Protective effects of female reproductive factors on gastric signet-ring cell carcinoma
INTRODUCTION
Gastric cancer (GC) is the fifth most common adenocarcinoma and ranks third in mortality worldwide[1]. In China, an estimated 390000 people die of GC annually, which accounts for > 50% of the global deaths and imposes a severe health burden[1-3]. Commonly, the age-standardized incidence rates of GC have shown a male predominance with the male-to-female rate of more than 2:1 in most populations around the world[1,4]. The difference, however, cannot be entirely attributed to the different prevalence of established major risk factors, such as tobacco smoking[5] and
infection[6] between the sexes. A study reported that incidence of intestinal GC after menopause increased with time, and the incidence 10 years after menopause was comparable to that of men[7]. An umbrella review including 616630 women in six observational studies showed that menopausal hormonal therapy was associated with decreased risk of GC[8]. The female reproductive factors and sex hormones may have protective effects on gastric adenocarcinoma.
Ethical approval was obtained through the Chinese Academy of Medical Science (Beijing, China).
Moreover, various reproductive factors have provided contradictory results in relation to the risk of GCs. The protective effects of female reproductive factors imply the potential role of sex hormones in carcinogenesis. Importantly, the explanation for the predominance of GSRC in young women might provide significant clues to the etiology of the tumors and pave the way for research on innovative prevention and treatment. The effect of female reproductive factors on GSRC tumorigenesis and tumor development remains unclear despite the advances in the understanding of its epidemiology and clinicopathology. The purpose of this study was to estimate the effects of female reproductive factors on the prognosis and combined modality treatment of GSRC.
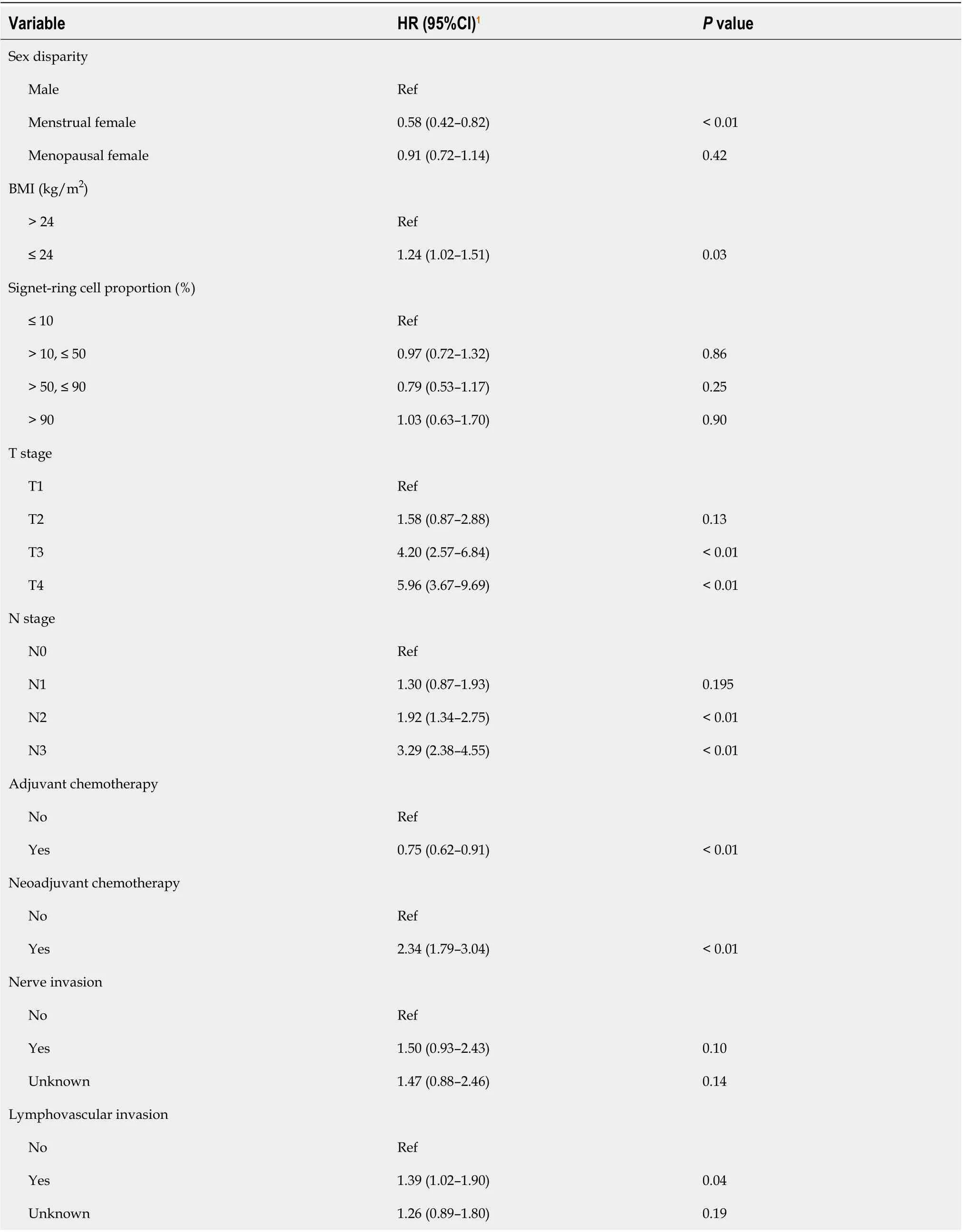
The Cox proportional hazards regression model results are presented in Table 2. Overall, the menstrual women had a significantly lower risk of mortality (HR = 0.58, 95%CI: 0.42–0.82) than male patients in the multivariable model. We did not observe this protective effect in menopausal women (HR = 0.91, 95%CI: 0.72–1.14).
