中藥續(xù)斷的化學(xué)成分研究
張成剛,楊昌貴,肖承鴻,凡迪,龔安慧,黃艷,周濤*
(1.貴州中醫(yī)藥大學(xué), 貴州 貴陽(yáng) 550025;2.貴州省農(nóng)作物技術(shù)推廣總站, 貴州 貴陽(yáng) 550001)
續(xù)斷始載于《神農(nóng)本草經(jīng)》,列為上品,是續(xù)斷科植物川續(xù)斷(DipsacusasperWall. ex Henry)的干燥根,具有補(bǔ)肝腎、強(qiáng)筋骨、續(xù)折傷、止崩漏之功效[1-2]。續(xù)斷主要含有三萜皂苷類、環(huán)烯醚萜類、生物堿類等化學(xué)成分,其中三萜皂苷類、環(huán)烯醚萜類成分含量較高[3-4],藥理研究表明續(xù)斷具有抗骨折、抗骨質(zhì)疏松、心肌保護(hù)、抗衰老及生殖系統(tǒng)保護(hù)等作用[5-6]。
“發(fā)汗”炮制法是常用的中藥材產(chǎn)地加工方法,對(duì)于保證中藥材質(zhì)量具有重要作用[7]。《中國(guó)藥典》規(guī)定續(xù)斷等5種藥材必須進(jìn)行“發(fā)汗”加工處理[2,8],許多學(xué)者對(duì)續(xù)斷“發(fā)汗”前后的化學(xué)成分及其含量變化做了大量研究,但結(jié)論各異。有的學(xué)者發(fā)現(xiàn)續(xù)斷“發(fā)汗”后其川續(xù)斷皂苷VI、川續(xù)斷苷A、異綠原酸B、異綠原酸C等成分含量升高,有的學(xué)者實(shí)驗(yàn)得出續(xù)斷“發(fā)汗”后其總皂苷、川續(xù)斷皂苷VI、異綠原酸A含量降低[9-12],也有學(xué)者研究發(fā)現(xiàn)續(xù)斷“發(fā)汗”前后其川續(xù)斷皂苷VI的含量無(wú)變化[13],還有學(xué)者比較續(xù)斷“發(fā)汗”前后的28種酚酸和脂類,發(fā)現(xiàn)順-13-十八碳烯酸和十七烷醇僅存在于“發(fā)汗”前續(xù)斷中,而“發(fā)汗”后檢測(cè)到的9,12,15-十八碳三烯酸甲酯和9-亞甲基-9H-芴是“發(fā)汗”前續(xù)斷中沒(méi)有的新化學(xué)成分[14]。
為進(jìn)一步闡釋續(xù)斷“發(fā)汗”前后化學(xué)成分及其含量的變化,并為續(xù)斷“發(fā)汗”加工前后化學(xué)成分轉(zhuǎn)化機(jī)制研究提供更豐富的化學(xué)成分物質(zhì)基礎(chǔ),本文對(duì)續(xù)斷藥材70%乙醇提取物進(jìn)行了研究,從中共分離得到13個(gè)化合物,包括5個(gè)三萜皂苷類化合物:川續(xù)斷皂苷Ⅵ(1)、威嚴(yán)仙皂苷A(2)、續(xù)斷皂苷A(3)、3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷(4)、3-O-(4-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷(5),6個(gè)環(huán)烯醚萜苷類化合物:馬錢(qián)苷酸(6)、馬錢(qián)子苷(7)、當(dāng)藥苷(8)、續(xù)斷苷A(9)、續(xù)斷苷B(10)、林生續(xù)斷苷I(11),以及2個(gè)其他類成分:5-羥甲基-2-呋喃甲醛(12)和α-亞麻酸(13),其中化合物4、12、13為首次從續(xù)斷中分離,也是首次從川續(xù)斷屬植物中分離得到。
1 儀器與材料
1.1 儀器
Bruker-400核磁共振波譜儀(德國(guó)布魯克公司),Bruker Impact II高分辨飛行時(shí)間質(zhì)譜儀(德國(guó)布魯克公司),ACCHROM S6000高效液相色譜儀(華譜科儀(北京)科技有限公司),RIGOL L-3000四元泵(蘇州普源精電科技有限公司),F(xiàn)lash System MP100中壓系統(tǒng)(天津博納艾杰爾科技有限公司);島津LC-20AR液相色譜儀(日本島津公司);BSA2202S-CW電子天平(德國(guó)賽多利斯公司);R-100旋轉(zhuǎn)蒸發(fā)儀(瑞士步琦有限公司)。乙腈為色譜純,95%乙醇、甲醇為分析純(天津康科德科技有限公司);YMC-Pack ODS-A C18反相色譜柱(日本維美希公司);D101大孔吸附樹(shù)脂(上海源葉生物科技有限公司);MCI小孔吸附樹(shù)脂(日本三菱化學(xué)公司);水為去離子水。
1.2 材料
實(shí)驗(yàn)所用續(xù)斷藥材購(gòu)自貴州省貴陽(yáng)市太升中藥材市場(chǎng),經(jīng)貴州中醫(yī)藥大學(xué)藥學(xué)院江維克教授鑒定為川續(xù)斷科植物川續(xù)斷DipsacusasperWall. ex Henry的干燥根。
2 方法與結(jié)果
2.1 方法
