Prognostic value of claudin 18.2 expression in gastric adenocarcinoma
Erkan Kayikcioglu, Ramazan O?uz Yüceer, Bulent Cetin, Kamuran Yüceer, Nermin Karahan
Erkan Kayikcioglu, Department of Medical Oncology, Suleyman Demirel University, Isparta 32260, Turkey
Ramazan O?uz Yüceer, Department of Pathology, Isparta City Hospital, Isparta 32360, Turkey
Bulent Cetin, Kamuran Yüceer, Department of Internal Medicine, Suleyman Demirel University, Faculty of Medicine, Isparta 32360, Turkey
Nermin Karahan, Department of Pathology, Suleyman Demirel University, Isparta 32360, Turkey
Abstract BACKGROUND Claudin 18.2 (CLDN18.2) is a cell surface protein expressed by gastric cancer cells. The monoclonal antibody zolbetuximab binds CLDN18.2-positive cancer cells and causes cancer cell death. A few studies researched the prognostic effect of CLDN18.2 expression in metastatic gastric adenocarcinoma.AIM To identify the prognostic value of CLDN18.2 expression in patients with metastatic gastric adenocarcinoma.METHODS This study was conducted with 65 patients over the age of 18 who were diagnosed with metastatic gastric adenocarcinoma. We investigated the effect of CLDN18.2 expression on clinicopathological characteristics (age, sex, histological grade, Lauren classification, family history, metastatic site, HER2 expression) and prognosis for patients with metastatic gastric adenocarcinoma.RESULTS CLDN18.2 expression was positive in 73.8% (48) of the patients. During the median 17.7-mo follow-up period, 89.2% (58) of the patients died. Median progression-free survival and overall survival (OS) were 6 mo (95% confidence interval: 1.6-10.4) and 12 mo (95% confidence interval: 7.5-16.5). There was no statistically significant correlation between CLDN18.2 expression and clinicopathological characteristics of the patients. In univariate and multivariate Cox regression analysis, there was no correlation between clinicopathological characteristics of patients and progression-free survival or OS.CONCLUSION CLDN18.2 expression was quite high in patients with gastric adenocarcinoma, identifying the proportion of the patients in whom zolbetuximab would be efficacious. There is no statistically significant correlation with clinicopathological characteristics and OS. CLDN18.2 is not a prognostic marker in patients with gastric adenocarcinoma, although it is predictive.
Key Words: Gastric adenocarcinoma; Claudin 18.2; Overall survival; Clinicopathological characteristics
INTRODUCTION
Stomach cancer represents the third most common cause of cancer-related mortality globally and caused 768793 deaths in 2020 (7.7% of all cancer deaths)[1]. Most people with stomach cancer in its early stages show no symptoms. The majority of patients (60%) receive diagnosis at the advanced stage following the emergence of symptoms[2]. In light of phase 2 and 3 studies from Europe, perioperative chemotherapy (ChT) has become standard for patients with stage 2 and 3 gastric cancer, but the 5-year overall survival (OS) is still approximately 36%[3,4]. The prognosis for locally advanced, unresectable, or metastatic gastric cancer is poor; in clinical trials evaluating the effectiveness of ChT, the median survival time was typically less than 1 year[5].
Claudin (CLDN) 18, a member of the cell surface protein claudin family, has two isoforms: CLDN18.1 expressed in lung tissue and CLDN18.2 expressed specifically in gastric tissue. CLDN18.2 is also expressed by gastric cancer cells, showing that it is not lost during malignant transformation[6]. The monoclonal antibody zolbetuximab binds CLDN18.2-positive cancer cells and causes cancer cell death by antibody-dependent cellular toxicity and complement-dependent cytotoxicity. In MONO phase 2a study of zolbetuximab as a single agent, CLDN18.2-positive patients with metastatic gastric and gastroesophageal junction (G/GEJ) adenocarcinoma received a minimum of one line of ChT and showed a 23% response rate[7]. The phase 2 FAST study of zolbetuximab plus ChT (epirubicin, oxaliplatin, capecitabine)vsChT (epirubicin, oxaliplatin, capecitabine) showed superior OS and progression-free survival (PFS), defining CLDN18.2 as a new target for cancer therapy[8].
We investigated the effect of CLDN18.2 expression on clinicopathological characteristics and prognosis of patients with metastatic gastric adenocarcinoma undergoing ChT.