真正做好黨建組織保障。企業(yè)黨建高質(zhì)量發(fā)展離不開(kāi)制度和人才的雙重保障。海陵藥業(yè)共有員工459人,黨員39人,成立一個(gè)黨支部,兩個(gè)黨小組。在制度層面,海陵藥業(yè)將支委會(huì)研究討論、提出意見(jiàn)明確為管理層決策的前置程序,確保黨組織有效參與企業(yè)重大決策,真正做到黨建工作與行政工作合心、合力、合拍。在組織構(gòu)架層面,由總經(jīng)理兼任黨支部書(shū)記,由黨員高管兼任支部委員,配備黨務(wù)副書(shū)記和黨務(wù)專(zhuān)員,專(zhuān)職協(xié)助書(shū)記工作,組建兼具組織力和領(lǐng)導(dǎo)力的高效團(tuán)隊(duì)。
MATERIALS AND METHODS
Study population and follow-up duration
Our study involved 1431 participants who were histologically confirmed with GC with signet-ring cells and underwent curative resection between January 2011 and December 2018 at the Cancer Hospital, Chinese Academy of Medical Sciences, China. Subtotal gastrectomy was performed for distal GCs, whereas total gastrectomy was conducted for proximal-third GCs. Patients with definitive signs of distant organ or peritoneal seeding metastases did not undergo gastrectomy and were referred for evaluation for chemotherapy instead. Based on the National Comprehensive Cancer Network Clinical Practice Guidelines, standard D2 lymphadenectomy was achieved in patients with curative intention[15]. All the surgical specimens were confirmed separately and independently by at least two experienced pathologists. Disagreements were resolved by discussion, especially on the proportion of signet-ring cells. The follow-up data were prospectively collected and regularly updated by surgeons every 6 mo after surgery. The overall survival was defined from the date of gastrectomy to the date of death or the end of follow-up period (April 30, 2020).
Measurements
Despite the aforementioned strengths of our study, several limitations have to be acknowledged. First, the data concerning the expression levels of ER and progesterone receptor (PR) were unavailable. Thus, we cannot conduct further analysis on the effects of ER and PR on GSRC prognosis and other molecular mechanisms. Further investigations are needed to clarify the biological role of estrogen or ER in the carcinogenesis and chemoresistance of GSRC. Second, future studies with a more specific measure of signet-ring cells in GC and more comprehensive long-term outcomes (
, the recurrence or chemoresistance, could offer more information) will be needed to verify the conclusion in this study.
Although the incidence of GC has been decreasing, this disease remains the third cause of cancer mortality worldwide and in China. The proportion of GSRC in all GC cases has been increasing recently, especially in the young and female populations. There are obvious differences on morbidity of GSRC between the sexes. To find a new treatment and improve the overall survival of GSRC, this study has clinical significance to investigate the disparities of reproductive factors between male and female GSRC patients.
Statistical analysis
The continuous variables were expressed as mean ± SD, while categorical variables were expressed as number of observations and percentages (%). Differences in the potential covariates between the sex groups were assessed by Wilcoxon–Mann–Whitney test for continuous variables and the
test for categorical variables. The proportional hazards assumption was estimated, and the Cox multivariable model was used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between the sex factors and survival of GC patients with signet-ring cell carcinoma. The model was adjusted for following potential covariates: BMI, proportion of signet-ring cells, T stage, N stage, adjuvant chemotherapy, neoadjuvant chemotherapy, nerve invasion, and lymphatic vessel invasion. Subgroup analyses were conducted by (1) BMI; (2) Signet-ring cells proportion; (3) Adjuvant chemotherapy; (4) Neoadjuvant chemotherapy; (5) Nerve invasion; and (6) Lymphatic vessel invasion to explore if the impact of the sex difference was stronger in certain groups. Interaction terms between exposures and these covariates were added into the multivariable model, and Wald tests were used to examine whether the interaction terms were statistically significant. The survival curves were estimated by the Kaplan–Meier method. In the present analysis, two-sided
< 0.05 was considered to indicate statistically significant differences. All statistical analyses (and figures created) were performed with Stata 15.0 (StataCorp LLC: College Station, TX, United States).