干燥的續(xù)斷藥材10 kg,粉碎至適宜粒度后用70%乙醇回流提取3次,提取液回收溶劑后得到浸膏4 kg。浸膏用水溶解后注入D101大孔吸附樹(shù)脂柱,分別用20%、50%、95%乙醇梯度洗脫,減壓濃縮得到20%乙醇洗脫部位(Fr.1, 200 g)、50%乙醇洗脫部位(Fr.2, 500 g)以及95%乙醇洗脫部位(Fr.3, 200 g)。

2.2 結(jié)果
化合物1: 白色無(wú)定形粉末。1H-NMR (400 MHz, Piridine-d5)δ:4.22 (1H, m, H-3), 5.40 (1H, d,J= 3.0 Hz, H-12), 4.23 (1H, overlapped, H-23), 3.64 (1H, overlapped, H-23), 0.90 (3H, s, H-24), 0.95 (3H, s, H-25), 1.10 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (6H, s, H-29, H-30)。4.91 (1H, d,J= 7.2 Hz, H-1′), 4.39 (1H, m, H-2′), 4.15 (1H, overlapped, H-3′), 4.30 (1H, overlapped, H-4′), 3.70 (1H, m, H-5′)。6.22 (1H, d,J= 7.8 Hz, H-1″), 4.08 (1H, m, H-2″), 4.16 (1H, overlapped, H-3″), 4.04 (1H, overlapped, H-4″), 4.05 (1H, overlapped, H-5″), 4.26 (1H, m, H-6″), 4.66 (1H, m, H-6″)。5.00 (1H, d,J= 7.8 Hz, H-1?), 3.96 (1H, m, H-2?), 4.29 (1H, overlapped, H-3?), 4.15 (1H, overlapped, H-4?), 3.83 (1H, m, H-5?), 4.30 (1H, overlapped, H-6?), 4.41 (1H, m, H-6?)。13C-NMR (100 MHz, Piridine-d5)δ: 38.6 (C-1), 25.9 (C-2), 81.7 (C-3), 43.3 (C-4), 47.3 (C-5), 18.0 (C-6), 32.3 (C-7), 39.7 (C-8), 48.0 (C-9), 36.7 (C-10), 23.7 (C-11), 122.7 (C-12), 144.0 (C-13), 41.9 (C-14), 28.1 (C-15), 23.1 (C-16), 46.8 (C-17), 41.4 (C-18), 46.0 (C-19), 30.5 (C-20), 33.9 (C-21), 32.6 (C-22), 64.2 (C-23), 14.0 (C-24), 16.0 (C-25), 17.4 (C-26), 25.9 (C-27), 176.4 (C-28), 32.9 (C-29), 23.5 (C-30), 106.4 (C-1′), 72.8 (C-2′), 74.4 (C-3′), 69.4 (C-4′), 66.7 (C-5′), 95.4 (C-1″), 73.6 (C-2″), 78.4 (C-3″), 70.6 (C-4″), 77.7 (C-5″), 69.1 (C-6″), 104.9 (C-1?), 74.9 (C-2?), 78.1 (C-3?), 71.3 (C-4?), 78.1 (C-5?), 62.3 (C-6?)。以上數(shù)據(jù)與文獻(xiàn)[15]報(bào)道基本一致,故將該化合物鑒定為川續(xù)斷皂苷Ⅵ。
化合物2: 白色無(wú)定形粉末。HR-ESI-MS給出分子式C35H56O8(測(cè)量值m/z603.392 3 [M-H]-,計(jì)算值m/z603.390 2 [M-H]-)。1H-NMR (400 MHz, Piridine-d5)δ: 4.25 (1H, m, H-3), 5.46 (1H, br s, H-12), 3.68 (1H, overlapped, H-23), 4.26 (1H, overlapped, H-23), 0.90 (3H, s, H-24), 0.92 (3H, s, H-25), 1.00 (3H, s, H-26), 1.22 (3H, s, H-27), 0.92 (3H, s, H-29), 0.98 (3H, s, H-30)。4.96 (1H, d,J= 7.2 Hz, H-1′), 4.40 (1H, t,J= 8.0 Hz, H-2′), 3.27 (1H, dd,J= 3.0, 13.2, H-3′), 4.23 (1H, m, H-4′), 3.71 (1H, overlapped, H-5′), 4.27 (1H, overlapped, H-5′)。