聚對苯撐苯并二噁唑纖維共聚改性研究進展
摘 要:聚對苯撐苯并二噁唑(PBO)纖維是目前綜合性能最好的高性能有機纖維之一,但PBO纖維存在抗紫外光老化性能差、壓縮強度低和界面粘結性能差等缺陷,無法滿足航天、兵器等領域的嚴苛需求,因此需對其進行改性以提高性能。根據改性目的,從提升拉伸力學性能、提升壓縮強度、改善界面粘接性能和提升抗紫外老化性能4個方面,綜述了近年來國內外PBO纖維共聚改性技術的研究進展,提出共聚改性應充分考慮化學結構、凝聚態結構和紡絲工藝對最終纖維性能的綜合影響,并對未來PBO纖維的共聚改性技術進行了展望。
關鍵詞:PBO纖維;共聚改性;力學性能;界面粘結性能;耐紫外光老化性能
中圖分類號:TQ342
文獻標志碼:A
文章編號:1009-265X(2024)11-0123-11
聚對苯撐苯并二噁唑(Poly (p-phenylene benzo-bisoxazole),PBO)纖維是繼芳綸纖維之后出現的新一代高性能化學纖維,其重復單元由苯并雙噁唑環和苯環構成,分子結構高度共軛且無弱鍵,剛性極強,因此具有優良的力學性能和耐熱性。PBO纖維強度可達5.8 GPa,模量可達280 GPa,極限氧指數為68,最高分解溫度為650 ℃,并且擁有優良的耐化學腐蝕性和抗沖擊性,綜合性能為有機纖維之最,被譽為新一代超級纖維[1]。
PBO纖維因其具有優良的綜合性能,在航天航空、兵器艦船、建筑增強、高溫過濾以及特種防護等領域有廣闊的應用潛力。因其優異的強度、模量和韌性,PBO纖維可用于防彈衣、防彈頭盔以及防彈插板等抗沖擊領域[2],也可以作為光纜補強、橡膠增強、高強繩索、高壓氣瓶、體育器材和建筑增強等領域的增強材料;根據其耐熱、阻燃性好的特點,可以應用于消防服、防火服、隔熱手套、隔熱襯墊、高溫濾材等領域[3]。
盡管PBO纖維力學性能和耐熱性良好,但仍然存在一定的性能缺陷,限制了其應用和推廣。目前商品化的PBO纖維模量為280 GPa,與其460~478 GPa的理論模量相差較遠[4],強度跟T1000碳纖維相比仍有不足。PBO分子鏈高度對稱,無極性側基,分子鏈間作用力弱,纖維壓縮強度較差[5]。PBO纖維表面光滑,呈現出極強的惰性,與樹脂基體的界面粘結性能較差[6]。PBO纖維受紫外光照射易老化降解,導致各項性能下降[7-8]。
為了改善PBO纖維的上述性能缺陷,需對其進行改性處理。根據改性的方法不同,PBO纖維改性可分為表面改性、物理共混和共聚改性。表面改性包括輻射改性、化學試劑處理、等離子體表面處理、納米粒子涂敷或表面接枝等[9],一般都是作用于纖維的表面,表面改性的PBO纖維與負載介質間常存在結合牢度差的問題[10];物理共混法是將納米粒子填料以共混的方式加入到纖維基體中,工藝簡單,但納米粒子在纖維基體中分散性較差[11];共聚改性是指PBO聚合物聚合過程中除常規反應單體外,在大分子主鏈上引入第三單體,并通過調節共聚物分子量、組成及序列結構制備性能更優異的共聚物。