MATERIALS AND METHODS
Patients admitted to the medical oncology clinic of Suleyman Demirel University hospital between January 2013 and December 2021 with metastatic gastric adenocarcinoma were enrolled in this study. All cases were histopathologically confirmed according to the 5thedition of the World Health Organization classification of digestive system tumors[9]. The Protocol for the Examination of Specimens from Patients with Cancers of the Stomach 2022 of the College of American Pathologists was used to identify histopathologic subtype, tumor location, tumor grade, and HER2 for gastric adenocarcinoma[10]. From the hospital database, the following clinical data were obtained: age, sex, histological type and grade, family history of gastric cancer, metastatic site, HER2 expression, PFS, and OS. The ethics committee of Suleyman Demirel University approved the study with date and number 01/04/2022-102. Patients who accepted participation in the study, who were older than 18-years-old, followed up in the medical oncology clinic of Suleyman Demirel University hospital, and whose paraffin blocks for diagnosis of gastric adenocarcinoma could be reached were enrolled in the study.
Immunohistochemistry
Hematoxylin and eosin sections representing the tumor of patients diagnosed with gastric adenocarcinoma were re-examined. The best paraffin block was selected for immunohistochemistry staining. Sections with 4-micron thickness were taken from paraffin blocks and transferred onto an adhesive coated slide system. The following method was used for immunohistochemical staining with streptavidin-biotin. Sections were incubated at 56 °C for 12 h for deparaffinization. Three percent hydrogen peroxide was used to block endogenous peroxidase. Antigen retrieval was performed in a microwave oven for 20 min using 0.01 mol/L Tris/EDTA buffer pH 9.0. Sections were coated with primary antibodies including CLDN18.2 (rabbit monoclonal antibody, Clone EPR19202, at 1:500 dilution, Abcam, United Kingdom) and incubated at room temperature for 2 h. Sections were incubated for another 20 min at room temperature after the addition of binding (secondary) antibody (Goat Anti-Rabbit IgG H&L (HRP) kit, Abcam, United Kingdom). The streptavidin-biotin complex was added. 3,3′-Diaminobenzidine was used as chromogen for visualization. CLDN18.2 non-tumor gastric tissues were used as positive controls for each staining session.
Evaluation of immunohistochemical staining
Pathology slides were reviewed by two expert pathologists (ROY and NK) who did not know patient treatments and outcomes. Tumor cells were scored positive for CLDN18.2 if they showed definite membranous staining and negative if tumor nuclei and cell membrane did not have immunoreactivity. Staining intensity was scored between 0 and 3 (absent: 0, weak: 1, moderate: 2, strong: 3).
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences 26.0 (SPSS Inc., Chicago, IL, United States). Age and clinical characteristics were compared between patients with expression of CLDN18.2 using the Mann-WhitneyU-test for individual samples. In patient tumor samples with expression of CLDN18.2, sex, localization, family history, Lauren classification, grade, sites of metastasis, liver and lung metastases, and history of adjuvant and neoadjuvant ChT were compared using Pearson’sχ2test. The correlation between CLDN18.2 and HER2 was determined with the Spearman correlation test. OS and PFS were estimated using the Kaplan-Meier method, and a logrank test was used to compare study groups in terms of survival. Multivariate analyses were performed using Cox regression analysis. APvalue of < 0.05 was considered statistically significant.
RESULTS
Sixty-nine patients were screened, and sixty-five were included in the study. The mean age was 64.6 years ± 12.9 years. Among the patients, 49 (75.4%) were male, and 16 (24.6%) were female. Table 1 shows the demographic and clinicopathologic characteristics of the patients according to CLDN18.2 expression. Immunohistochemical staining was used to screen 65 metastatic gastric adenocarcinoma cases for the pathological significance of CLDN18.2 expression (Figure 1). CLDN18.2 expression was positive in 73.8% (48) of the patients.