13C-NMR (100 MHz, Piridine-d5)δ: 38.6 (C-1), 25.9 (C-2), 81.7 (C-3), 43.3 (C-4), 47.4 (C-5), 17.9 (C-6), 32.7 (C-7), 39.6 (C-8), 49.9 (C-9), 36.7 (C-10), 23.6 (C-11), 122.4 (C-12), 144.6 (C-13), 41.9 (C-14), 28.1 (C-15), 23.6 (C-16), 46.4 (C-17), 41.7 (C-18), 46.2 (C-19), 30.7 (C-20), 34.0 (C-21), 33.0 (C-22), 64.3 (C-23), 13.4 (C-24),15.9 (C-25),17.3 (C-26), 26.9 (C-27), 180.0 (C-28), 33.0 (C-29), 23.5 (C-30), 106.4 (C-1′), 72.9 (C-2′), 74.5 (C-3′), 69.4 (C-4′), 66.70 (C-5′)。以上數(shù)據(jù)與文獻(xiàn)[16]報(bào)道基本一致,故將該化合物鑒定為威嚴(yán)仙皂苷A。
化合物3: 白色無(wú)定形粉末。1H-NMR (400 MHz, Piridine-d5)δ: 4.18 (1H, m, H-3), 5.42 (1H, br s, H-12), 3.69 (1H, d,J= 10.4 Hz, H-23), 4.15 (1H, overlapped, H-23), 1.04 (3H, s, H-24), 1.00 (3H, s, H-25), 1.14 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (3H, s, H-29), 0.85 (3H, s, H-30)。6.25 (1H, d,J= 8.0 Hz, H-1′) 4.12 (1H, m, H-2′), 4.19 (1H, overlapped, H-3′), 4.32 (1H, m, H-4′), 4.09 (1H, m, H-5′), 4.34 (1H, m, H-6′), 4.70 (1H, d,J= 10.0 Hz, H-6′)。5.02 (1H, d,J= 8.0 Hz, H-1″), 3.99 (1H, t,J= 8.4 Hz, H-2″), 3.87 (1H, m, H-3″), 4.19 (2H, overlapped, H-4″, H-5″), 3.18 (1H, dd,J= 3.6, 13.6 Hz, H-6″), 4.15 (1H, overlapped, H-6″)。13C-NMR (100 MHz, Piridine-d5)δ:38.6 (C-1), 27.5 (C-2), 73.2 (C-3), 42.7 (C-4), 48.4 (C-5), 18.4 (C-6), 32.7 (C-7), 39.7 (C-8), 48.0 (C-9), 37.0 (C-10), 23.6 (C-11), 122.7 (C-12), 144.0 (C-13), 41.9 (C-14), 28.1 (C-15), 23.2 (C-16), 46.8 (C-17), 41.5 (C-18), 46.0 (C-19), 30.5 (C-20), 33.8 (C-21), 32.3 (C-22), 67.7 (C-23), 12.9 (C-24), 15.9 (C-25), 17.4 (C-26), 25.9 (C-27), 176.3 (C-28), 32.9(C-29), 23.5 (C-30), 95.5 (C-1′), 73.7 (C-2′), 78.5 (C-3′), 70.7 (C-4′), 77.8 (C-5′), 69.2 (C-6′), 105.1 (C-1″), 74.9 (C-2″), 78.2 (C-3″), 71.3 (C-4″), 78.2 (C-5″), 62.4 (C-6″)。以上數(shù)據(jù)與文獻(xiàn)[16]報(bào)道基本相同,故將該化合物確定為續(xù)斷皂苷A。
化合物4: 白色無(wú)定形粉末。1H-NMR (400 MHz, Piridine-d5)δ:4.21 (1H, m, H-3), 5.39 (1H, m, H-12), 3.68 (1H, overlapped, H-23), 3.87 (H, d,J= 10.4 Hz, H-23), 0.90 (3H, s, H-24), 0.95 (3H, s, H-25), 1.10 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (6H, s, H-29, H-30)。