相比于表面改性與物理共混,共聚改性從分子結構角度設計大分子主鏈,根本上提升PBO纖維的本征性能且改性效果具有長效穩定性,具有較高的研究價值和良好的發展前景。近年來,國內外科研人員針對PBO纖維的合成及改性開展了大量的研究工作,但缺乏系統性的歸納總結報告。本文介紹PBO聚合物的合成及共聚改性技術研究進展,根據改性的效果進行分類梳理,總結不同單體結構對纖維性能的貢獻,對并對共聚改性方法存在的問題及后續改進方向進行展望。
1 PBO聚合物合成方法
根據不同的聚合工藝,PBO聚合物的合成方法主要有脫氯化氫法、絡合鹽法、AB型自縮合法、中間相聚合法、三甲基硅烷基化法等。
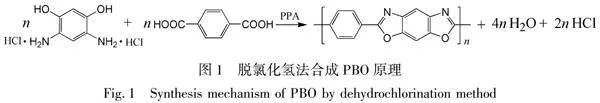
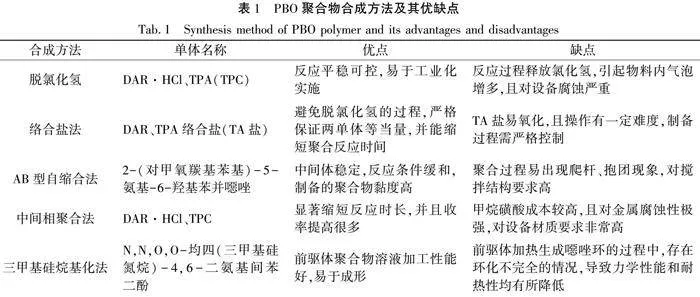
脫氯化氫法是目前合成PBO聚合物的經典方法。Wolfe等[12]報道了PBO聚合物的合成工藝,其方法是將4,6-二氨基間苯二酚鹽酸鹽
(DAR·HCl)和對苯二甲酸(TPA)在多聚磷酸(PPA)中縮聚制得PBO聚合物,PBO聚合物縮聚反應式如圖1所示。其中PPA既是溶劑又是催化劑,五氧化二磷(P2O5)為脫水劑。首先單體DAR·HCl脫出氯化氫增加活性,然后與TPA發生縮聚反應,期間補加P2O5,并控制最終PPA中P2O5的濃度,經程序升溫反應,最終得到PBO聚合物。脫氯化氫法主要受單體純度、反應溫度、攪拌速度、固含量、最終P2O5含量的影響。該方法操作簡便、易于實施[13],至今仍是PBO聚合物的主流合成方法。Wolfe等[14]用對苯二甲酰氯(TPC)代替TPA,制備了PBO聚合物。TPC在PPA中的溶解性遠大于TPA,聚合反應時間短、速度快,缺點是反應放出的氯化氫氣體增加,發泡現象嚴重,對設備腐蝕增加,設備利用率下降。
絡合鹽法[15]是將DAR和TPA反應成絡合鹽(TA鹽),然后進行聚合反應。該方法可避免脫氯化氫的過程,嚴格保證兩單體等當量,并能縮短聚合反應時間。TA鹽的質量直接決定了合成PBO聚合物時的反應速度和氧化程度。TA鹽的粒徑和白度是最重要的兩個控制指標,TA鹽的直徑應控制在15~50 μm,白度不應低于85,控制好上述兩個指標才能得到優質PBO聚合物。但因TA鹽易氧化、難操作,目前國內生產企業未采用該工藝。
AB型自縮合法[1]以4,6-二硝基間苯二酚為原料,還原得到4-氨基-6-硝基間苯二酚鹽酸鹽,然后與對甲氧羰基苯甲酰氯進行反應,再催化加氫得到AB型新單體2-(對甲氧羰基苯基)-5-氨基-6-羥基苯并噁唑,該單體兩端帶有可發生縮合反應的不同官能團,可以自縮聚生成PBO聚合物。該方法具有中間體穩定、反應條件緩和等技術優勢,但縮聚過程隨著聚合物黏度的上升有出現爬桿、抱團現象,應采用具有更高分散能力的攪拌裝置。
