Sr6Lu2Al4O15∶Tb3+熒光粉的發光特性
李 敏,孫曉園*,范小暄,劉椿淼,婁文靜,田宛鷺,李昊翔,駱永石
(1.長春師范大學 物理學院,吉林 長春 130032;2.中國科學院長春光學精密機械與物理研究所 發光學及應用國家重點實驗室,吉林 長春 130033)
1 引言
稀土材料被廣泛應用在綠色照明、航空航天、新能源汽車、高品質顯示器、光學防偽等領域[1-5]。在稀土材料的發展過程中,稀土發光材料得到了廣泛研究[6-12]。稀土發光材料因其豐富的軌道能級和獨特的4f 電子躍遷,在發光性質上遠勝于其他材料[13-18]。其中,Tb3+激活的發光材料已得到了廣泛的應用,如燈用熒光粉LaPO4∶Ce3+,Tb3+[19]、增感屏熒光粉Gd2O2S∶Tb3+[20]、三基色熒光燈用熒光粉CeMgAl11O19∶Tb3+[21]等。鋁酸鹽體系熒光粉因其化學和物理性質穩定、發光性能高、激發發射峰寬、制備工藝簡易等特點被廣泛研究[22-24]。Wang等[25]首次報道了Sr6Y2Al4O15的晶體結構,并研究了Sr6Ln2Al4O15(Ln=Tb,Dy,Ho,Er,Tm,Yb,Lu)的晶胞參數。Yang 等[26]研究了Yb3+/Ho3+共摻Sr3YAl2O7.5和Sr3LuAl2O7.5熒光粉的發光性質和能量傳遞機理。Tao 等[27]研究了Sr3LuAl2O7.5∶Ce3+熒光粉的發光性質和熱穩定性。Wang 等[28]研究了Sr3YAl2O7.5∶Bi3+,Eu3+的發光性質、能量傳遞機理和熱穩定性。Dalal 等[29]研究了Sr6Y2Al4O15∶Eu3+的發光性質和熱穩定性。但是,Tb3+摻雜的Sr6Lu2Al4O15熒光粉迄今為止尚未有研究報道。
本文成功制備了一系列適于紫外光激發的Sr6Lu2-2xAl4O15∶xTb3+樣品,通過調節樣品中Tb3+的摻雜濃度,樣品的發光顏色可以從藍光變為黃綠光。研究了其晶體結構、發光性質、濃度猝滅機理、熱穩定性和量子效率。
2 實 驗
主要實驗原料有:SrCO3(AR)、Lu2O3(4N5)、Al2O3(高純)、Tb4O7(4N5)。通過高溫固相法制備了一系列Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)樣品。首先,按化學計量比稱取相應的原料,在瑪瑙研缽中充分研磨,將研磨均勻的原料裝入剛玉坩堝,置于高溫箱式馬弗爐中,在還原氣氛下,1 500 ℃恒溫4 h,隨爐冷卻至室溫,將樣品取出,研磨得到粉末樣品。
物相分析采用DX-2700 型號X 射線衍射儀,采用日立F-4600 測樣品的激發光譜、發射光譜(用Xe 燈作激發光源)。熒光壽命測量由Tektronix-TDS 3052 數字示波器記錄,利用Continuum Surelite Nd∶YAG 激光器泵浦Horizon OPO(光參量振蕩器)輸出268 nm 脈沖激光激發。使用日立F-4600 測試樣品的熱穩定性(用TCB1402C 高溫粉末檢測選配件控制溫度)。樣品的量子效率采用日立F-7000 測得。
3 結果與討論
3.1 樣品的晶體結構分析