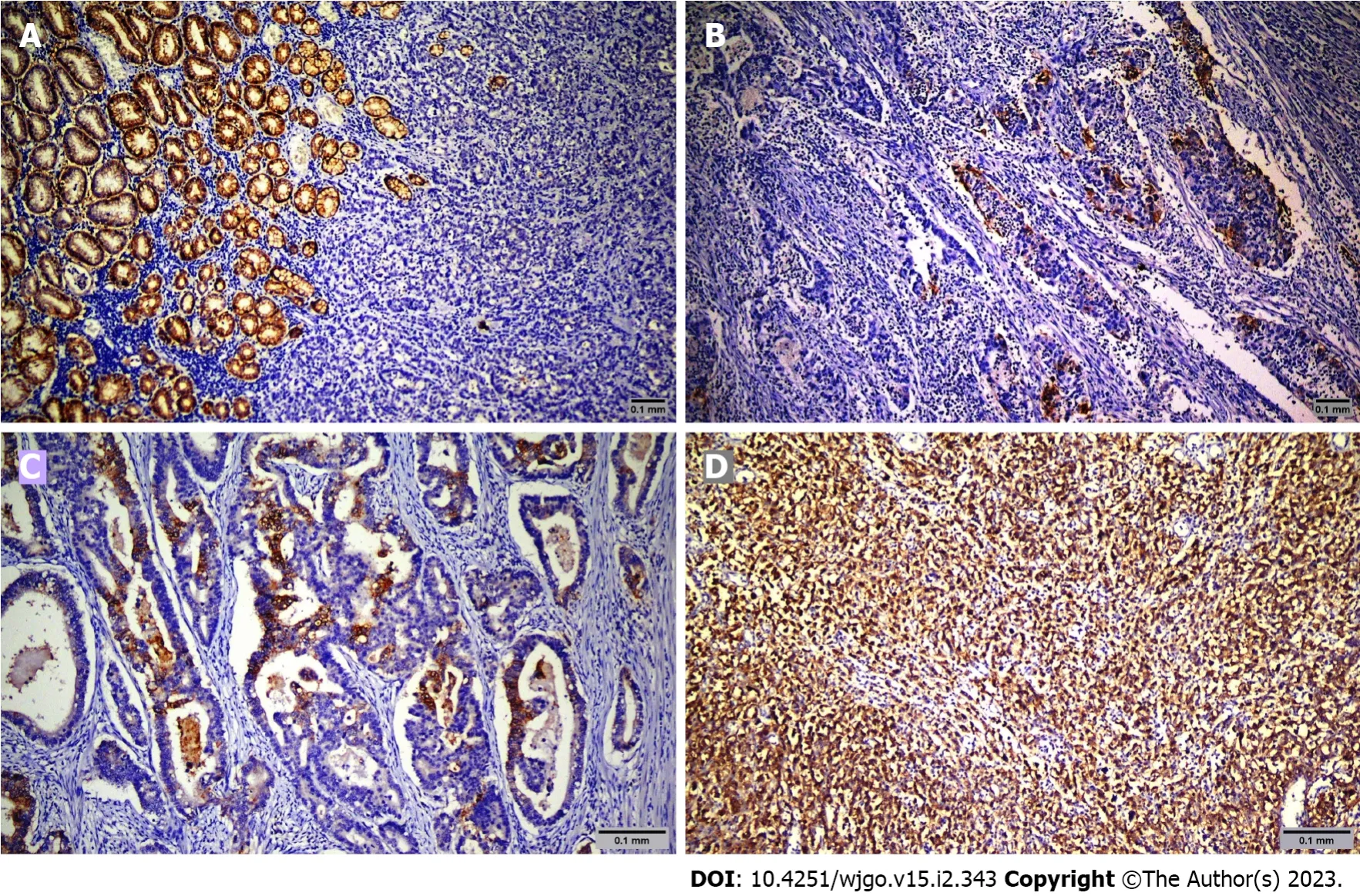
Figure 1 Representative images of claudin 18.2 immunohistochemical staining in gastric adenocarcinoma. A: Score 0; B: Score 1+; C: Score 2+; D: Score 3+.
During the median 17.7-mo follow-up period, 89.2% (58) of the patients died. Median PFS and OS were 6 mo (95% confidence interval: 1.6-10.4) and 12 mo (95% confidence interval: 7.5-16.5). There was no statistically significant correlation between CLDN18.2 expression and clinicopathological characteristics of the patients (Figure 2). In univariate and multivariate Cox regression analysis for PFS, there was no correlation between clinicopathological characteristics of patients and PFS (Table 2). In univariate and multivariate Cox regression analysis for OS, older age was an independent risk factor for poor OS (Table 3).