中間相聚合法[16]采用甲烷磺酸為溶劑和縮聚劑,加入質量分數為40%~45%的五氧化二磷,將單體4,6-二氨基間苯二酚鹽酸鹽和對苯二甲酰氯進行縮聚反應,得到PBO聚合物。該方法可以將聚合物時間從100 h縮短至10 h,顯著提高收率。但甲烷磺酸成本較高,且對金屬腐蝕性極強,對設備材質要求非常高。
三甲基硅烷基化法[17]先制備N,N,O,O-均四(三甲基硅氮烷)取代的中間體,再使中間體與對苯二甲酰氯在低溫下進行反應得到聚合物,然后進行高溫環化制得PBO纖維。該方法的優點是前驅體聚合物溶液黏度較低、可紡性好便于加工成型PBO纖維;缺點是前驅體纖維熱環化制備PBO纖維的過程中,可能因存在環化不完全的情況,導致PBO纖維中分子鏈的弱鍵增多,力學性能和耐熱性均有所降低。
具體PBO聚合物合成方法及優缺點如表1所示。從PBO聚合工藝發展來看,脫氯化氫法仍然是目前工業領域的主流工藝技術,已實現產業化應用。絡合鹽法和AB型自縮合法具有一定的技術先進性,單體配比容易控制且可以避免脫氯化氫過程,聚合反應控制難度低。但其聚合前驅體TA鹽和AB鹽制備工藝復雜,過程控制有一定難度。如能克服上述工藝問題,絡合鹽法或AB型自縮合法有望取代目前的脫氯化氫法,實現工業化應用,進一步推動PBO聚合物制備技術發展。
2 PBO纖維紡絲成形
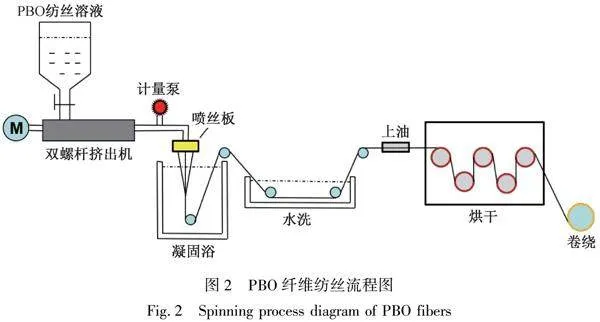
PBO聚合物的紡絲成形采用液晶相濃溶液的干噴濕紡工藝。高黏度PBO紡絲原液在一定壓力和溫度下從噴絲孔擠出,剛性棒狀PBO大分子鏈在噴絲孔內剪切應力和出口拉伸應力的作用下,沿應力方向取向呈現向列型液晶態。液晶態的PBO紡絲原液從噴絲孔擠出進入空氣層,在空氣層發生高倍牽伸。液晶態的PBO紡絲原液在應力下更容易運動和取向,因此PBO紡絲原液在空氣層可以進行高倍取向和拉伸。拉伸取向后的PBO絲條進入凝固浴發生雙擴散,絲條中的PPA溶劑擴散進入凝固浴,凝固浴中的水擴散進入PBO絲條內部,PBO大分子在凝固劑的作用下迅速凝固成形,取向結構得以保存下來。成形后的PBO纖維再經水洗、上油、烘干和卷繞得到PBO纖維,具體紡絲流程如圖2所示。
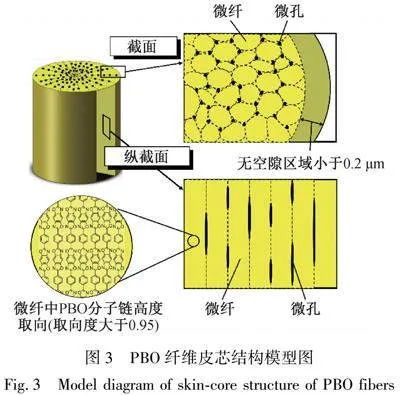
經紡絲成形后的PBO纖維是典型的皮芯結構。Kitagawa等[18]研究表明,PBO纖維表層為0.2 μm的致密皮層,皮層高度取向且無孔隙;PBO纖維芯層為直徑10~50 nm的微原纖,微原纖由沿纖維軸高度延伸取向的PBO大分子構成,微原纖間存在20~30 的毛細管狀微孔,這些微孔通過微原纖之間的裂縫或空隙相互連通,結構模型如圖3所示。PBO纖維的皮芯結構是在紡絲成形過程中形成的[19]。PBO聚合物溶液從噴絲頭擠出,會出現明顯的出口脹大,紡絲細流外表層承受拉伸應力,取向程度高于芯層;紡絲細流進入空氣層后,細流表面隨著溫度的降低形成凍膠層,失去流動能力,此時芯層處于黏流狀態,因此紡絲張力主要由表層的凍膠層承擔,表層的取向度遠高于芯層,因此表層結構致密[20]。進入凝固浴后,纖維成形時表層的凝固速度快于芯層,表層的溶劑在凝固浴中快速脫除,形成致密的皮層結構,成形的皮層阻礙了芯層溶劑的擴散速度,因此芯層溶劑擴散速度慢,凝固成形慢。表層成形后芯層繼續凝固收縮,因此易于形成微孔。