圖1 為樣品Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)的X 射線衍射圖。樣品的衍射峰與國際標準卡片JCPDS 00-057-0779(Sr6Al4Lu2O15)衍射峰的位置基本吻合,說明制備的樣品晶體結構與Sr6Al4Lu2O15一致。圖2 為Sr6Al4M2O15(M=Lu,Tb)的晶體結構,Sr6Al4Lu2O15為單斜晶系,晶胞參數為a=1.748 7 nm,b=0.571 4 nm,c=0.763 7 nm,α=90.0°,β=90.914°,γ=90.0°。

圖1 Sr6Lu2-2xAl4O15∶xTb3+熒光粉的XRD 衍射圖Fig.1 X-ray diffraction patterns of the Sr6Lu2-2xAl4O15∶xTb3+phosphors
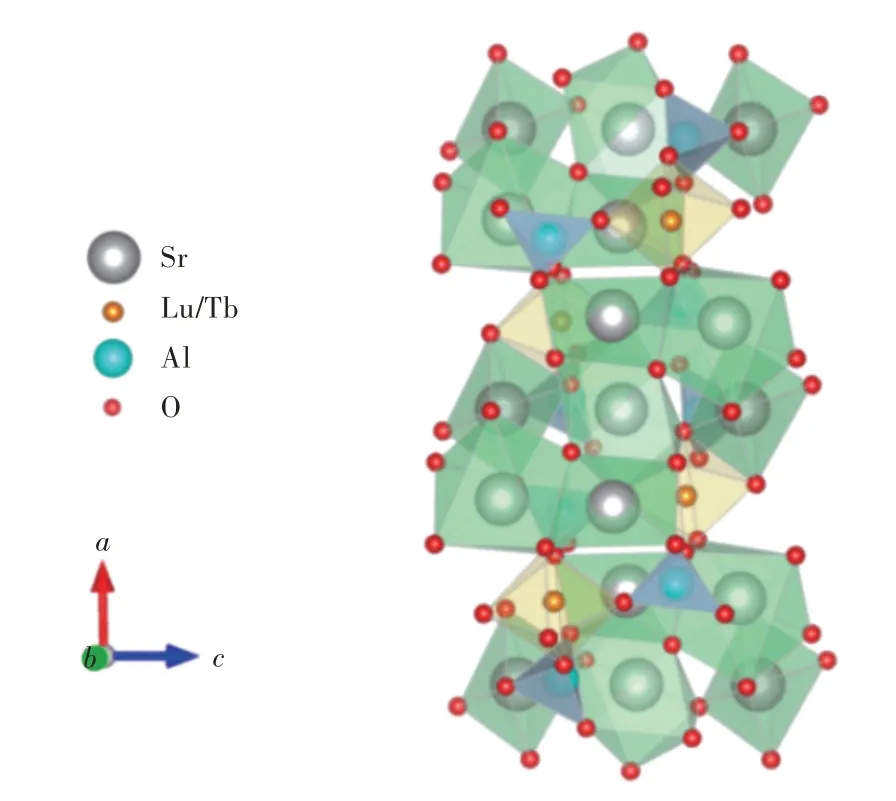
圖2 Sr6Al4M2O15(M=Lu,Tb)的晶體結構圖Fig.2 Crystal structure of Sr6Al4M2O15(M=Lu,Tb)
Lu3+與摻雜離子Tb3+具有相同的電荷和相近的離子半徑,當配位數為6 時,Tb3+半徑(r=0.092 nm)大于Lu3+半徑(r=0.086 nm)[30],因此,摻雜的Tb3+可能會占據Lu3+格位。從圖1 中可以看出,樣品的XRD 衍射峰逐漸向小角度偏移。在本樣品中,較大離子半徑的Tb3+取代了較小離子半徑的Lu3+的格位,所以,樣品的晶面間距d隨Tb3+摻雜濃度增加而增大。根據布拉格方程:
可以得出隨樣品晶面間距d增大,θ角變小。
3.2 發光性質
圖3 為Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)的激發光譜,監測波長為381 nm,監測范圍為200~360 nm,峰值位于268,303,315 nm,源于Tb3+的4f8-4f75d 躍遷。當Tb3+的一個電子從4f8組態躍遷至4f75d1激發態時,它會產生兩種不同的f-d 躍遷:自旋允許和自旋禁戒的躍遷。通常自旋允許的7FJ-7DJ躍遷能量高、強度大,而自旋禁戒的7FJ-9DJ躍遷能量低、強度小。因此,圖3 中位于268 nm 的激發峰來自于Tb3+自旋允許的7FJ-7DJ躍遷,而處于303,315 nm 的激發峰來自于Tb3+自旋禁戒的7FJ-9DJ躍遷[31-32]。隨Tb3+摻雜濃度的升高,樣品激發峰的強度先增強后減弱。x=0.01 的樣品位于268 nm 附近的激發峰強度最強。
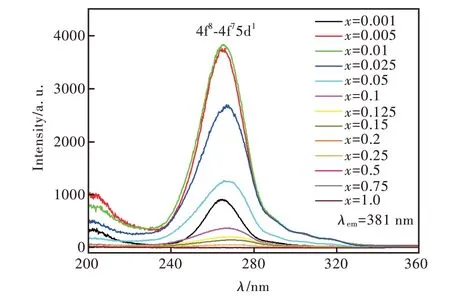
圖3 Sr6Lu2-2xAl4O15∶xTb3+(x =0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)樣品的激發光譜,λem=381 nm。Fig.3 Excitation spectra of the Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)phosphors,λem=381 nm.