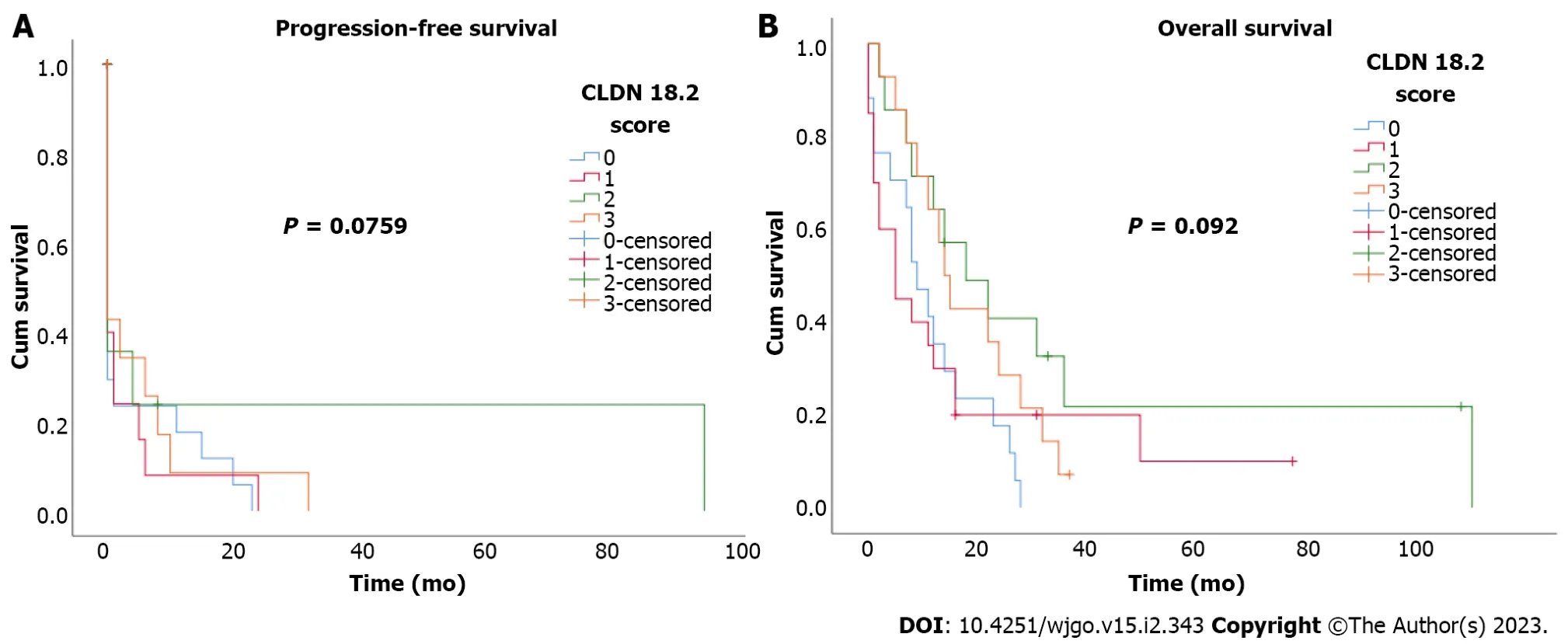
Figure 2 Kaplan-Meier curves for according to claudin 18.2 expression scores. A: Progression-free survival; B: Overall survival according to claudin 18.2 expression (CLDN 18.2) scores.