3 PBO纖維共聚改性
為了提升PBO纖維的性能,國內外學者從分子結構設計角度出發,開發了新型苯并噁唑基聚合物纖維。根據共聚方法賦予性能的不同,可分為改善拉伸力學性能、提升壓縮強度、改善界面粘結性能和提高耐紫外光老化性能4個方面。
3.1 提升拉伸力學性能
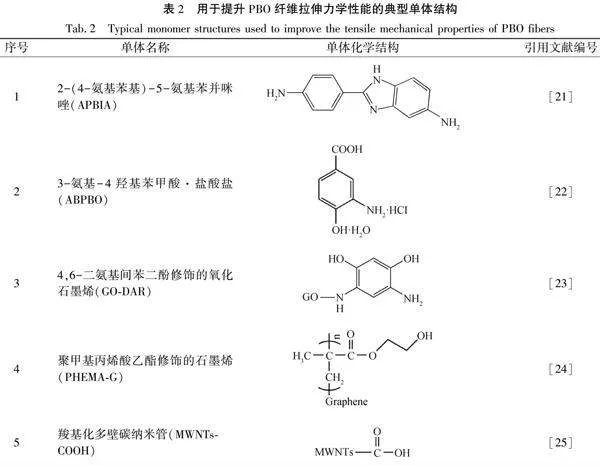
采用共聚的方法引入第三單體,改善聚合物溶液的可紡性,可以提高分子鏈取向度,進而提升纖維力學強度。王陽等[21]將單體2-(4-氨基苯基)-5-氨基苯并咪唑(APBIA)引入PBO聚合體系,采用原位聚合法制備了PBO/APBIA共聚物,并采用干噴濕紡工藝得到了共聚改性纖維。加入APBIA后的PBO聚合物溶液具備更好的可紡性,纖維表面更光滑,并且APBIA的加入改變了纖維成形過程中的結晶速度,提高了分子鏈取向。測試結果表明:PBO/APBIA纖維與純PBO纖維相比,拉伸強度提高了30.3%,拉伸模量提高了19.4%。田雪等[22]在PBO大分子鏈中引入半剛性3-氨基-4羥基苯甲酸鹽酸鹽(ABPBO),采用共聚法制備了一系列不同組成的PBO-b-ABPBO纖維,新單體的引入顯著增加了分子鏈柔性,降低了聚合物溶液的表觀黏度。PBO-b-ABPBO多嵌段共聚物的鏈間橫向距離比PBO聚合物大,因此分子鏈運動能力增加,更易排列取向,聚合物溶液的流動性和可紡性都有改善,制備的PBO-b-ABPBO纖維的耐熱性和力學性能沒有明顯降低,達到了低成本制備高性能纖維的目的。碳納米管或石墨烯是有機纖維的理想增強劑[23],經功能化后可與PBO分子鏈進行共聚,得到納米復合纖維,可進一步提高PBO纖維力學性能[24]。石墨烯或碳納米管常見的功能化方式有氨基化和羧基化兩種方式。Wang等[25]將氧化石墨烯(GO)與4,6-二氨基間苯二酚(DAR)進行反應,制備了化學改性的GO-DAR,然后在GO-DAR表面接枝PBO大分子,制備了含GO的PBO復合纖維。GO-DAR在PBO纖維基體中分散良好,當復合纖維中GO-DAR添加量為質量分數為1%時,拉伸強度和模量分別比純PBO纖維增加35.9%和19.6%。Hu等[26]制備了聚甲基丙烯酸乙酯修飾的石墨烯(PHEMA-G),然后原位共聚、干噴濕紡,制備了PHEMA-G/PBO復合纖維,PHEMA-G中的羥基可以與PBO分子形成共價鍵,當PHEMA-G加入量為質量分數為1%時,復合纖維強度和模量分別比純PBO纖維高51.2%和33.7%。Li等[27]制備了羧基化多壁碳納米管/PBO共聚物,多壁碳納米管表面羧基參與反應,接枝到PBO大分子上。經測試,當多壁碳納米管添加量為質量分數為5%時,復合纖維的拉伸強度比純PBO纖維提高了30%,耐熱性也更加優良。Zhou等[28]和Li等[29]分別制備了PBO納米復合纖維,納米碳材料表面的官能團參與了化學反應,制備的復合纖維的力學性能和耐熱性比原纖維均有一定程度的提升。下表2為用于提升PBO纖維拉伸力學性能的典型單體結構。