圖4 為Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)的激發光譜,監測波長為545 nm,監測范圍為200~400 nm。不同Tb3+摻雜濃度的樣品激發光譜形狀相似,都由較強的4f75d1寬帶吸收(200~336 nm)和較弱的4f-4f 電子躍遷吸收(336~400 nm)兩部分組成。峰值位于268 nm(7F6-7DJ),288,303,315 nm(7F6-9DJ),340 nm(7F6-5L7),351 nm(7F6-5L9),359 nm(7F6-5G5),367 nm(7F6-5L10)和376 nm(7F6-5G6)[31-40]。隨著Tb3+摻雜濃度提高,樣品激發峰的強度先增強后減弱。當x=0.15 時,位于268 nm 的激發峰的強度比其他樣品大。當x=0.5 時,位于302,316 nm 的激發峰的強度比其他樣品大。
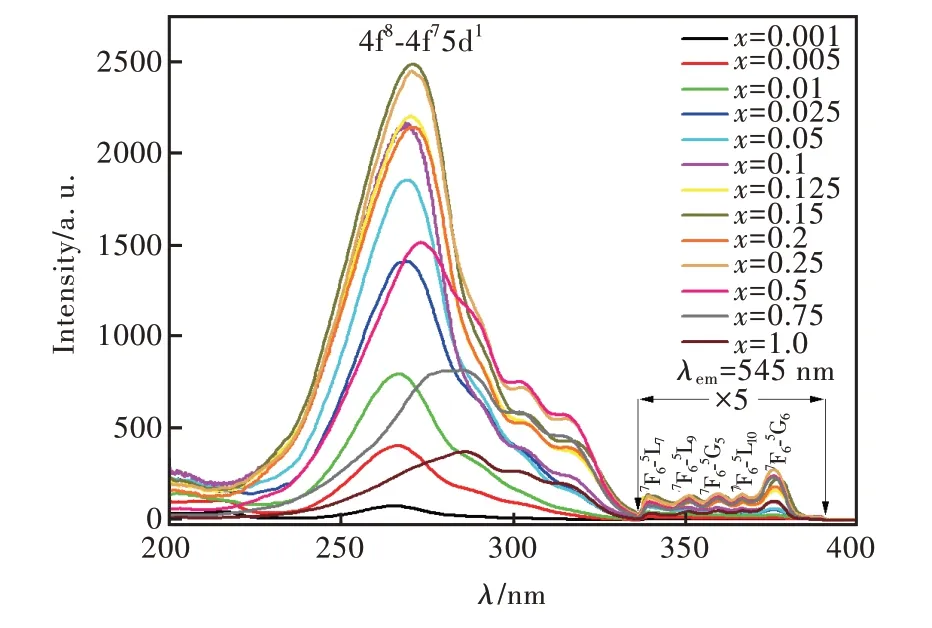
圖4 Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)樣品的激發光譜,λem=545 nm。Fig.4 Excitation spectra of the Sr6Lu2-2xAl4O15∶xTb3+ (x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0) phosphors,λem=545 nm.
圖5 為激發波長268 nm 時Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)的發射光譜。摻雜不同濃度Tb3+離子的樣品的發射光譜都由一系列銳峰組成。峰值位于381 nm、391 nm(5D3-7F6),423 nm(5D3-7F5),439 nm(5D3-7F4),450 nm(5D3-7F3),463 nm(5D3-7F2),489 nm、504 nm(5D4-7F6),545 nm、554 nm(5D4-7F5),581,586,596,604 nm(5D4-7F4),626 nm(5D4-7F3)[33-40]。x=0.005 和x=0.01 的樣品位于381 nm 的發射峰發光強度相近。x=0.15 的樣品位于545 nm 的發射峰發光強度最大。當Tb3+摻雜濃度較低時,可以同時看到5D3-7FJ(J=6,5,4,3,2)和5D4-7FJ(J=6,5,4,3)發射出的熒光;當x> 0.1 時,5D3-7FJ躍遷發光強度明顯減弱。這是由于5D3能級的粒子交叉弛豫過程被倒空到5D4能級的緣故。圖6 是Tb3+的能級圖。Tb3+主要猝滅過程是5D3+7F6→5D4+7F0的交叉弛豫過程,隨著Tb3+摻雜濃度的升高,5D3能級發出的熒光逐漸減弱,5D4能級發出的熒光逐漸增強[41-42]。
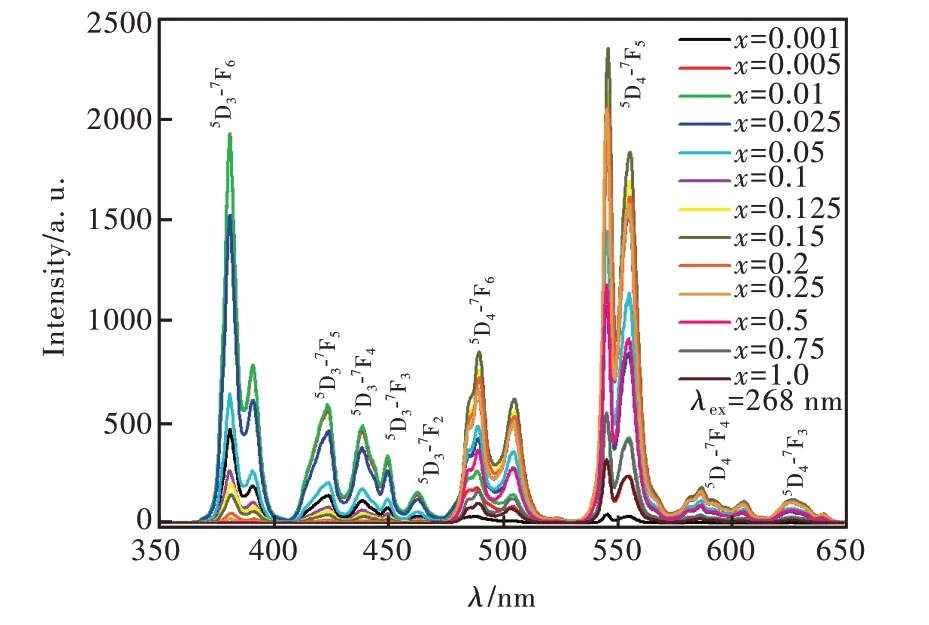
圖5 Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)樣品的發射光譜,λex=268 nm。Fig.5 Photoluminescence spectra of the Sr6Lu2-2xAl4O15∶xTb3+(x =0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)phosphors,λex=268 nm.