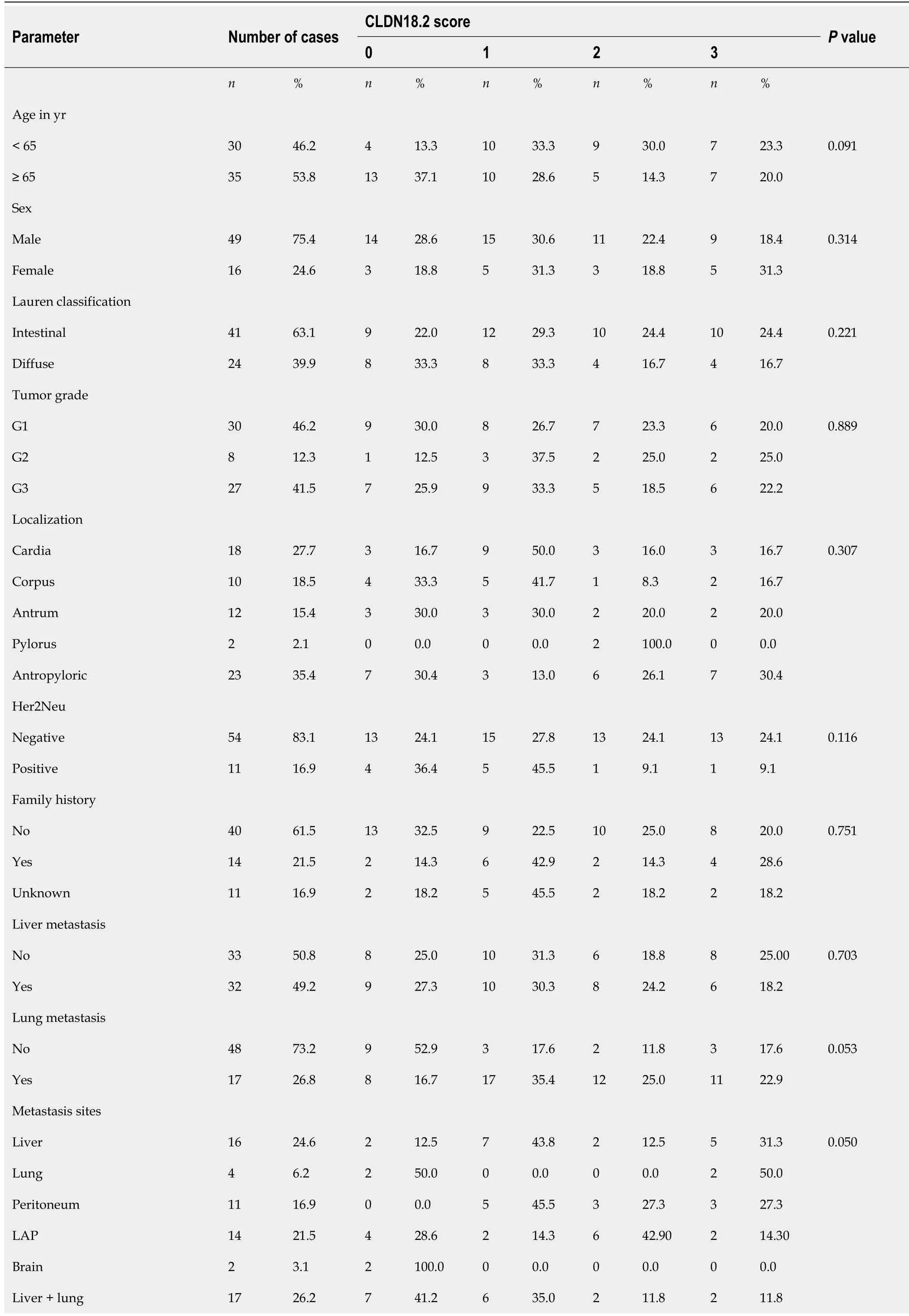
Table 1 Clinicopathological characteristics of patients with gastric adenocarcinoma based on claudin 18.2 expression
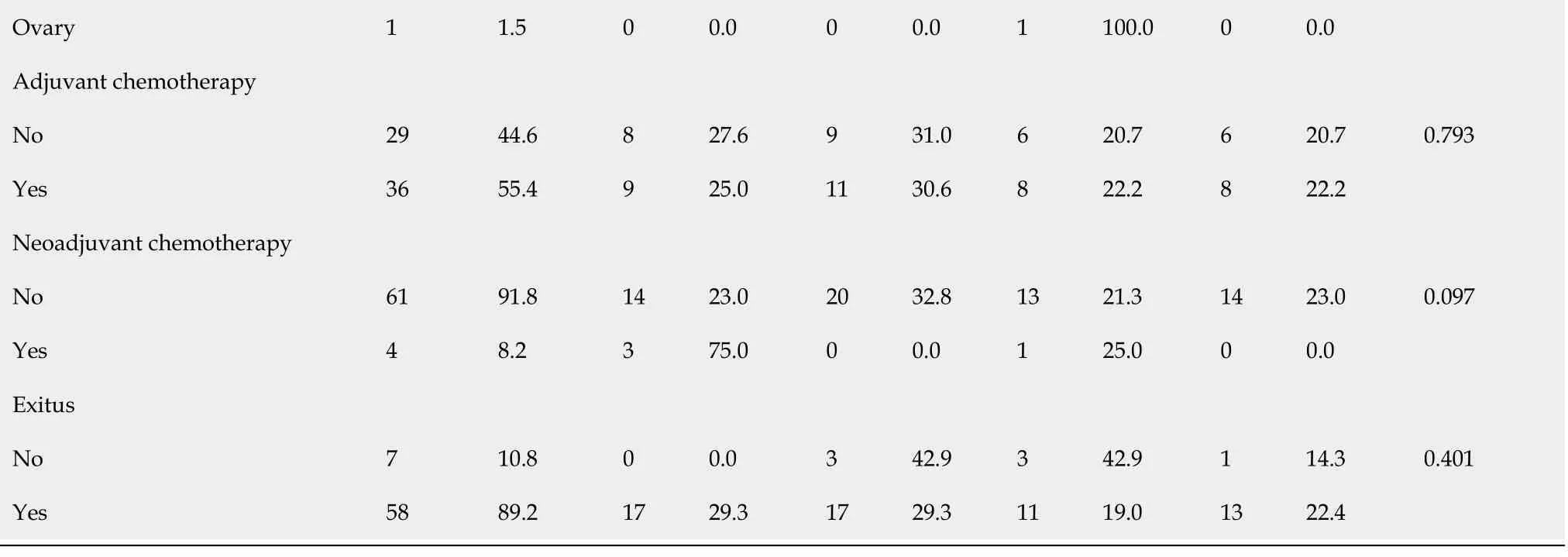
CLDN18.2: Claudin 18.2; LAP: Lymphadenopathy.