5.01 (1H, d,J=8.0 Hz, H-1′), 5.88 (1H, dd,J= 7.6, 13.2 Hz, H-2′), 4.21 (1H, overlapped, H-3′), 4.02 (1H, m, H-4′), 3.68 (1H, overlapped, H-5′), 4.24 (1H, m, H-5′)。6.24 (1H, d,J= 8.0 Hz, H-1″), 4.11 (1H, m, H-2″), 4.19 (1H, overlapped, H-3″), 4.32 (1H, overlapped, H-4″), 4.08 (1H, overlapped, H-5″), 4.33 (1H, overlapped, H-6″), 4.70 (1H, d,J= 10.0 Hz, H-6″)。4.95 (1H, d,J= 8.0 Hz, H-1?), 4.00 (1H, m, H-2?), 4.32 (1H, overlapped, H-3?), 4.15 (1H, overlapped, H-4?), 3.87 (1H, overlapped, H-5?), 4.34 (1H, overlapped, H-6?), 4.46 (1H, dd,J= 2.0, 11.6 Hz, H-6?), 2.10 (3H, s, H-COCH3)。13C-NMR (100 MHz, Piridine-d5)δ:38.5 (C-1), 26.0 (C-2), 80.9 (C-3), 43.1 (C-4), 46.9(C-5), 17.9 (C-6), 32.5 (C-7), 39.7 (C-8), 47.9 (C-9), 36.6 (C-10), 23.6 (C-11), 122.7 (C-12), 143.9 (C-13), 41.9 (C-14), 28.1 (C-15), 23.1 (C-16), 46.8 (C-17), 41.4 (C-18), 46.0 (C-19), 30.5 (C-20), 33.7 (C-21), 32.3 (C-22), 63.4 (C-23), 13.3 (C-24), 15.9 (C-25), 17.3 (C-26), 25.8 (C-27), 176.3 (C-28), 32.9 (C-29), 23.5 (C-30)。105.0 (C-1′), 74.1 (C-2′), 69.5 (C-3′), 72.3 (C-4′), 66.9 (C-5′), 95.5 (C-1″), 73.7 (C-2″), 78.5 (C-3″), 70.7 (C-4″), 77.7 (C-5″), 69.1 (C-6″), 103.8 (C-1?), 74.9 (C-2?), 78.2 (C-3?), 71.3 (C-4?), 78.2 (C-5?), 62.4 (C-6?), 169.9 (C-COCH3), 21.0 (C-COCH3)。以上數(shù)據(jù)與文獻(xiàn)[17]報(bào)道基本相同,故將該化合物確定為3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷。
化合物5: 白色無(wú)定形粉末。HR-ESI-MS給出分子式C49H78O19(測(cè)量值m/z993.501 8 [M+Na]+,計(jì)算值m/z993.503 5 [M+Na]+)。1H-NMR (400 MHz, Piridine-d5)δ: 4.29 (1H, m, H-3), 5.43 (1H, br s, H-12), 3.73 (2H, m, H-23), 0.90 (3H, s, H-24), 0.95 (3H, s, H-25), 1.10 (3H, s, H-26), 1.16 (3H, s, H-27), 0.84 (6H, s, H-29, H-30)。4.99 (1H, d,J= 7.2 Hz, H-1′), 4.37 (1H, overlapped, H-2′), 4.17 (1H, overlapped, H-3′), 5.54 (1H, br s, H-4′), 4.25 (1H, overlapped, H-5′), 4.34 (1H, overlapped, H-5′)。6.27 (1H, d,J= 8.0 Hz, H-1″), 4.14 (1H, overlapped, H-2″), 4.23 (1H, overlapped, H-3″), 4.33 (1H, overlapped, H-4″), 4.12 (1H, overlapped, H-5″), 4.37 (1H, overlapped, H-6″), 4.73(1H, d,J=10.0 Hz, H-6″)。5.05 (1H, d,J= 7.2 Hz, H-1?), 4.01 (1H, dd,J= 7.6, 7.6 Hz, H-2?), 4.21 (1H, overlapped, H-3?), 4.21 (1H, overlapped, H-4?), 3.90 (1H, m, H-5?), 4.37 (1H, overlapped, H-6?), 4.49 (1H, d,J= 11.6 Hz, H-6?), 1.98 (3H, s, H-COCH3)。