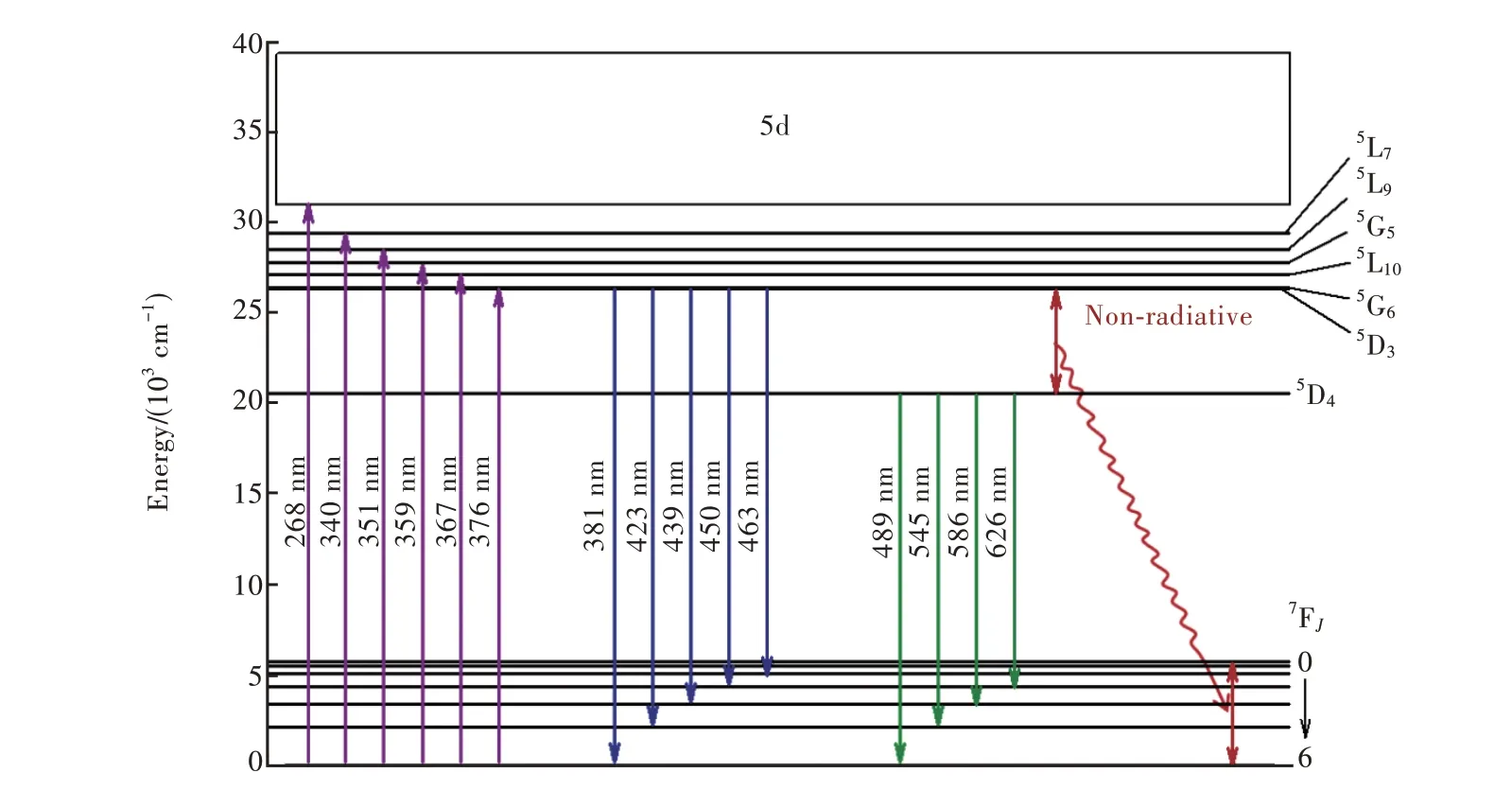
圖6 Tb3+的能級圖Fig.6 Energy level diagram of Tb3+
表1 為樣品Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)在268 nm 激發下的CIE 坐標。圖7為樣品的色坐標圖,可以看出,在紫外光激發下,隨著Tb3+摻雜濃度的升高,樣品的發光顏色從藍光變為黃綠光,說明通過改變Tb3+的摻雜濃度,可以實現Sr6Lu2-2xAl4O15∶xTb3+體系樣品發光顏色的調控。
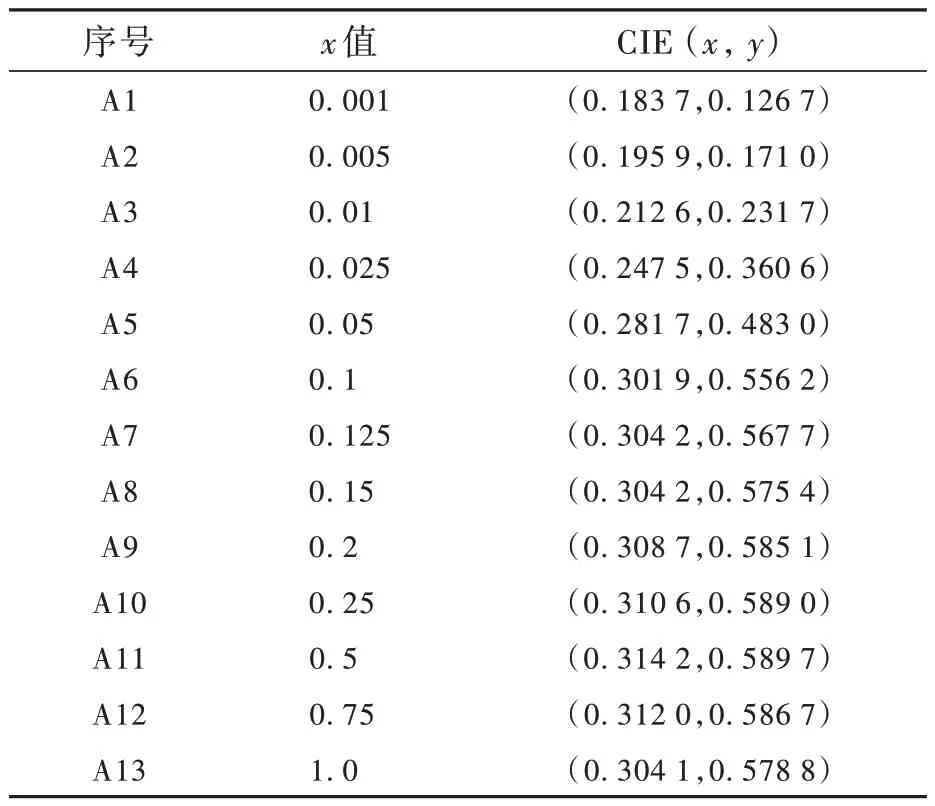
表1 Sr6Lu2-2xAl4O15∶xTb3+樣品的CIE 坐標Tab.1 CIE of Sr6Lu2-2xAl4O15∶xTb3+ phosphors

圖7 Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)CIE 坐標圖,λex=268 nm;插圖為樣品在254 nm 紫外燈下的發光照片。Fig.7 CIE chromaticity coordinates of Sr6Lu2-2xAl4O15∶xTb3+(x =0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)phosphors under 268 nm excitation.Inset shows the images of samples under 254 nm UV lamp.
3.3 濃度猝滅機理