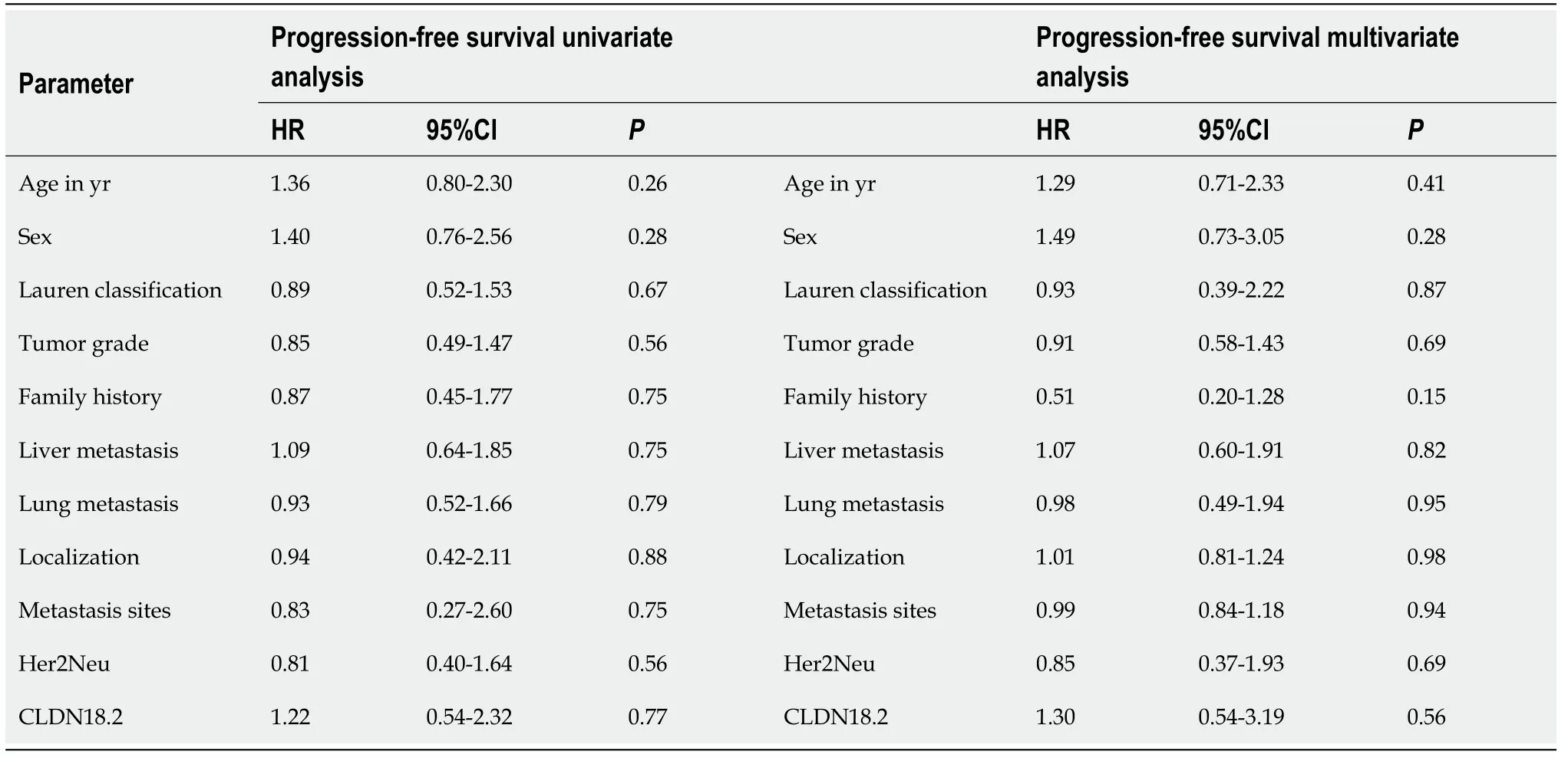
Table 2 Univariate and multivariate analysis of baseline characteristics for progression-free survival
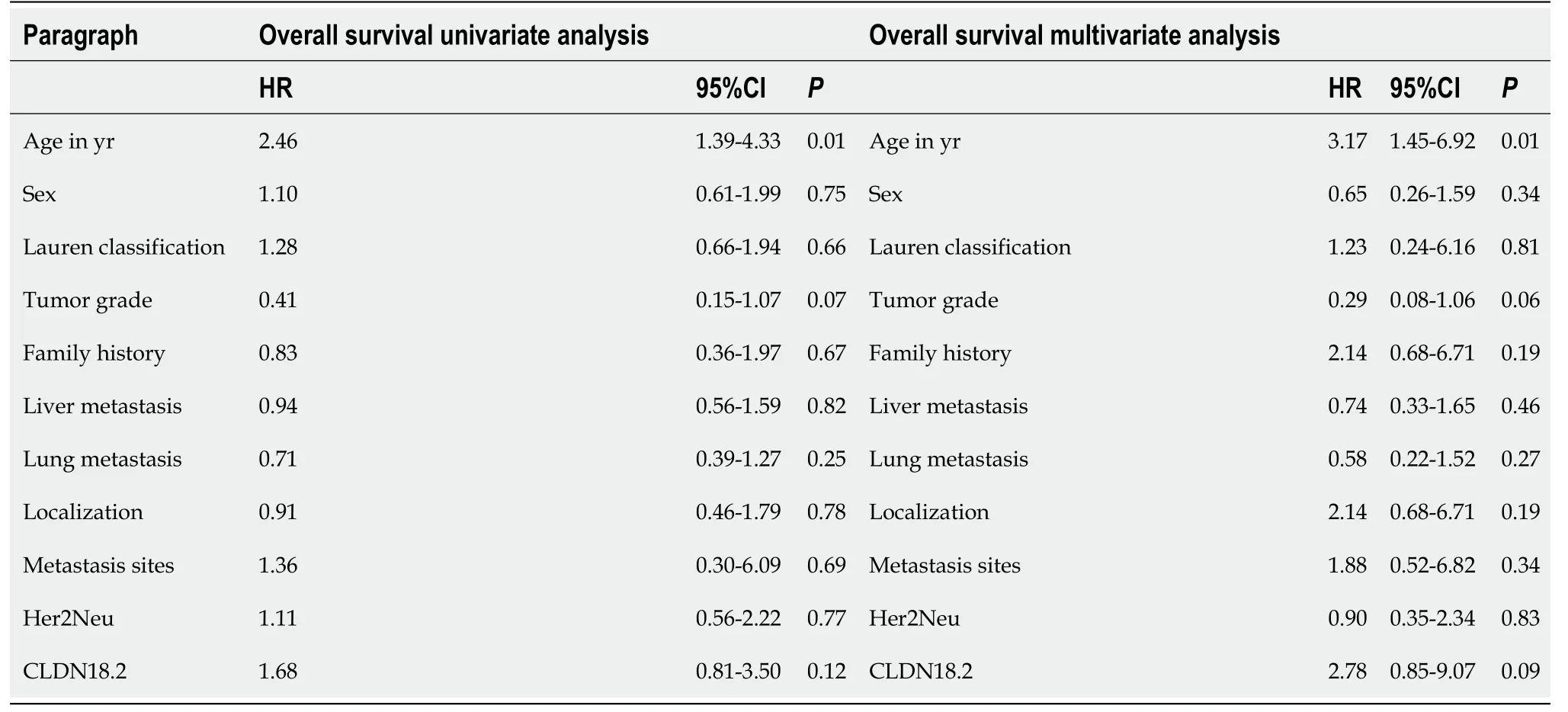
Table 3 Univariate and multivariate analysis of baseline characteristics for overall survival
DISCUSSION
Gastric cancer is common and fatal. With targeted agents and immunotherapy, the median OS of patients with metastatic gastric cancer has reached 13.8-14.4 mo[11,12]. Novel therapies are critical for extending the survival of gastric adenocarcinoma patients. CLDN18.2 is a tight junction molecule found on the surface of gastric mucosa epithelium and gastric cancer cells[6]. In metastatic gastric cancer patients, the monoclonal antibody zolbetuximab targeting CLDN18.2 contributes to OS alone and when combined with ChT. It had tolerable side effects such as nausea and vomiting[7,8]. Worldwide clinical trials of zolbetuximab in the first-line setting, in combination with ChT and immunotherapy, are ongoing for G/GEJ adenocarcinoma (NCT03505320, NCT03504397, and NCT03653507).
Histopathological subtype was diffuse in 36.9% (24) of patients and intestinal in 63.1% (41), and there was no correlation with CLDN18.2 expression. In a study including 481 patients with gastric cancer, there was no correlation between histopathological subtype per Lauren classification and CLDN18.2 expression, as in our study[13]. However, in a study