao W, Liang S, et al. Metagenomic characterization of bacterial community and antibiotic resistance genes in representative ready-to-eat food in southern China[J]. Sci Rep, 2020, 10(1): 15175.
許女, 李雅茹, 王超宇, 等. 傳統發酵食品中乳酸菌的抗生素耐藥性評估及耐藥基因分析[J]. 中國食品學報, 2020, 20(7): 160-171.
向文良, 張慶, 盧倩文, 等. 獲得性抗生素抗性基因:威脅發酵蔬菜食品安全的一種新型污染物[J]. 食品安全質量檢測學報, 2015. 6(10): 3917-3922.
Liao X Y, Ma Y N, Daliri E B M, et al. Interplay of antibiotic resistance and food-associated stress tolerance in foodborne pathogens[J]. Trends Food Sci Technol, 2020, 95(1): 97-106.
蔡婷, 徐顧榕, 林凱, 等. 發酵用鮮辣椒中乳酸菌抗生素耐藥性與耐藥基因[J]. 食品與生物技術學報, 2016, 35(9): 941-949.
Lopez C M, Callegari M L, Patrone V, et al. Assessment of antibiotic resistance in Staphylococci involved in fermented meat product processing[J]. Curr Opin Food Sci, 2020, 31(4): 17-23.
Pisacane V, Callegari M L, Puglisi E, et al. Microbial analyses of traditional Italian salami reveal microorganisms transfer from the natural casing to the meat matrix[J]. Int J Food Microbiol, 2015, 207(16): 57-65.
Zeng H, Li C, Ling N, et al. Prevalence, genetic analysis and CRISPR typing of Cronobacter spp. isolated from meat and meat products in China[J]. Int J Food Microbiol, 2020, 321(3): 108549.
Wang X, Zhang Y, Ren H, et al. Comparison of bacterial diversity profiles and microbial safety assessment of salami, Chinese dry-cured sausage and Chinese smoked-cured sausage by high-throughput sequencing[J]. Lwt-food Sci Technol, 2018, 90(12): 108-115.
Gibson M K, Crofts T S, Dantas G. Antibiotics and the developing infant gut microbiota and resistome[J]. Curr Opin Microbiol, 2015, 27(20): 51-56.
Wei R, He T, Zhang S, et al. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China[J]. Chemosphere, 2019, 215(1): 234-240.
徐秋桐, 顧國平, 章明奎. 土壤中獸用抗生素污染對水稻生長的影響[J]. 農業資源與環境學報, 2016, 33(1): 60-65.
McKinney C W, Dungan R S, Moore A, et al. Occurrence and abundance of antibiotic resistance genes in agricultural soil receiving dairy manure[J]. Fems Microbiol Ecol, 2018, 94(3): 10.
Wang H, Chu Y, Fang C. Occurrence of Veterinary Antibiotics in Swine Manure from Large-scale Feedlots in Zhejiang Province, China[J]. Bull Environ Contam Toxicol, 2017, 98(4): 472-477.
Zhao X, Wang J, Zhu L, et al. Field-based evidence for enrichment of antibiotic resistance genes and mobile genetic elements in manure-amended vegetable soils[J]. Sci Total Environ, 2019, 654(5): 906-913.
Xiang W, Lu K, Zhang N, et al. Organic Houttuynia cordata Thunb harbors higher abundance and diversity of antibiotic resistance genes than non-organic origin, suggesting a potential food safe risk[J]. Food Res Int, 2019, 120(11): 733-739.
常旭卉, 賈書剛, 王淑平, 等. 糞源環丙沙星對潮土中抗生素抗性基因的影響[J]. 農業環境科學學報, 2018, 37(12): 2727-2737.
Zhu N, Jin H M, Ye X M, et al. Fate and driving factors of antibiotic resistance genes in an integrated swine wastewater treatment system: From wastewater to soil[J]. Sci Total Environ, 2020, 721(4): 137654.
Ma Q, Fu Y, Sun H, et al. Antimicrobial resistance of Lactobacillus spp. from fermented foods and human gut[J]. Lwt-food Sci Technol, 2017, 86(12): 201-208.
Losasso C, Di Cesare A, Mastrorilli E, et al. Assessing antimicrobial resistance gene load in vegan, vegetarian and omnivore human gut microbiota[J]. Int J Antimicrob Agents, 2018, 52(5): 702-705.
Onaran B, Goncuoglu M, Bilir Ormanci F S. Antibiotic resistance profiles of vancomycin resistant enterococci in chicken meat samples[J]. Ankara Univ Vet Fak, 2019, 66(4): 331-336.
Talley J L, Wayadande A C, Wasala L P, et al. Association of Escherichia coli O157:H7 with filth flies (Muscidae and Calliphoridae) captured in leafy greens fields and experimental transmission of E. coli O157:H7 to spinach leaves by house flies (Diptera: Muscidae)[J]. J Food Prot, 2009, 72(7): 1547-1552.