圖8(a)給出了Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)在268 nm 激發下,樣品源于5D3能級躍遷發光的積分發光強度與Tb3+濃度的關系,積分范圍為360~470 nm。隨Tb3+摻雜濃度的增加,樣品的積分發光強度先增強后減弱。當x=0.01 時,樣品的積分發光強度最強;當0.01 <x<0.1 時,樣品5D3-7FJ的發光強度急劇下降,這是由于發生了濃度猝滅。當x≥ 0.25 時,樣品的5D3-7FJ躍遷幾乎完全猝滅。猝滅臨界濃度為0.01。圖8(b)給出了Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)在268 nm 激發下,471~650 nm 范圍內5D4能級躍遷發光的積分發光強度與Tb3+濃度的關系。隨Tb3+摻雜濃度的增加,樣品的積分發光強度先增強后減弱。當x< 0.15 時,5D4-7FJ的發光強度逐漸增強;當x> 0.15 時,5D4-7FJ的發光強度逐漸減弱,這是由于發生了濃度猝滅。猝滅臨界濃度為0.15。
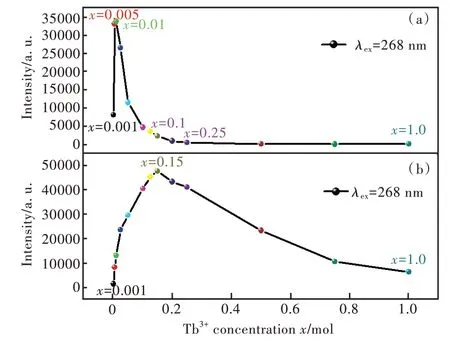
圖8 (a)Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)樣品5D3 能級躍遷強度隨Tb3+濃度的變化關系;(b)Sr6Lu2-2xAl4O15∶xTb3+樣品5D4能級躍遷強度隨Tb3+濃度的變化關系。Fig.8 (a)5D3 energy level emission intensity of Sr6Lu2-2xAl4-O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)with the Tb3+concentrations.(b)5D4 energy level emission intensity of Sr6Lu2-2xAl4O15∶xTb3+ with the Tb3+ concentrations.