3.2 提升壓縮強度
PBO纖維具有較高的拉伸強度和模量,但其橫向的壓縮強度較弱。PBO纖維是典型的皮芯結構,纖維外層是致密的皮層,厚度約為0.2 μm;內層是直徑約為10~50 nm的微原纖。Charles等[30]認為微原纖的不穩定性是剛性鏈有機纖維壓縮強度低的主要原因之一,纖維在壓縮下的扭結來自相互作用最弱的區域。Dang等[31]同樣認為微原纖結構導致橫向作用弱,在壓應力作用下易被彎曲。
So等[32]制備了分子鏈中側基含聚苯硫醚(PPS)的PBO纖維,熱處理工藝為600 ℃/30s時,聚苯硫醚基團可形成交聯,交聯后的纖維在甲烷磺酸(MSA)中不溶解,抗壓強度比未改性的PBO纖維提高約20%。So等[33]將間苯二甲酰雙-4-苯并環丁烯引入PBO大分子中,加熱固化后制備了熱固性PBO復合纖維,固化后的纖維在MSA中膨脹但不溶解,復合纖維的抗壓強度比未改性PBO纖維高20%~30%。Harris等[34]提出:在PBO大分子中引入
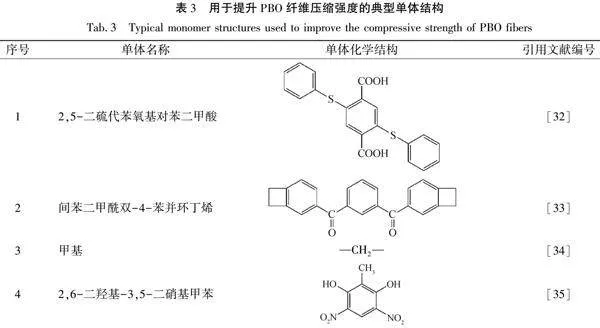
甲基側基,甲基受熱后形成分子鏈間—CH2—共價鍵交聯,可提升PBO纖維軸向抗壓性能。毛婷婷等[35]合成了單體2,6-二羥基-3,5-二硝基甲苯,該單體可合成含甲基的PBO大分子,提高壓縮強度。Dean等[36]以提升分子鏈間作用力為出發點,制備了一系列二維和三維結構的改性PBO纖維,雖然壓縮性能并沒有得到改善,但為交聯型PBO大分子合成提供了解決思路。在PBO纖維結構中引入物理鍵或化學鍵交聯,提高微原纖間作用力,是提升其壓縮性能的有效途徑。用于提升PBO纖維壓縮強度的典型單體結構見表3。
3.3 改善界面粘結性能
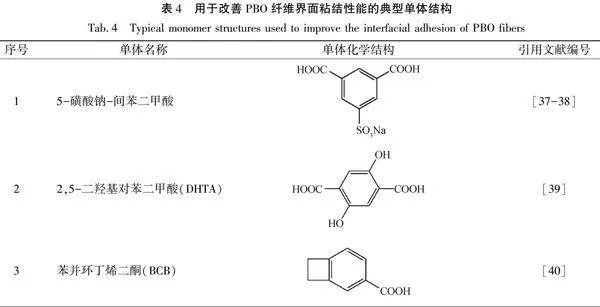
PBO纖維表面光滑,不含極性基團,作為復合材料的增強纖維與樹脂基體的界面粘結性能差,界面剪切強度較低,限制了其在復合材料領域的應用[12]。用于改善PBO纖維界面粘結性能的典型單體結構見表4。
Jiang等[37]和金俊弘等[38]采用5-磺酸鈉-間苯二甲酸代替部分對苯二甲酸,制備了含離子基團的共聚改性PBO纖維(SPBO)。測試結果表明:引入離子基團后,PBO纖維的表面含氮、氧量均增加,與水和乙醇的接觸角更小,浸潤過程更快,表面浸潤性能得到提高,改性SPBO纖維與樹脂的界面剪切強度(IFSS)從未改性的8.2MPa增加至10.1MPa,提高了23%。