RESULTS
Clinicopathological features of GSRC in male and female patients
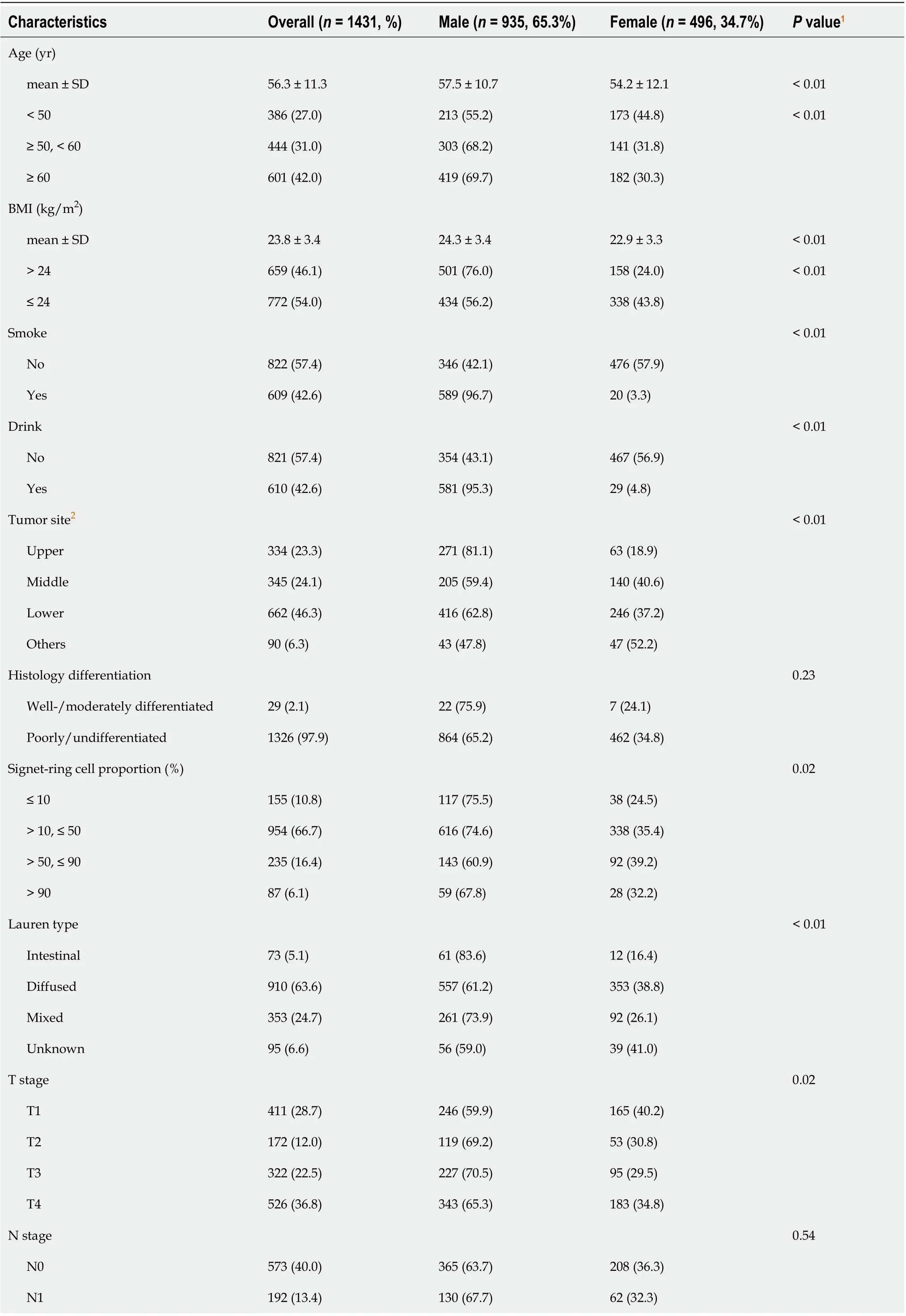
Table 1 displays the distributions of the demographic and potential risk factors by sex difference. Of the 1431 GC patients with signet-ring cell carcinoma, 935 (65.3%) were male, and 496 (34.7%) were female. Overall, over one-third of the participants were aged ≥ 60 (42.0%) years, with a mean age of 56.3 (SD: 11.3) years. There were no significant differences between the sex groups in terms of histological differentiation, N stage, and adjuvant or neoadjuvant chemotherapy (
> 0.05). The female subjects were more likely to be: younger, nonsmokers and nondrinkers; with diffuse Lauren type, T1 stage and metastasis; without nerve and lymphovascular invasion; and have middle and lower tumor location, lower BMI, higher signet-ring cells proportion, and more lymph nodes removed (
< 0.05).
為患者開(kāi)展醫(yī)療安全培訓(xùn),使患者的自我防護(hù)意識(shí)獲得提高,治療期間,需要對(duì)患者積極配合治療意識(shí)加以培養(yǎng),為患者提供安全健康的培訓(xùn)工作,充分尊重患者自身權(quán)力,同時(shí)和患者家屬保持良好的交流,使其更為詳細(xì)并且全面的掌握患者病情,提高患者的康復(fù)速度[3] 。
Subgroup analysis in the different sex groups with GSRC
Since chemoresistance is a distinct feature of GSRC, we noticed that the adjuvant chemotherapy was a protective factor in multivariate analysis. Then, we performed further subgroup analysis on adjuvant chemotherapy effects. Interestingly, protective effects exerted by female reproductive factors were observed only in the nonadjuvant chemotherapy group (HR = 0.38, 95%CI: 0.22–0.56) and the nonneoadjuvant chemotherapy group (HR = 0.53, 95%CI: 0.36–0.77). However, female reproductive factors lost their advantages and did not improve the survival after chemotherapy. It is possible that these conditions of poor survival and chemoresistance are mediated by female reproductive factors, such as ERα and ERβ, with unknown mechanism. Recently, Wang
[30] proposed that the loss of ERβ in GSRC cells might increase the potential for malignant invasion into the deep tissues easier through the mTOR signaling pathway. Therefore, ERβ might inhibit the malignancy of GSRC and can thus become a potential target in its adjuvant treatment. The investigation of Wang did not discuss the connection between ERβ and chemotherapy. We noticed that the prognostic trends are similar in all three studied groups. However, in the subgroup analysis of adjuvant chemotherapy, the mean survival time in the menstrual female group (41.4 mo) was the shortest compared to those of the male (47.6 mo) and menopausal female (45.1 mo) groups. We considered that the probable reason was the proportion of signet-ring cells in GCs. However, no association was identified between the signet-ring cell proportion and influence of female reproductive factors on the prognosis of subjects who received chemotherapy. The results of recent studies confirm the difficulties to understand the possible mechanism between sex hormones and chemoresistance in GSRC. However, more research on this topic needs to be undertaken to clearly elucidate the association between ER and chemotherapy outcomes.