Tb3+離子的濃度猝滅現象可以歸因于Tb3+離子之間的能量傳遞。能量傳遞主要有兩種機制:電多極相互作用和交換相互作用,主要由臨界距離Rc決定。Blasse[43]推測晶體中能量傳遞的臨界距離Rc的計算公式為:
V(V=0.763 nm3)為單位晶胞體積,N(N=4)為單位晶胞中激活劑離子占據的陽離子格點數,Xc是猝滅臨界濃度。一般而言,當Rc≤0.5 nm 時,能量傳遞以交換相互作用為主;當Rc>0.5 nm 時,能量傳遞主要歸因于電多極相互作用。將上述數值代入公式(2)中,計算得到Rc1=3.32 nm > 0.5 nm,Rc2=1.34 nm > 0.5 nm。所以Sr6Lu2-2xAl4O15∶xTb3+中Tb3+的5D3能級和5D4能級濃度猝滅的能量損失均為電多極-電多極相互作用。
Dexter[44]曾指出,在無機非導電性材料中,激活劑的電多極-電多極相互作用所導致的濃度猝滅中,發射光強度I與摻雜摩爾濃度x之間存在著以下關系:
其中,C為常數,θ=6,8,10 分別對應于電偶極-電偶極(d-d)、電偶極-電四極(d-q)、電四極-電四極(q-q)相互作用。作出lg(I/x)與lgx的關系曲線,如圖9 所示,通過線性擬合得出lg(I/x)與lgx呈線性關系。5D3能級的擬合斜率為-2.289 53,θ1=6.87,近似等于6;5D4能級的擬合斜率為-2.053 47,θ2=6.16,近似等于6。因此,樣品中Tb3+的5D3能級和5D4能級的猝滅機理均為電偶極-電偶極相互作用。
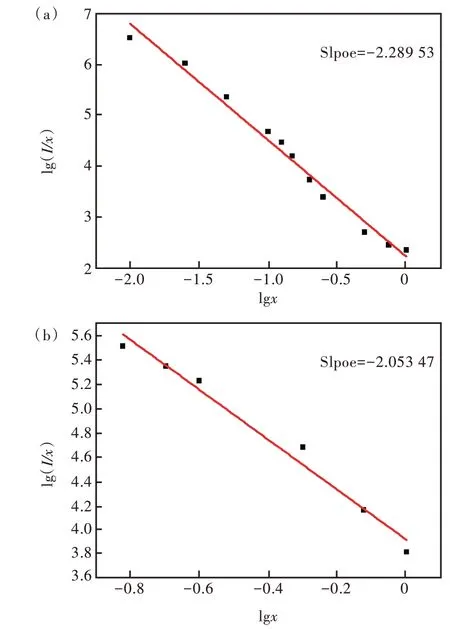
圖9 (a)Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)樣品5D3能級的lg(I/x)與lgx 關系曲線;(b)Sr6Lu2-2xAl4O15∶xTb3+樣品5D4能級的lg(I/x)與lgx關系曲線。Fig.9 (a)The plot of lg(I/x) versus lgx in Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)phosphors for 5D3 energy level.(b)The plot of lg(I/x)versus lgx in Sr6Lu2-2xAl4O15∶xTb3+ phosphors for 5D4 energy level.
在268 nm 光激發下,監測381 nm(5D3-7F6)和545 nm(5D4-7F5)兩個位置,測得Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)的熒光衰減曲線,如圖10 和圖11 所示。壽命由公式(4)計算得到:
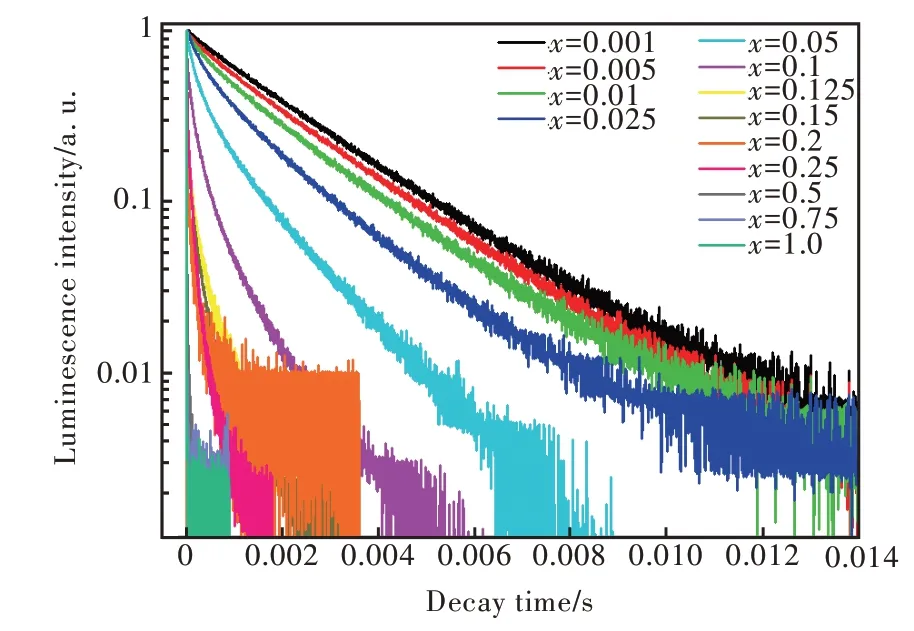
圖10 Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)熒光壽命衰減曲線,監測波長為381 nm,λex=268 nm。Fig.10 PL decay curves of the Tb3+ ions in Sr6Lu2-2xAl4O15∶xTb3+ (x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0) phosphors monitored at 381 nm.λex=268 nm.