including 263 Japanese patients with gastric adenocarcinoma, diffuse histopathological subtype was associated with strong CLDN18.2 expression[14]. In another study of 85 patients with gastric adenocarcinoma, intestinal subtype was associated with strong CLDN18.2 expression[15]. There was no correlation between grades of tumors and CLDN18.2 expression in a study including 485 patients with esophageal adenocarcinoma[16]; however, grade 3 tumors were associated with strong CLDN18.2 expression in two studies[13,14].
HER2 expression was positive in 16.9% (11) of patients, and there was no correlation between HER2 and CLDN18.2 expression. In three different studies, there was no correlation between HER2 and CLDN18.2 expression[13,15,17], while there was an inverse correlation in a study including patients with esophageal adenocarcinoma[16].
In the present study, CLDN18.2 expression was detected in 73.8% (48) of patients, with moderate to strong expression (≥ 2+) in 43.1% (n= 28). CLDN18.2 expression was detected in 87%, with moderate and strong expression in 51.5%, of Japanese patients in a study conducted by Rohdeet al[14], and moderate to strong expression was present in 49% of patients with G/GEJ adenocarcinoma in the FAST study conducted by Sahinet al[6]. There was no correlation between clinicopathological characteristics of the patients and OS in the present study, consistent with other studies[13,15,16].