13C-NMR (100 MHz, Piridine-d5)δ: 38.6 (C-1), 25.9 (C-2), 82.0 (C-3), 43.3 (C-4), 47.4 (C-5), 18.0 (C-6), 32.9 (C-7), 39.7 (C-8), 48.0 (C-9), 36.7 (C-10),23.6 (C-11),122.3 (C-12),143.9 (C-13),41.9(C-14), 28.1 (C-15), 23.1 (C-16), 46.8 (C-17), 41.5 (C-18), 46.0 (C-19), 30.5 (C-20), 33.8 (C-21), 32.8 (C-22), 64.2 (C-23), 13.4 (C-24), 16.0 (C-25), 17.4 (C-26), 25.8 (C-27), 176.3 (C-28), 32.9 (C-29), 23.5 (C-30)。106.5 (C-1′), 73.1 (C-2′), 72.2 (C-3′), 72.3 (C-4′), 64.2 (C-5′), 95.4 (C-1″), 73.7 (C-2″), 78.5 (C-3″), 70.7 (C-4″), 77.7 (C-5″), 69.2 (C-6″), 105.0 (C-1?), 74.9 (C-2?), 78.2 (C-3?), 71.3 (C-4?), 78.2 (C-5?), 62.4 (C-6?), 170.6 (C-COCH3), 20.9 (C-COCH3)。以上數(shù)據(jù)和文獻(xiàn)[15]報(bào)道的大體一致,從而將該化合物確定為3-O-(4-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷。
化合物6: 白色無(wú)定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.26 (1H, d,J= 5.5 Hz, H-1), 7.41 (1H, s, H-3), 3.09 (1H, dd,J= 7.6, 16.0 Hz, H-5), 1.65 (1H, m, H-6a), 2.03 (1H, m, H-6b), 4.06(1H, m,H-7),1.87(1H,m,H-8),2.24(1H,dd,J= 8.4, 14.0 Hz, H-9), 1.09 (3H, d,J=6.8 Hz, H-10), 4.67 (1H, d,J= 8.0 Hz, H-1′), 3.22 (1H, m, H-2′), 3.41 (1H, m, H-3′), 3.31 (1H, m, H-4′), 3.33 (1H, m, H-5′), 3.68 (1H, dd,J= 5.2, 12.0 Hz, H-6′a), 3.85 (1H, d,J= 12.0 Hz, H-6′b)。13C-NMR (100 MHz, CD3OD)δ: 96.3 (C-1), 150.9 (C-3), 112.7 (C-4), 31.0 (C-5), 41.5 (C-6), 73.8 (C-7), 41.0 (C-8), 45.3 (C-9), 12.2 (C-10), 169.7 (C-11), 98.6 (C-1′), 73.3 (C-2′), 76.6 (C-3′), 70.2 (C-4′), 76.8 (C-5′), 61.4 (C-6′)。以上數(shù)據(jù)和文獻(xiàn)[18]報(bào)道的基本相同,從而將該化合物確定為馬錢(qián)苷酸。
化合物7: 白色無(wú)定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.26 (1H, d,J= 5.5 Hz, H-1), 7.39 (1H, s, H-3), 3.10 (1H, dd,J= 8.0, 16.0 Hz, H-5), 1.61 (1H, m, H-6a), 2.02 (1H, m, H-6b), 4.04 (1H, m, H-7), 1.87 (1H, m, H-8), 2.22 (1H, dd,J= 8.0, 14.0 Hz, H-9), 1.09 (3H, d,J=6.8 Hz, H-10), 3.68 (3H, s, H-COOCH3), 4.65 (1H, d,J= 8.0 Hz, H-1′), 3.19 (1H, m, H-2′), 3.37 (1H, m, H-3′), 3.27 (1H, m, H-4′), 3.29 (1H, m, H-5′), 3.66 (1H, dd,J= 5.6, 12.0 Hz, H-6a′), 3.89 (1H, d,J= 12.0 Hz, H-6b′)。13C-NMR (100 MHz, CD3OD)δ: 96.3 (C-1), 150.7 (C-3), 112.6 (C-4), 30.8 (C-5), 41.3 (C-6), 73.3 (C-7), 40.8 (C-8), 45.1 (C-9), 12.0 (C-10), 168.2 (C-11), 50.3 (C-COOCH3), 98.7 (C-1′), 73.7 (C-2′), 77.0 (C-3′), 70.2 (C-4′), 76.6 (C-5′), 61.4 (C-6′)。以上數(shù)據(jù)與文獻(xiàn)[19]報(bào)道大體一致,故將該化合物確定為馬錢(qián)子苷。