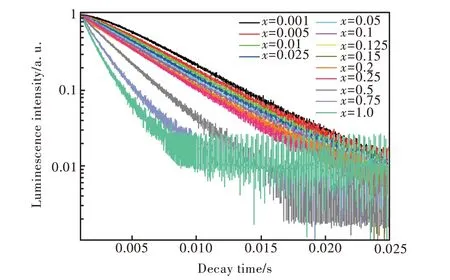
圖11 Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)熒光壽命衰減曲線,監測波長為545 nm,λex=268 nm。Fig.11 PL decay curves of the Tb3+ ions in Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)phosphors monitored at 545 nm.λex=268 nm.
其中,I(t)代表t時刻的發光強度,τ是熒光壽命。用公式(4)計算得出的壽命如圖12所示。從圖12(a)中可以看出,隨Tb3+濃度的增加,Sr6Lu2-2xAl4O15∶xTb3+樣品5D3-7F6躍遷發射的壽命總體呈現衰減的趨勢,當x≤ 0.1 時,樣品熒光壽命衰減較快。從圖12(b)中可以看出,隨Tb3+濃度的增加,樣品5D4-7F5躍遷發射的壽命逐漸衰減。當x≤ 0.05時,樣品熒光壽命衰減較快;當0.1≤x≤0.2 時,樣品熒光壽命衰減緩慢。
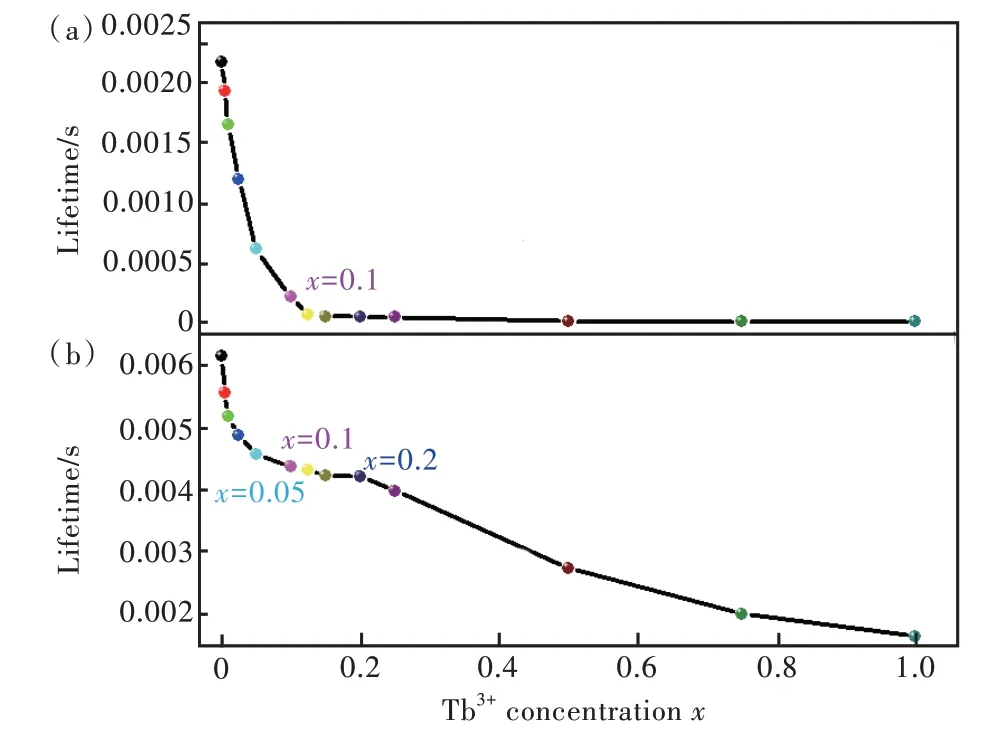
圖12 (a)監測381 nm 時Sr6Lu2-2xAl4O15∶xTb3+樣品的壽命隨Tb3+濃度的變化關系;(b)監測545 nm 時樣品的壽命隨Tb3+濃度的變化關系。λex=268 nm。Fig.12 (a) Lifetime for Sr6Lu2-2xAl4O15∶xTb3+ phosphors with the Tb3+ concentrations monitored at 381 nm.(b) Lifetime for Sr6Lu2-2xAl4O15∶xTb3+ phosphors with the Tb3+concentrations monitored at 545 nm.λex=268 nm.