Inconsistent with the present study, Türeciet al[7] and Sahinet al[8] detected CLDN18.2 expression in only 17.1% and 14.1% of patients, respectively. This could be due to the different patient cohorts in the studies as well as the different kits used to detect CLDN18.2 expression. Few studies have been published regarding the expression of CLDN18.2 in gastric adenocarcinoma. Conflicting results exist about the CLDN18.2 expression ratios and the relationship between these parameters and the clinicopathological characteristics of patients with gastric adenocarcinoma; however, the studies are consistent in showing there is no correlation between CLDN18.2 expression and OS, as in the present study. The proportion of patients with gastric adenocarcinoma in whom zolbetuximab was efficacious was determined by the MONO and FAST trials. Our findings are consistent with these studies.
The limitations of this study included the relatively small number of patients analyzed and the retrospective character. Additional studies with a larger number of patients are needed to define the effect of CLDN18.2 expression on OS.
CONCLUSION
CLDN18.2 expression is quite high in patients with gastric adenocarcinoma, identifying the proportion of the patients in whom zolbetuximab would be efficacious. There is no statistically significant correlation with clinicopathological characteristics and OS. CLDN18.2 is not a prognostic marker in patients with gastric adenocarcinoma.
ARTICLE HIGHLIGHTS
Research background
Claudin 18.2 (CLDN18.2) is a cell surface protein expressed by gastric cancer cells and a new target for the monoclonal antibody named zolbetuximab.
Research motivation
It is unknown whether CLDN18.2 expression on gastric cancer cells is prognostic.
Research objectives
To identify the prognostic value of CLDN18.2 expression in patients with metastatic gastric adenocarcinoma.
Research methods
We investigated the effect of CLDN18.2 expression on clinicopathological characteristics (age, sex, histological grade, Lauren classification, family history, metastatic site, and HER2 expression) and prognosis for patients with metastatic gastric adenocarcinoma.
Research results
There was no statistically significant correlation between CLDN18.2 expression and clinicopathological characteristics of the patients. In univariate and multivariate Cox regression analysis, there was no correlation between clinicopathological characteristics of patients and progression-free survival or overall survival. The expression of CLDN18.2 was predictive for zolbetuximab in metastatic gastric adenocarcinoma, but it is not prognostic.
Research conclusions
CLDN18.2 expression is high in metastatic gastric adenocarcinoma and predictive for zolbetuximab, but it is not prognostic.
Research perspectives
Detection of new prognostic and predictive markers will make gastric cancer more manageable.
FOOTNOTES
Author contributions:Kayikcioglu E contributed to conceptualization; Yüceer RO, Kayikcioglu E, Karahan N, and Cetin B contributed to formal analysis and investigation; Kayikcioglu E and Cetin B contributed to supervision; Kayikcioglu E, Yüceer RO, and Yüceer K contributed to resources; Yüceer K contributed to visualization; Kayikcioglu E, Yüceer RO, Yüceer K, Karahan N, and Cetin B contributed to writing and original draft preparation; Kayikcioglu E, Karahan N, and Cetin B contributed to reviewing and editing.
Institutional review board statement:The study was reviewed and approved by the Ethics Committee of Suleyman Demirel University (Approval No. 01/04/2022-102).
Informed consent statement:The informed consent was obtained from the patients.
Conflict-of-interest statement:The authors declare no conflicts of interest.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Turkey
ORCID number:Erkan Kayikcioglu 0000-0002-7401-5446; Ramazan O?uz Yüceer 0000-0002-9418-8862; Bulent Cetin 0000-0001-8628-0864; Kamuran Yüceer 0000-0002-7721-5646; Nermin Karahan 0000-0003-0883-4037.
S-Editor:Chen YL
L-Editor:Filipodia
P-Editor:Chen YL
 World Journal of Gastrointestinal Oncology2023年2期
World Journal of Gastrointestinal Oncology2023年2期
- World Journal of Gastrointestinal Oncology的其它文章
- Comment on “Crosstalk between gut microbiota and COVID-19 impacts pancreatic cancer progression”
- Potent bromodomain and extraterminal domain inhibitor JAB-8263 suppresses MYC expression and exerts anti-tumor activity in colorectal cancer models
- microRNA-627-5p inhibits colorectal cancer cell proliferation,migration and invasion by targeting Wnt2
- Increased CD4/CD8 Lymphocyte ratio predicts favourable neoadjuvant treatment response in gastric cancer: A prospective pilot study
- Cancerous inhibitor of protein phosphatase 2A enhances chemoresistance of gastric cancer cells to oxaliplatin
- Evaluation of polygenic risk score for risk prediction of gastric cancer