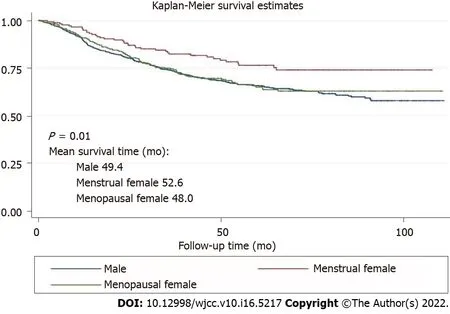
Comparison of overall survival in GSRC between the sexes
The survival curves in Figure 1 depict the survival probability based on the sex difference. Menstrual female patients had a significantly better overall survival than the male and menopausal female groups (
< 0.01). Survival analysis showed better prognosis of menstrual female patients in the non-adjuvant chemotherapy group (
< 0.01) and the inadequate survival advantages of menstrual female patients in the adjuvant chemotherapy group (
= 0.73) (Figure 2). We compared overall survival between the sexes with different levels of signet-ring cells (Figure 3). Menstrual female patients had a survival advantage compared with male and menopausal female groups; however, this advantage was not significant in the GSRC group with > 50% signet-ring cells (Supplementary Figure 1).
DISCUSSION
在工業(yè)化發(fā)展的進(jìn)程中,三次產(chǎn)業(yè)產(chǎn)值在GDP中的占比呈現(xiàn)出一定的規(guī)律性:在前工業(yè)化階段,農(nóng)業(yè)作為經(jīng)濟(jì)的主導(dǎo)產(chǎn)業(yè),GDP主要來(lái)自于農(nóng)業(yè)產(chǎn)值;在工業(yè)化初期,農(nóng)業(yè)產(chǎn)值在GDP中的占比仍大幅高于工業(yè)產(chǎn)值;在工業(yè)化中期,隨著經(jīng)濟(jì)的發(fā)展和工業(yè)化的推進(jìn),農(nóng)業(yè)產(chǎn)值在GDP中的比重有所下降,工業(yè)產(chǎn)值占比大幅上升,第三產(chǎn)業(yè)不斷涌現(xiàn);在工業(yè)化后期,第三產(chǎn)業(yè)產(chǎn)值在GDP中的占比將會(huì)高于第二產(chǎn)業(yè);進(jìn)入發(fā)達(dá)經(jīng)濟(jì)初級(jí)期和發(fā)達(dá)經(jīng)濟(jì)高級(jí)期,依然是第三產(chǎn)業(yè)為主導(dǎo)產(chǎn)業(yè),其產(chǎn)值在三次產(chǎn)業(yè)中的占比最大。根據(jù)庫(kù)茲涅茨對(duì)于三次產(chǎn)業(yè)結(jié)構(gòu)和工業(yè)化發(fā)展階段關(guān)系的研究結(jié)果,得出三次產(chǎn)業(yè)結(jié)構(gòu)指標(biāo)衡量工業(yè)化發(fā)展水平的標(biāo)準(zhǔn)(見(jiàn)表2)。
要發(fā)揮園林綠化企業(yè)檔案管理的科學(xué)性和有效性,就要分析園林綠化企業(yè)檔案管理的主要特點(diǎn),才能夠有的放矢地推進(jìn)企業(yè)的檔案管理上一個(gè)更新的臺(tái)階。
In this study, we focused on the influence of the sex-specific differences on the prognosis of GSRC. Our results showed that there was a stronger positive association with overall survival in menstrual female patients with GSRC, compared to that of the male or menopause female patients. The findings of the present large patient-based retrospective study may provide valuable insights into pathways for reducing GSRC mortality in the future.
The multivariate analysis results in our study showed that being a menstrual woman was a protective factor (HR = 0.58, 95%CI: 0.42–0.82) against GSRC. A similar effect was previously reported in various types of GCs[17,18]. In a study with 758 patients, Kim
[7] proposed that female reproductive hormones might be a potentially protective factor against intestinal-type GC, and the incidence of intestinal-type GC after the menopause increased and became comparable to that in men. A Japanese study revealed that the risk of GC was lower in menstrual women (HR = 0.33, 95%CI: 0.23–0.49). Protective effects were observed in differentiated histological types (HR = 0.25, 95%CI: 0.11–0.55) and undifferentiated histological types (HR = 0.39, 95%CI: 0.23–0.63)[19]. The aforementioned consistent results demonstrate the effects of female reproductive factors in reducing the risks of different GCs. In a large Chinese prospective study with female subjects, the risks of GC increased with age of menopausal women (HR = 0.80 per 5-year increase in the menopausal age, 95%CI: 0.66–0.97)[20]. A similar finding was also obtained in a cohort study including 1 million women whose risks of GC in the menopausal age were considerably higher than those in the menstrual age (RR = 1.46, 95%CI: 1.07–2.0 and RR = 1.59, 95%CI: 1.15–2.20, respectively) from the United Kingdom[21]. Our results indicate that the effect of female reproductive factors on GSRC development is steady and robust.