表2 給出了在監測381 nm 和545 nm 時Sr6Lu2-2xAl4O15∶xTb3+樣品的壽命。監測381 nm時,樣品5D3-7F6躍遷發射的壽命為4.88×10-3~2.18 ms;監測545 nm 時,樣品5D4-7F5躍遷發射的壽命為1.65~6.15 ms。隨Tb3+濃度的增加,5D3能級的壽命變短趨勢比5D4能級更顯著,并且5D3能級的壽命比5D4能級的壽命短,這與熒光強度隨濃度的變化情況一致。
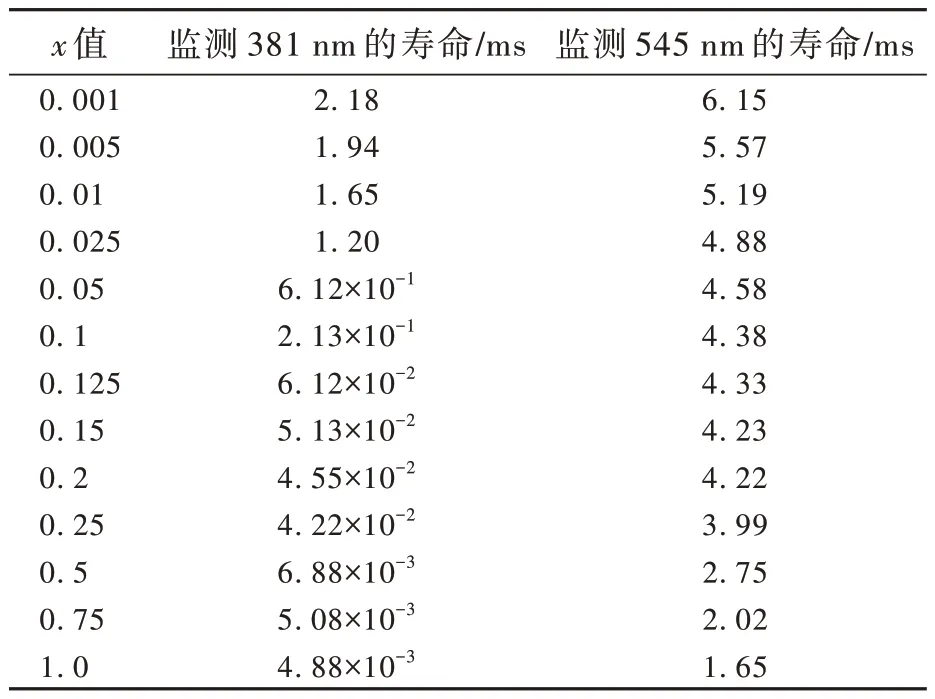
表2 監測381 nm 和545 nm 時Sr6Lu2-2xAl4O15∶xTb3+樣品的壽命Tab.2 Lifetime for Sr6Lu2-2xAl4O15∶xTb3+ phosphors monitored at 381 nm and 545 nm
3.4 熱穩定性和量子效率
為研究樣品的熱穩定性,對Sr6Lu1.7Al4O15∶0.3Tb3+樣品在298~483 K 不同溫度下的發射光譜進行了測試。如圖13 所示,隨著溫度升高,樣品的發光強度先增強后減弱。當溫度為323 K 和343 K 時,樣品的發光強度分別為初始發光強度的102.4% 和101.5%,說明樣品具有負熱猝滅的特性。當溫度為423 K 時,樣品的發光強度為初始溫度的80.9%,說明樣品具有良好的熱穩定性。
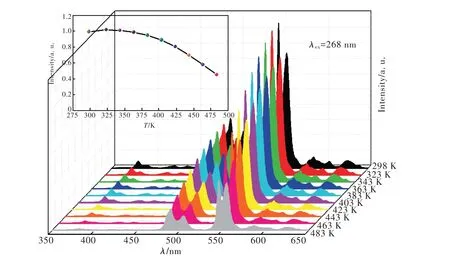
圖13 Sr6Lu1.7Al4O15∶0.3Tb3+在不同溫度下的發射光譜,插圖為不同溫度下的積分發光強度。Fig.13 The temperature-dependent luminescence intensities of Sr6Lu1.7Al4O15∶0.3Tb3+ phosphor.Inset shows the intensities on temperature.
為研究樣品的量子效率,對Sr6Lu1.7Al4O15∶0.3Tb3+樣品的量子效率進行了測量。樣品的內量子效率為53.5%,外量子效率為39.3%。
4 結論
本文采用高溫固相法合成了Sr6Lu2-2xAl4O15∶xTb3+(x=0.001,0.005,0.01,0.025,0.05,0.1,0.125,0.15,0.2,0.25,0.5,0.75,1.0)系列熒光粉。研究了其晶體結構、發光性質、濃度猝滅機理、熱穩定性和量子效率。在紫外光激發下,x=0.01 的樣品,Tb3+的5D3-7F6躍遷強度最大,位于381 nm;x=0.15的樣品,Tb3+的5D4-7F5躍遷強度最大,位于545 nm。通過改變Tb3+摻雜濃度,樣品的發光顏色可以從藍光變為黃綠光,色坐標從(0.183 7,0.126 7)變化到(0.314 2,0.589 7)。樣品5D3和5D4能級濃度猝滅機理均為電偶極-電偶極相互作用。通過測量樣品的熒光壽命衰減曲線,發現隨著Tb3+濃度的增加,5D3能級的壽命變短趨勢比5D4能級更顯著,這與熒光強度隨濃度的變化情況一致。Sr6Lu1.7Al4O15∶0.3Tb3+樣品具有良好的熱穩定性,423 K時,樣品的發光強度為初始發光強度的80.9%。Sr6Lu1.7Al4O15∶0.3Tb3+內量子效率為53.5%,外量子效率為39.3%。Sr6Lu2-2xAl4O15∶xTb3+是一種適合于紫外激發的發光顏色可調的熒光粉。Sr6Lu2-2xAl4O15∶xTb3+是潛在的可應用于黑光燈、彩色熒光燈的新型熒光粉。
本文專家審稿意見及作者回復內容的下載地址:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20230147.