Liu Z, Klumper U, Shi L, et al. From pig breeding environment to subsequently produced pork: Comparative analysis of antibiotic resistance genes and bacterial community composition[J]. Front Microbiol, 2019, 10(10): 43.
Chen Y, Shen W T, Wang B, et al. Occurrence and fate of antibiotics, antimicrobial resistance determinants and potential human pathogens in a wastewater treatment plant and their effects on receiving waters in Nanjing, China[J]. Ecotoxicol Environ Saf, 2020, 206(12): 483-490.
Bai L, Lan R, Zhang X, et al. Prevalence of Salmonella isolates from chicken and pig Slaughterhouses and emergence of ciprofloxacin and cefotaxime co-resistant S. enterica serovar Indiana in Henan, China[J]. PLoS One, 2015, 10(12): e0144532.
Molechan C, Amoako D G, Abia A L K, et al. Molecular epidemiology of antibiotic-resistant Enterococcus spp. from the farm-to-fork continuum in intensive poultry production in KwaZulu-Natal, South Africa[J]. Sci Total Environ, 2019, 692(14): 868-878.
Mascaro V, Squillace L, Nobile C G, et al. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) carriage and pattern of antibiotic resistance among sheep farmers from Southern Italy[J]. Infect Drug Resist, 2019, 12: 2561-2571.
Smith K E, Besser J M, Hedberg C W, et al. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. Investigation Team[J]. N Engl J Med, 1999, 340(20): 1525-1532.
Lu X, Zeng M, Xu J, et al. Epidemiologic and genomic insights on mcr-1-harbouring Salmonella from diarrhoeal outpatients in Shanghai, China, 2006-2016[J]. EBio Medicine, 2019, 42: 133-144.
Otto S J, Carson C A, Finley R L, et al. Estimating the number of human cases of ceftiofur-resistant Salmonella enterica serovar Heidelberg in Quebec and Ontario, Canada[J]. Clin Infect Dis, 2014, 59(9): 1281-1290.
Al-Nabulsi A A, Osaili T M, Shaker R R, et al. Effects of osmotic pressure, acid, or cold stresses on antibiotic susceptibility of Listeria monocytogenes[J]. Food Microbiol, 2015, 46(4): 154-160.
Yu T, Jiang X, Zhang Y, et al. Effect of benzalkonium chloride adaptation on sensitivity to antimicrobialagents and tolerance to environmental stresses in Listeria monocytogenes[J]. Front Microbiol, 2018, 9(11): 2906.
Thapa S P, Shrestha S, Anal A K. Addressing the antibiotic resistance and improving the food safety in food supply chain (farm-to-fork) in Southeast Asia[J]. Food Control, 2020, 108(2): 106809.
Cavaco L M, Hendriksen R S, Aarestrup F M. Plasmid-mediated quinolone resistance determinant qnrS1 detected in Salmonella enterica serovar Corvallis strains isolated in Denmark and Thailand[J]. J Antimicrob Chemother, 2007, 60(3): 704-706.
Ma Y, Xu X, Gao Y, et al. Antimicrobial resistance and molecular characterization of Salmonella enterica serovar Corvallis isolated from human patients and animal source foods in China[J]. Int J Food Microbiol, 2020, 335(24): 108859.
Vounba P, Rhouma M, Arsenault J, et al. Prevalence of colistin resistance and mcr-1/mcr-2 genes in extended-spectrum beta-lactamase/AmpC-producing Escherichia coli isolated from chickens in Canada, Senegal and Vietnam[J]. J Glob Antimicrob Resist, 2019, 19(4): 222-227.
Van T T H, Yidana Z, Smooker P M, et al. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses[J]. J Glob Antimicrob Resist, 2020, 20(1): 170-177.
Roth N, Kasbohrer A, Mayrhofer S, et al. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview[J]. Poult Sci, 2019, 98(4): 1791-1804.
Van T T, Nguyen H N, Smooker P M, et al. The antibiotic resistance characteristics of non-typhoidal Salmonella enterica isolated from food-producing animals, retail meat and humans in South East Asia[J]. Int J Food Microbiol, 2012, 154(3): 98-106.
Aarestrup F M, Hendriksen R S, Lockett J, et al. International spread of multidrug-resistant Salmonella schwarzengruna in food products[J]. Emerg Infect Dis, 2007, 13(5): 726-731.
Chen Y, Hammer E E, Richards V P. Phylogenetic signature of lateral exchange of genes for antibiotic production and resistance among bacteria highlights a pattern of global transmission of pathogens between humans and livestock[J]. Mol Phylogenet Evol, 2018, 125(8): 255-264.
Barrow P A, Jones M A, Smith A L, et al. The long view: Salmonella-the last forty years[J]. Avian Pathol, 2012, 41(5): 413-420.