Here, we hypothesize that sex hormones or their receptors, such as estrogen and estrogen receptor (ER), are the key inducers of the protective effects of female reproductive factors against GSRC. The influence of estrogen has been studied by other researchers, but the results were controversial[22]. Estrogens regulate the tissue growth, differentiation, and function, which is mediated by ER-α and ER-β. The oncological importance of estrogen and ER in carcinomas occurring in the breast and ovaries has been investigated but not in GCs. The expression of ER in the stomach is a basis on which to explore the relationship between sex hormones and survival of GC. Some researchers have demonstrated the expression of ER in gastric tissues, which provides a basis for the probable functions of estrogen in this respect[23-25]. Hess
[26] established that ERs and female sex hormones were expressed in the male reproductive tract and may have some functions. The protective effect of estrogen was discovered also in men who received hormonal therapy for prostate cancer; notably, the results showed a decrease risk of GC[27].
ER has several types, including ERα, ERβ and ERγ. The biological actions of estrogen are mediated through two specific ERs, ERα and ERβ, which belong to the nuclear receptor superfamily[28]. Zhao
[25] and Matsuyama
[29] reported that both ERα, ERβ were expressed in poorly differentiated adenocarcinoma specifically in gastric signet-ring cell adenocarcinomas with characteristics of sex hormone dependency. Different effects of these two ER types were found in various studies. For example, Kameda
[27] proposed that ERα expressed in diffuse-type GC promoted the proliferation of ERα-positive GC cells. The suggested mechanism was that the activation of the ERα pathway stimulated cancer cell proliferation by activating the hedgehog pathway in a ligand-dependent but dose-independent manner
Shh induction of diffuse-type GC. ERβ is homologous to ERα, particularly in the DNA-binding domain, but it is structurally and functionally different. ERβ manifested strong cytoplasmic staining with anti-ERβ antibody in addition to the stained nuclei[29]. This result provides evidence for the potential of GSRC treatment targets. Recently, using genomic analysis, Kang
[29] identified ERγ as a potential tumor suppressor in GC. The molecular mechanisms of its action suggest that the activation of ERγ by the antagonizing Wnt signaling through DY131 could suppress GC cell growth and tumorigenesis. Studies on tumorigenesis regulated by estrogens or estrogen receptors are rare but will be urgently needed in the future.

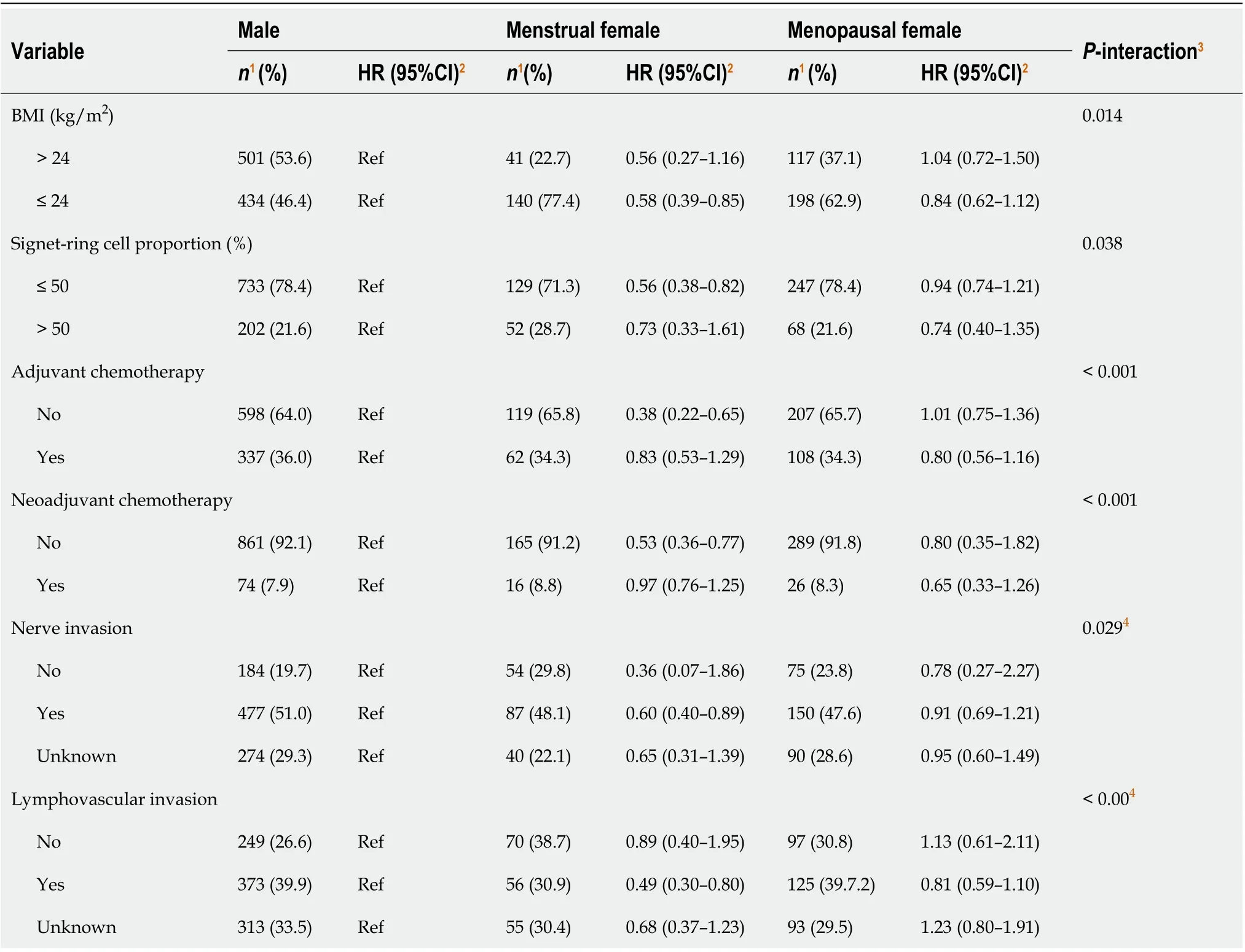
Other variables associated with the overall survival included BMI, T stage, N stage, adjuvant or neoadjuvant chemotherapy, and lymphovascular invasion. The results of the subgroup analyses (Table 3) showed that the impact of menstruation was more significant in the female participants with lower levels of signet-ring cells (HR = 0.56, 95%CI: 0.38–0.82,
-interaction = 0.038), nerve invasion (yes
no, HR = 0.60, 95%CI: 0.40–0.89,
-interaction = 0.029), or lymphovascular invasion (yes
no, HR = 0.49, 95%CI: 0.30–0.80,
-interaction < 0.001), or without adjuvant chemotherapy (HR = 0.38, 95%CI: 0.22–0.65,
-interaction < 0.001) or neoadjuvant chemotherapy (HR = 0.53, 95%CI: 0.36–0.77,
interaction < 0.001). There was a reversal effect across the strata of BMI for menopausal women (crossover interactions).