Zhang等[39]將2,5-二羥基對苯二甲酸(DHTA)引入PBO大分子鏈,并采用干噴濕紡法制備了二羥基聚對苯撐苯并雙噁唑(DHPBO)纖。加入雙羥基極性基團后的DHPBO纖維與水的接觸角由71.4°降至到50.7°,與乙醇的接觸角從37.2°降至27.4°,并且浸潤時間大幅縮短。當DHTA摩爾含量為10%時,DHPBO纖維與環氧樹脂之間的IFSS為18.87 MPa,比純PBO纖維高92.55%。Yalvac等[40]將苯并環丁烯二酮(BCB)添加到PBO紡絲原液中,制備含BCB結構的PBO纖維。BCB的引入提升了PBO纖維與樹脂基體的界面剪切強度,當BCB摩爾含量為18%時,其界面剪切強度比純PBO纖維提高74%。
3.4 提升抗紫外老化性能
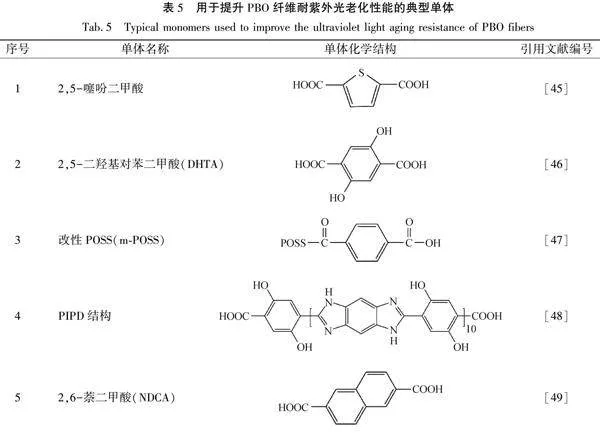
PBO纖維的抗紫外老化性能較弱,在紫外光照射下易吸收能量造成噁唑環開環[41]和分子鏈斷裂[42]。PBO纖維在340 nm紫外線照射150 h后,其強度由最初的33 g/den降為5 g/den,強度損失高達85%[43]。PBO纖維的光降解過程可分為兩個階段,第一階段為物理階段,主要表現為纖維皮層結構缺陷的產生和發展,該階段纖維強度下降較為緩慢;第二階段為化學階段,主要表現為PBO大分子鏈的化學斷裂,分子量迅速降低,纖維皮層和結晶結構受到嚴重破壞,纖維強度降低速度較快[44]。用于提升PBO纖維耐紫外光老化性能的典型單體見表5。
張利等[45]以改進PBO纖維抗紫外光老化性能為出發點,在PBO結構中引入第三單體2,5-噻吩二甲酸,通過干噴濕紡制備了纖維,并將一系列不同結構PBO纖維在313 nm紫外光下輻照240 h。該研究結果表明:2,5-噻吩二甲酸單體含量越高,特性黏度保持率越高,說明PBO結構中引入噻吩結構單元,有助于提升PBO纖維的耐光老化性能。Zhang等[46]通過添加2,5-二羥基對苯二甲酸(DHTA)進行共聚,得到了DHPBO纖維,共聚改性后的PBO纖維經過412 h的紫外照射后,其強度保持率為70.3%,而純PBO纖維同等條件下的強度保持率僅為35.8%,其中DHPBO中氫鍵的形成是抗紫外能力提升的主要原因。Jang等[47]將4-甲酰基苯甲酸與多面低聚倍半硅氧烷(POSS)進行反應,合成了改性POSS(m-POSS),并將m-POSS加入到PBO纖維中,通過干噴濕紡工藝制備了納米復合PBO纖維。該測試結果表明:m-POSS添加量為質"量分數為2%的PBO復合纖維的強度比純PBO纖維增加了16%,16h紫外光照射后的強度保持率從48%增加到62%。