Hu Y, Yang X, Qin J, et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota[J]. Nat Commun, 2013, 4(7): 2151.
Forslund K, Sunagawa S, Kultima J R, et al. Country-specific antibiotic use practices impact the human gut resistome[J]. Genome Res, 2013, 23(7): 1163-1169.
Takewaki D, Suda W, Sato W, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis[J]. Proc Natl Acad Sci U S A, 2020, 117(36): 22402-22412.
Haidar G, Green M, American society of transplantation infectious diseases community of practice Intra-abdomina infections in solid organ transplant recipients: Guidelines from the American society of transplantation infectious diseases community of practice[J]. Clin Transplant, 2019, 33(9): e13595.
Requena T, Cotter P, Shahar D R, et al. Interactions between gut microbiota, food and the obese host[J]. Trends Food Sci Tech, 2013, 34(1): 44-53.
Dunn S J, Connor C, McNally A. The evolution and transmission of multi-drug resistant Escherichia coli and Klebsiella pneumoniae: The complexity of clones and plasmids[J]. Curr Opin Microbiol, 2019, 51(20): 51-56.
Esmaeil A? M, Shomali N, Bakhshi A, et al. Gut microbiome and multiple sclerosis: New insights and perspective[J]. Int Immunopharmacol, 2020, 88(9): 107024.
Alban L, Nielsen E O, Dahl J. A human health risk assessment for macrolide-resistant Campylobacter associated with the use of macrolides in Danish pig production[J]. Prev Vet Med, 2008, 83(2): 115-129.
Schoen M E, Peckham T K, Shirai J H, et al. Risk of nasal colonization of methicillin-resistant Staphylococcus aureus during preparation of contaminated retail pork meat[J]. Microbial Risk Anal, 2020, 16(3): 100136.
Presi P, Stark K D, Stephan R, et al. Risk scoring for setting priorities in a monitoring of antimicrobial resistance in meat and meat products[J]. Int J Food Microbiol, 2009, 130(2): 94-100.
Zhu B, Chen Q, Chen S, et al. Does organically produced lettuce harbor higher abundance of antibiotic resistance genes than conventionally produced?[J]. Environ Int, 2017, 98(1): 152-159.
OFlaherty E, Solimini A G, Pantanella F, et al. Human exposure to antibiotic resistant-Escherichia coli through irrigated lettuce[J]. Environ Int, 2019, 122(1): 270-280.
Njage P M, Buys E M. Quantitative assessment of human exposure to extended spectrum and AmpC beta-lactamases bearing E. coli in lettuce attributable to irrigation water and subsequent horizontal gene transfer[J]. Int J Food Microbiol, 2017, 240(1): 141-151.
Lakhanpal P, Panda A K, Chahota R, et al. Incidence and antimicrobial susceptibility of Staphylococcus aureus isolated from ready-to-eat foods of animal origin from tourist destinations of North-western Himalayas, Himachal Pradesh, India[J]. J Food Sci Technol, 2019, 56(2): 1078-1083.
Kim J, Park H, Kim J, et al. Comparative analysis of aerotolerance, antibiotic resistance, and virulence gene prevalence in Campylobacter jejuni isolates from retail raw chicken and duckmeat in South Korea[J]. Microorganisms, 2019, 7(10): 433.
Maktabi S, Ghorbanpoor M, Hossaini M, et al. Detection of multi-antibiotic resistant Campylobacter coli and Campylobacter jejuni in beef, mutton, chicken and water buffalo meat in Ahvaz, Iran[J]. Vet Res Forum, 2019, 10(1): 37-42.
Abd-Elghany S M, Sallam K I, Abd-Elkhalek A, et al. Occurrence, genetic characterization and antimicrobial resistance of Salmonella isolated from chicken meat and giblets[J]. Epidemiol Infect, 2015, 143(5): 997-1003.
Narvaez-Bravo C, Taboada E N, Mutschall S K, et al. Epidemiology of antimicrobial resistant Campylobacter spp. isolated from retail meats in Canada[J]. Int J Food Microbiol, 2017, 253(8): 43-47.
Beier R C, Byrd J A, Andrews K, et al. Disinfectant and antimicrobial susceptibility studies of the foodborne pathogen Campylobacter jejuni isolated from the litter of broiler chicken houses[J]. Poultry Science, 2020, 100(2): 1024-1033.
收稿日期:2020-12-29
基金項目:國家自然科學基金(No. 42077393和No. 41703088);河北省重點研發計劃項目(No.19273707D);
河北省自然科學基金青年基金(No.C2018402255)
作者簡介:曹弘揚,男,生于1993年,在讀碩士研究生,研究方向為環境微生物,E-mail: caohongyang086@163.com
*通訊作者, E-mail: wangqing@hebeu.edu.cn