We investigated the effects of female reproductive factors on the prognosis of the GSRC with various proportions of signet-ring cells. Being a menstrual female was a protective factor (HR = 0.56, 95%CI: 0.38–0.82) and was associated with better overall survival than that of menopausal women and men with GSRC cells < 50%. However, menstrual women lost their GSRC-associated advantages over menopausal women at a proportion of the signet-ring cells > 50%. We supposed that ERβ downregulation or ectopic expression in the cytoplasm could lead to a worse prognosis of GSRC with a higher proportion of signet-ring cells. Nevertheless, the probable mechanism remains unclear. Future studies on the topic are therefore highly recommended.
This is the first large population study focused on the relationship between female reproductive factors and GSRC with an 8-year follow-up. Our results highlight the significant effect of female reproductive factors on GSRC prognosis. The clinical importance of our present findings is critical as they have outlined the prognostic role of female reproductive factors in GSRC. Therefore, further strong evidence is provided which will increase the GSRC treatment effectiveness. In this study, we have also investigated the effect of female reproductive factors on survival in a multidimensional aspect with robust statistics such as multivariable Cox proportional hazards model and different subgroups, which could considerably diminish the impact of confounders and explore the potential effect in a specific group.

The demographic characteristics included age and body mass index (BMI). We considered age as an ordinal variable (< 50, 50?60, and ≥ 60 years) to approximate tertiles. We defined BMI based on the standard cutoff points established by the Work Group on Obesity in China in categories of underweight and normal: ≤ 24 kg/m
, overweight and obese: > 24.0 kg/m
[16]. Health-related lifestyle indicators, including alcohol consumption (yes: Any alcoholic beverage consumption in the last 12 mo, or no: No alcohol consumption in the last 12 mo) and smoking (yes: Any regular tobacco consumption, or no: Never smoked), were excluded from the analysis based on the variable selection outcome of best-subset selection approach and the uneven distribution. We categorized the proportion of signet-ring cells as ≤ 10%, 10%–50%, 50%–90%, and > 90%, but we used the proportion of signet-ring cells as a dichotomous variable (≤ 50% and > 50%) in the adjustments for confounding variables and to ensure that each level had a sufficient number of observations. Clinicopathological characteristics, such as BMI, proportion of signet-ring cells, T stage, N stage, adjuvant chemotherapy, neoadjuvant chemotherapy, nerve invasion, and lymphatic vessel invasion were subjected to analysis since they were closely associated with the survival of GC patients with signet-ring cell carcinoma based on
knowledge.
CONCLUSION
Sex differences, especially female reproductive factors, might be protective against GSRC and serve as a significant prognostic factor. Further studies including more comprehensive measures will be essential to elucidate the mechanism of action of female reproductive factors on GSRC.
ARTICLE HIGHLIGHTS
FOOTNOTES
All of the authors contributed to this work; Li Y and Tian YT designed the research study and performed the research; Li Y collected the data, performed the statistical analysis, and drafted the manuscript; Li Y, Zhong YX, Xu Q, and Tian YT participated in discussion and manuscript revision; All authors have read and approve the final version of the manuscript and agreed to be accountable for all aspects of the work.
National Natural Science Foundation of China, No. 82072734.
安:謝謝你的贊揚(yáng)!其實(shí)如我在訪談一開(kāi)始提到的一般,這樣的能力絕非天生,而必須得益于帕內(nèi)拉教授和齊科里尼教授對(duì)我的言傳身授。是他們反復(fù)為我提供這樣的解決方案,并一直告訴我,多數(shù)的所謂“技術(shù)問(wèn)題”,其實(shí)大多來(lái)自對(duì)樂(lè)句音樂(lè)性的不理解。在跟隨他們學(xué)習(xí)的幾年之中,我一直讓自己保持思考,以充分吸收他們的觀點(diǎn)。而他們的藝術(shù)觀點(diǎn)解鎖了我對(duì)肢體的運(yùn)用,以及我對(duì)音樂(lè)的理解。
Gastric signet-ring cell carcinoma (GSRC) is a distinct type of GC, and its incidence has been steadily increasing in Asia, Europe, and the United States, accounting for > 30% of new gastric adenocarcinoma cases[9]. GSRC belongs to the diffused, undifferentiated, and poorly differentiated types in the Laurén classification, Nakamura’s classification, and Japanese Gastric Cancer Association, respectively[10-13]. GSRC in early and advanced stages is more frequently observed in younger female patients than gastric adenocarcinoma[14]. A contradiction with the protective effect of female reproductive factors on gastric adenocarcinoma seems to exist. There is also evidence that female reproductive factors induce diffusetype GC through estrogen activity[7].