Li等[48]將PIPD纖維(聚2,5-二羥基-1,4-苯撐吡啶并二咪唑)的結構引入到PBO中,制備了PBO/PIPD嵌段共聚物,然后采用干噴濕紡工藝制備了PBO/PIPD纖維。PBO/PIPD共聚物纖維(摩爾比為7∶1)具有更高的表面潤濕性能和抗紫外光老化性能,在480 h紫外光老化后的強度比純PBO纖維高30.8%。Wang等[49]在PBO結構中引入2,6-萘二甲酸(NDCA),制備了一系列共聚改性PBO纖維,研究發現纖維直徑隨紫外光照射時間的延長而成比例減小,說明紫外照射在纖維表面形成刻蝕,當NDCA添加比例為摩爾分數9%時,PBO纖維在紫外光照射后的強度保持率從49%提升至60%,并且纖維的刻蝕程度隨NDCA含量增加而降低,說明萘結構的引入顯著提升了PBO纖維的耐紫外光老化性能。Li等[50]采用原位聚合法將PBO與α-氨基酞菁銅(α-TDMACuPc)共聚,并在液晶狀態下通過干噴濕法紡絲獲得纖維。將α-TDMACuPc引入到PBO大分子中,提高了PBO纖維的拉伸強度,并且該共聚PBO纖維可以將吸收的紫外光能量以熱能的形式釋放出去,因此表現出優異的抗紫外光老化性能。研究數據表明在紫外線照射100 h后,改性后PBO纖維的拉伸強度保持率從66.44%提高到88.80%。
4 結論
本文針對PBO纖維的性能缺陷,對PBO纖維的共聚改性技術進行了系統綜述,總結了目前國內外重要的研究進展,主要得出以下結論:
a)在PBO纖維結構中引進新型芳雜環單體結構,可以改善PBO纖維的強度和模量,但新單體的加入也可能破壞PBO大分子的結構對稱性,降低纖維凝聚態結構規整性,導致力學性能下降。
b)在PBO纖維結構中引入物理鍵或化學鍵交聯,提高微原纖間作用力,可以提升其壓縮強度。
c)在PBO纖維結構中加入含離子基團或強極性基團的單體結構,可以提高纖維與樹脂基體的界面粘結性能,提高其在復合材料領域的應用價值。
d)在PBO纖維結構中增加耐紫外性能良好的稠環、雜環結構或引入氫鍵,可以顯著提升PBO纖維的耐紫外光老化性能,拓展其應用場景。
參考文獻:
[1]ZHANG J T, JIN N R, GAO J R. Superior comprehensive performance of a rigid-rod poly(hydroxy-p-phenyleneben-zobisoxazole) fiber[J]. Polymer, 2018,149: 325-333.
[2]劉姝瑞, 譚艷君, 霍倩, 等. PBO纖維力學強度表征其耐酸性的研究[J]. 現代紡織技術, 2017, 25(4):15-19.
LIU Shurui, TAN Yanjun, HUO Qian, et al. Research on acid resistance property of PBO fiber characterized by its mechanical strength[J]. Advanced Textile Technology, 2017, 25(4): 15-19.
[3]白金旺, 張殿波, 鐘蔚華, 等. 耐紫外老化PBO纖維改性技術研究進展[J]. 化工新型材料, 2024, 52(3): 22-27.