化合物8: 白色無(wú)定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.55 (1H, overlapped, H-1), 7.60 (1H, d,J= 2.0 Hz, H-3), 3.15 (1H, m, H-5), 1.63~1.81 (2H, m, H-6), 4.45 (1H, m, H-7a), 4.37 (1H, m, H-7b), 5.55 (1H, overlapped, H-8), 3.71 (1H, m, H-9), 5.29 (2H, overlapped, H-10), 4.70 (1H, d,J= 8.4 Hz, H-1′), 3.22 (1H, m, H-2′), 3.40 (1H, m, H-3′), 3.29 (1H, m, H-4′), 3.33 (1H, overlapped, H-5′), 3.67 (1H, dd,J= 6.0, 12.0 Hz, H-6′a), 3.89 (1H, d,J= 10.8 Hz, H-6′b)。13C-NMR (100 MHz, CD3OD)δ: 96.7 (C-1), 152.7 (C-3), 104.7 (C-4), 27.0 (C-5), 24.5 (C-6), 68.4 (C-7), 131.9 (C-8), 42.4 (C-9), 119.6 (C-10), 167.2 (C-11), 98.4 (C-1′), 73.3 (C-2′), 76.5 (C-3′), 70.1 (C-4′), 76.9 (C-5′), 61.3 (C-6′)。上述數(shù)據(jù)與文獻(xiàn)[20]報(bào)道大體相同,從而將該化合物確定為當(dāng)藥苷。
化合物9: 白色無(wú)定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.47 (1H, d,J= 4.8 Hz, H-1a1), 7.38 (1H, s, H-3a1), 2.70 (1H, m, H-5a1), 3.02 (1H, dd,J= 8.4, 7.8 Hz, H-6a1), 2.33 (1H, dd,J= 7.8, 6.6 Hz, H-6a1), 6.67 (1H, t,J=6.6 Hz, H-7a1), 5.68 (1H, ddd,J=7.8, 9.0, 9.6, 10.2, 16.8 Hz, H-8a1), 2.30 (1H, br m, H-9a1), 5.28 (1H, d,J= 16.8 Hz, H-10a1), 5.21 (1H, d,J= 10.8 Hz, H-10a1), 4.59 (1H, d,J= 7.8 Hz, H-1′a1), 3.10~3.29 (1H, H-2′a1), 3.10~3.29 (1H, H-3′a1), 3.10~3.29 (1H, H-4′a1), 3.10~3.29 (1H, H-5′a1), 3.54~3.58 (1H, H-6′a1), 3.78~3.82 (1H, H-6′a1)。5.39 (1H, d,J= 1.8 Hz, H-1a2), 7.45 (1H, s, H-3a2), 4.01 (1H, d,J= 4.2 Hz, H-5a2), 9.32 (1H, s, H-7a2), 5.54 (1H, ddd,J= 9.6, 7.2, 10.2, 9.6, 17.4 Hz, H-8a2), 2.48 (1H, br s, H-9a2), 4.96(1H,d,J= 17.4 Hz, H-10a2), 4.93(1H,d,J=10.2 Hz, H-10a2), 4.56 (1H, d,J= 7.8 Hz, H-1′a2), 3.10~3.29 (1H, H-2′a2), 3.10~3.29 (1H, H-3′a2), 3.10~3.29 (1H, H-4′a2), 3.10~3.29 (1H, H-5′a2), 3.54~3.58 (1H, H-6′a2), 3.78~3.82 (1H, H-6′a2)。