All participants gave written informed consent.
The authors declare that they have no conflict of interest.
All data included in this study are available on reasonable request from the corresponding author.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
《大學(xué)》中又寫(xiě)道:為人君,止于仁;為人臣,止于敬;為人子,止于孝;為人父,止于慈;與國(guó)人交,止于信。”《尚書(shū)》中的“民為邦本,本固邦寧”,“仁者愛(ài)人”,“出入相友,守望相助。”“忠信,禮之本也;義理,禮之文也。無(wú)本不正,無(wú)文不行(《禮記·禮器》)。”“禮形于外”,“道誠(chéng)于心”。以忠信、誠(chéng)意按照一定的禮節(jié)規(guī)程相互表達(dá)“仁義”則“智”(意指思想道德,在今天也可延伸為科學(xué)與人文精神)成。
China
Yang Li 0000-0002-4549-1087; Yu-Xin Zhong 0000-0002-8865-3297; Quan Xu 0000-0001-6177-9503; Yan-Tao Tian 0000-0001-6479-7547.
Ma YJ
人民警察在實(shí)際執(zhí)法過(guò)程中,存在著對(duì)繼續(xù)盤(pán)問(wèn)、傳喚、先行拘留和拘傳等強(qiáng)制措施混淆適用的情形。對(duì)已經(jīng)確認(rèn)相對(duì)人存在著違法犯罪嫌疑的,按照法律規(guī)定應(yīng)當(dāng)采取傳喚、先行拘留或拘傳等強(qiáng)制措施,并不辨明清楚具體情形,統(tǒng)統(tǒng)以繼續(xù)盤(pán)問(wèn)為由予以處置。
Kerr C
他們連滾帶爬地出現(xiàn)在后排。一切都恢復(fù)了人性的本源,一切都那么的順其自然,一切都開(kāi)始了,沒(méi)有回頭的路徑了。往前,唯有秉持著向前的動(dòng)力,沖破內(nèi)心的桎梏,去尋找去探尋甚至去醉臥在銷(xiāo)魂蝕骨的風(fēng)水寶地中。
Ma YJ
1 Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018 : GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018 ; 68 : 394 -424 [PMID: 30207593 DOI: 10 .3322 /caac.21492 ]
2 Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, Yang Z, Zou X, He J. Cancer incidence and mortality in China, 2013 .
2017 ; 401 : 63 -71 [PMID: 28476483 DOI: 10 .1016 /j.canlet.2017 .04 .024 ]
3 Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015 .
2016 ; 66 : 115 -132 [PMID: 26808342 DOI: 10 .3322 /caac.21338 ]
4 Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer 2008 ; 44 :2397 -2403 [PMID: 18755583 DOI: 10 .1016 /j.ejca.2008 .07 .031 ]
5 Freedman ND, Derakhshan MH, Abnet CC, Schatzkin A, Hollenbeck AR, McColl KE. Male predominance of upper gastrointestinal adenocarcinoma cannot be explained by differences in tobacco smoking in men versus women.
2010 ; 46 : 2473 -2478 [PMID: 20605442 DOI: 10 .1016 /j.ejca.2010 .05 .005 ]
6 Ibrahim A, Morais S, Ferro A, Lunet N, Peleteiro B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: Systematic review and meta-analysis of 244 studies. Dig Liver Dis 2017 ; 49 : 742 -749 [PMID: 28495503 DOI: 10 .1016 /j.dld.2017 .03 .019 ]
7 Kim SM, Min BH, Lee J, An JY, Lee JH, Sohn TS, Bae JM, Kim JJ, Kang WK, Kim S, Choi MG. Protective Effects of Female Reproductive Factors on Lauren Intestinal-Type Gastric Adenocarcinoma.
2018 ; 59 : 28 -34 [PMID:29214773 DOI: 10 .3349 /ymj.2018 .59 .1 .28 ]
8 Zhang GQ, Chen JL, Luo Y, Mathur MB, Anagnostis P, Nurmatov U, Talibov M, Zhang J, Hawrylowicz CM, Lumsden MA, Critchley H, Sheikh A, Lundb?ck B, L?sser C, Kankaanranta H, Lee SH, Nwaru BI. Menopausal hormone therapy and women’s health: An umbrella review.
2021 ; 18 : e1003731 [PMID: 34339416 DOI:10 .1371 /journal.pmed.1003731 ]
9 Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge.
2015 ; 21 : 11428 -11438 [PMID: 26523107 DOI:10 .3748 /wjg.v21 .i40 .11428 ]
10 Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt at A Histo-Clinical Classification.
1965 ; 64 : 31 -49 [PMID: 14320675 ]
11 Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system.
2020 ; 76 : 182 -188 [PMID: 31433515 DOI: 10 .1111 /his.13975 ]
12 Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances.