5.06 (1H, br s, H-1b1), 7.34 (1H, s, H-3b1), 2.92 (1H, br q,J= 8.4 Hz, H-5b1), 1.67 (1H, ddd,J= 4.8, 3.6, 6.0 Hz, H-6b1), 2.07 (1H, m, H-6b1), 5.17 (1H, d,J= 4.8 Hz, H-7b1), 2.07 (1H, m, H-8b1), 1.97 (1H, m, H-9b1), 0.99 (3H, d,J= 6.6 Hz, H-10b1), 3.62 (3H, s, H-12b1), 4.57 (1H, d,J= 8.4 Hz, H-1′b1), 3.10~3.29(9H, H-2′b1,H-3′b1,H-4′b1,H-5′b1,H-6′b1,H-2′b2, H-3′b2,H-4′b2,H-5′b2),3.78~3.82(2H, H-6′b1, H-6′b2)。5.15 (1H, d,J=4.8 Hz, H-1b2),7.35(1H, s, H-3b2), 2.92(1H, br q,J=8.4 Hz, H-5b2), 1.62 (1H, ddd,J= 4.8, 4.2, 5.4 Hz, H-6b2), 1.97 (1H, m, H-6b2), 5.09 (1H, d,J= 6.0 Hz, H-7b2), 2.07 (1H, m, H-8b2), 1.87 (1H, m, H-9b2), 0.91 (3H, d,J= 7.2 Hz, H-10b2), 3.60 (3H, s, H-12b2), 4.53 (1H, d,J=7.2 Hz, H-1′b2), 3.54~3.58 (1H, H-6′b2)。13C-NMR(100 MHz, CD3OD)δ: 97.0 (C-1a1), 153.8 (C-3a1), 112.8(C-4a1), 32.6(C-5a1), 28.3(C-6a1), 155.7(C-7a1), 134.3(C-8a1), 45.3 (C-9a1), 119.3 (C-10a1), 166.9 (C-11a1), 99.1 (C-1′a1), 73.4 (C-2′a1), 76.7 (C-3′a1), 70.3 (C-4′a1), 77.1 (C-5′a1), 61.5 (C-6′a1)。96.9 (C-1a2), 151.4 (C-3a2), 110.0 (C-4a2), 29.4 (C-5a2), 142.4 (C-6a2), 196.8 (C-7a2), 134.0 (C-8a2), 44.0(C-9a2),118.1(C-10a2),167.0(C-11a2),98.5(C-1′a2),73.2(C-2′a2), 76.5 (C-3′a2), 70.2 (C-4′a2), 77.0 (C-5′a2), 61.4 (C-6′a2)。97.6 (C-1b1), 151.9 (C-3b1), 111.3 (C-4b1), 32.2 (C-5b1), 39.1 (C-6b1), 77.3 (C-7b1), 39.8 (C-8b1), 46.9 (C-9b1), 13.0 (C-10b1), 168.2 (C-11b1),50.6(C-12b1), 98.3(C-1′b1),74.5(C-2′b1), 76.9(C-3′b1),70.3(C-4′b1), 77.1 (C-5′b1), 61.4 (C-6′b1)。96.4 (C-1b2), 151.9 (C-3b2), 109.5 (C-4b2), 32.6 (C-5b2), 39.0 (C-6b2), 76.5 (C-7b2), 39.8 (C-8b2), 45.7(C-9b2), 12.9(C-10b2), 168.2(C-11b2), 50.5(C-12b2), 98.9(C-1′b2), 73.4 (C-2′b2), 76.7 (C-3′b2), 70.3 (C-4′b2), 77.1 (C-5′b2), 61.3 (C-6′b2)。上述數(shù)據(jù)與文獻(xiàn)[21]報(bào)道的基本一致,從而將該化合物確定為續(xù)斷苷A。
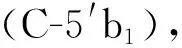
化合物11: 白色無(wú)定形粉末。1H-NMR (400 MHz, CD3OD)δ: 5.56 (1H, d,J= 6.4 Hz, H-1a), 7.50 (1H, s, H-3a), 2.84 (1H, m, H-5a), 1.86 (1H, m, H-6a), 1.74 (1H, m, H-6a), 3.57 (2H, m, H-7a), 5.78 (1H, m, H-8a), 2.65 (1H, m, H-9a), 5.26-5.31 (2H, overlapped, H-10a), 4.71/4.70 (1H, d,J=8.0 Hz, H-1′a/ H-1′b), 3.20 (2H, m, H-2′a, H-2′b), 3.31-3.38 (2H, m, H-3′a, H-3′b), 3.30 (2H, m, H-4′a, H-4′b), 3.31-3.38 (2H, m, H-5′a, H-5′b), 3.70 (2H, overlapped, H-6′a, H-6′b), 3.90 (2H, d,J= 15.6 Hz, H-6′a, H-6′b)。13C-NMRδ: 96.5 (C-1a), 152.2 (C-3a), 110.6 (C-4a), 30.0 (C-5a), 32.6 (C-6a), 59.9 (C-7a), 134.5 (C-8a), 44.1(C-9a), 118.0 (C-10a), 167.2 (C-11a), 98.8(C-1′a), 73.3(C-2′a), 76.9 (C-3′a), 70.2 (C-4′a), 77.0 (C-5′a), 61.4 (C-6′a)。96.2 (C-1b), 151.3 (C-3b), 111.8 (C-4b), 31.3 (C-5b), 38.9 (C-6b), 76.6 (C-7b), 39.7 (C-8b), 45.8 (C-9b), 12.5 (C-10b), 168.0 (C-11b), 50.4 (C-12b), 98.8 (C-1′b), 73.3 (C-2′b), 76.9 (C-3′b), 70.2 (C-4′b), 77.0 (C-5′b), 61.4 (C-6′b)。以上數(shù)據(jù)和文獻(xiàn)[22]報(bào)道基本一致,故將該化合物確定為林生續(xù)斷苷I。
化合物12: 棕色油狀物。1H-NMR (400 MHz, CD3OD)δ: 7.38 (1H, d,J= 3.6 Hz, H-3), 6.58 (1H, d,J= 3.6 Hz, H-4), 4.62 (2H, s, H-6), 9.53 (1H, s, H-7)。13C-NMR (100 MHz, CD3OD)δ: 152.5 (C-2), 123.6 (C-3), 109.6 (C-4), 161.8 (C-5), 56.3 (C-6), 178.1 (C-7)。以上數(shù)據(jù)和文獻(xiàn)[23]報(bào)道基本相同,從而將該化合物確定為5-羥甲基-2-呋喃甲醛。
化合物13: 棕色油狀物。HR-ESI-MS給出分子式C18H30O2(測(cè)量值m/z277.216 8 [M - H]-,計(jì)算值m/z277.217 3 [M- H]-)。1H-NMR (400 MHz, CD3OD)δ: 2.27 (2H, t,J= 7.6 Hz, H-2), 1.59 (2H, m, H-3), 1.33 (8H, overlapped, H-4, H-5, H-6, H-7), 2.07 (4H, overlapped, H-8, H-17), 5.33-5.41 (6H, overlapped, H-9, H-10, H-12, H-13, H-15, H-16), 2.81 (4H, t,J= 8.4 Hz, H-11, H-14), 0.97 (3H, t,J= 7.2 Hz, H-18)。13C-NMRδ: 176.3 (C-1), 33.6(C-2), 25.2(C-3), 28.8(C-4), 28.9(C-5), 28.9(C-6), 29.3 (C-7), 26.8 (C-8), 129.7 (C-9), 127.5 (C-10), 24.7 (C-11), 127.8 (C-12), 127.8 (C-13), 24.7(C-14), 126.9 (C-15), 131.4 (C-16), 20.1 (C-17), 13.2 (C-18)。以上數(shù)據(jù)和文獻(xiàn)[24]報(bào)道基本一致,從而將該化合物確定為α-亞麻酸(α-linolenic acid)。
3 討論與結(jié)論
本研究對(duì)續(xù)斷藥材化學(xué)成分進(jìn)行了進(jìn)一步研究,分離鑒定了13個(gè)化合物,其中3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷、5-羥甲基-2-呋喃甲醛、α-亞麻酸為首次從續(xù)斷藥材中分離得到,同時(shí)也是首次從川續(xù)斷屬植物中分離得到。
以川續(xù)斷皂苷Ⅵ為代表的三萜皂苷類化合物是續(xù)斷發(fā)揮藥效的主要化學(xué)成分群[12],3-O-(2-O-乙酰基)-α-L-吡喃阿拉伯糖常春藤皂苷元-28-O-β-D-吡喃葡萄糖-(1→6)-β-D-吡喃葡萄糖酯苷同川續(xù)斷皂苷Ⅵ一樣,也是具有相同化學(xué)骨架的三萜皂苷類化合物,由于化學(xué)結(jié)構(gòu)的相似性推測(cè)其也具有類似于川續(xù)斷皂苷Ⅵ的生物活性,該成分對(duì)于建立以質(zhì)量標(biāo)志物為藥材優(yōu)劣評(píng)價(jià)依據(jù)的續(xù)斷藥材質(zhì)量標(biāo)準(zhǔn)提供了物質(zhì)基礎(chǔ),同時(shí)為進(jìn)一步闡釋續(xù)斷“發(fā)汗”加工前后化學(xué)成分及其含量的變化、化學(xué)成分轉(zhuǎn)化機(jī)制研究提供了豐富的化學(xué)物質(zhì)基礎(chǔ)。