1968 ; 59 : 251 -258 [PMID: 5726267 ]
13 Arai T. Where does signet-ring cell carcinoma come from and where does it go? Gastric Cancer 2019 ; 22 : 651 -652 [PMID: 30963318 DOI: 10 .1007 /s10120 -019 -00960 -w]
14 Kao YC, Fang WL, Wang RF, Li AF, Yang MH, Wu CW, Shyr YM, Huang KH. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer.
2019 ; 22 : 255 -263 [PMID: 30069742 DOI: 10 .1007 /s10120 -018 -0860 -8 ]
15 Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H,Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN,Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA,Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3 .2016 , NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016 ; 14 : 1286 -1312 [PMID: 27697982 ]
16 Chen C, Lu FC; Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults.
2004 ; 17 Suppl: 1 -36 [PMID: 15807475 ]
17 Kaneko S, Tamakoshi A, Ohno Y, Mizoue T, Yoshimura T; JACC Study Group. Menstrual and reproductive factors and the mortality risk of gastric cancer in Japanese menopausal females.
2003 ; 14 : 53 -59 [PMID:12708725 DOI: 10 .1023 /a:1022596104796 ]
18 Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the anadian national enhanced cancer surveillance system.
2006 ;16 : 908 -916 [PMID: 16843679 DOI: 10 .1016 /j.annepidem.2006 .03 .001 ]
19 Persson C, Inoue M, Sasazuki S, Kurahashi N, Iwasaki M, Ye W, Tsugane S; JPHC Study Group. Female reproductive factors and the risk of gastric cancer in a large-scale population-based cohort study in Japan (JPHC study).
2008 ; 17 : 345 -353 [PMID: 18562960 DOI: 10 .1097 /CEJ.0 b013 e3282 f521 e4 ]
20 Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, Lubin JH, Li HL, Rothman N, Zheng W, Abnet CC.Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women.
2007 ; 56 : 1671 -1677[PMID: 17627962 DOI: 10 .1136 /gut.2007 .129411 ]
21 Green J, Roddam A, Pirie K, Kirichek O, Reeves G, Beral V; Million Women Study collaborators. Reproductive factors and risk of oesophageal and gastric cancer in the Million Women Study cohort.
2012 ; 106 : 210 -216 [PMID:22127287 DOI: 10 .1038 /bjc.2011 .525 ]
22 Brusselaers N, Maret-Ouda J, Konings P, El-Serag HB, Lagergren J. Menopausal hormone therapy and the risk of esophageal and gastric cancer.
2017 ; 140 : 1693 -1699 [PMID: 28006838 DOI: 10 .1002 /ijc.30588 ]
23 Zhang Y, Wang Y, Wan Z, Liu S, Cao Y, Zeng Z. Sphingosine kinase 1 and cancer: a systematic review and meta-analysis.
2014 ; 9 : e90362 [PMID: 24587339 DOI: 10 .1371 /journal.pone.0090362 ]
24 Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters.
2007 ;33 : 195 -201 [PMID: 17046193 DOI: 10 .1016 /j.ejso.2006 .09 .009 ]
25 Zhao XH, Gu SZ, Liu SX, Pan BR. Expression of estrogen receptor and estrogen receptor messenger RNA in gastric carcinoma tissues.
2003 ; 9 : 665 -669 [PMID: 12679906 DOI: 10 .3748 /wjg.v9 .i4 .665 ]
26 Hess RA, Cooke PS. Estrogen in the male: a historical perspective. Biol Reprod 2018 ; 99 : 27 -44 [PMID: 29438493 DOI:10 .1093 /biolre/ioy043 ]
27 Kameda C, Nakamura M, Tanaka H, Yamasaki A, Kubo M, Tanaka M, Onishi H, Katano M. Oestrogen receptor-alpha contributes to the regulation of the hedgehog signalling pathway in Eralpha-positive gastric cancer.
2010 ; 102 :738 -747 [PMID: 20087349 DOI: 10 .1038 /sj.bjc.6605517 ]
28 Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma.
2002 ; 128 : 319 -324 [PMID:12073050 DOI: 10 .1007 /s00432 -002 -0336 -3 ]
29 Kang MH, Choi H, Oshima M, Cheong JH, Kim S, Lee JH, Park YS, Choi HS, Kweon MN, Pack CG, Lee JS, Mills GB,Myung SJ, Park YY. Estrogen-related receptor gamma functions as a tumor suppressor in gastric cancer.
2018 ; 9 : 1920 [PMID: 29765046 DOI: 10 .1038 /s41467 -018 -04244 -2 ]
30 Wang X, Xia X, Xu E, Yang Z, Shen X, Du S, Chen X, Lu X, Jin W, Guan W. Estrogen Receptor Beta Prevents Signet Ring Cell Gastric Carcinoma Progression in Young Patients by Inhibiting Pseudopodia Formation
the mTORArpc1 b/EVL Signaling Pathway. Front Cell Dev Biol 2020 ; 8 : 592919 [PMID: 33553141 DOI: 10 .3389 /fcell.2020 .592919 ]
 World Journal of Clinical Cases2022年16期
World Journal of Clinical Cases2022年16期
- World Journal of Clinical Cases的其它文章
- Practical points that gastrointestinal fellows should know in management of COVID-19
- Electroconvulsive therapy plays an irreplaceable role in treatment of major depressive disorder
- Pleural involvement in cryptococcal infection
- Advances in the clinical application of oxycodone in the perioperative period
- Endoscopic surgery for intraventricular hemorrhage: A comparative study and single center surgical experience
- Pediatric acute myeloid leukemia patients with i(17)(q10) mimicking acute promyelocytic leukemia: Two case reports