BAI Jinwang, ZHANG Dianbo, ZHONG Weihua, et al. Advances in UV-resistant PBO fiber modification tech-nologies[J]. New Chemical Materials, 2024, 52(3): 22-27.
[4]LI N, HU Z, HUANG Y. Preparation and characterization of nanocomposites of poly(p-phenylene benzobisoxazole) with aminofunctionalized graphene[J]. Polymer Composites, 2018, 39(8): 2969-2976.
[5]SO Y H, Rigid-rod polymers with enhanced lateral interactions[J]. Progress in Polymer Science, 2000, 25(1): 137-157.
[6]CHEN L, LI Z, WU G, et al. In situ growth of TiO2 nanoparticles onto PBO fibers via a mussel-inspired strategy for enhancing interfacial properties and ultraviolet resistance[J]. Polymer Composites, 2021, 42(10): 5065-5074.
[7]CHEN, L, HU Z, LIU L, et al. A facile method to prepare multifunctional PBO fibers: Simultaneously enhanced interfacial properties and UV resistance[J]. RSC Advances, 2013, 3(46): 24664-24670.
[8]SHAO Q, LU F, YU L, et al. Facile immobilization of graphene nanosheets onto PBO fibers via MOF-mediated coagulation strategy: Multifunctional interface with self-healing and ultraviolet-resistance performance[J]. Journal of Colloid and Interface Science, 2021,587: 661-671.
[9]李芝華, 李慧, 劉夏清, 等. PBO纖維性能及表面改性的研究進展[J]. 包裝工程, 2016, 37(19): 146-151.
LI Zhihua, LI Hui, LIU Xiaqing, et al. Research progress of PBO fiber properties and surface modification[J]. Packaging Engineering, 2016, 37(19): 146-151.
[10]LIU Z, SONG B, WANG T, et al. Significant improved interfacial properties of PBO fibers composites by in situ constructing rigid dendritic polymers on fiber surface[J]. Applied Surface Science, 2020,512: 145719.
[11]GOUTIANOS S, PEIJS T. On the low reinforcing efficiency of carbon nanotubes in high-performance polymer fibres[J]. Nanocomposites, 2021, 7(1): 53-69.
[12]WOLFE J F, ARNOLD F E. Rigid-rod polymers. 1. Synthesis and thermal properties of Para-aromatic polymers with 2,6-benzobisoxazole units in the main chain[J]. Macromolecules, 1981, 14(4): 909-915.
[13]袁會齊, 張麗. 4,6-二氨基間苯二酚的研究進展[J]. 生物化工, 2019, 5(1): 136-138.
YUAN Qihui, ZHANG Li. Research progress of 4,6-diamino-resorcinol[J]. Biological Chemical Engineering, 2019, 5(1): 136-138.
[14]WOLFE J F, SYBERT P D, SYBERT J R. Liquid crystalline polymer compositions, process, and products: US4533693[P]. 1985-08-06.
[15]GAO Z C, WANG J Q, FENG L F, et al. Flow-accelerated polycondensation reaction to prepare rigid rodlike poly(p-phenylene-cis-benzobisoxazole)[J]. Chemical Engineering and Processing-Process Intensification, 2022(176): 108972.
[16]郭玲, 趙亮, 胡娟, 等. 國產PBO纖維研究現狀及發展趨勢[J]. 高科技纖維與應用, 2014, 39(2): 11-15.
GUO Ling, ZHAO Liang, HU Juan, et al. Research status development trend of domestic PBO fiber[J]. Hi-Tech Fiber and Application, 2014, 39(2): 11-15.
[17]IMAI Y, ITOYA K, KAKIMOTO M A. Synthesis of aromatic polybenzoxazoles by silylation method and their thermal and mechanical properties[J]. Macromolecular Chemistry and Physics, 2000, 201(17): 2251-2256.
[18]KITAGAWA T, MURASE H, YABUKI K. Morphological study on poly-p-phenylenebenzobisoxazole (PBO) fiber[J]. Journal of Polymer Science Part B Polymer Physics, 1998, 36(1): 39-48.
[19]KITAGAWA T. Novel fine structures in poly-p-phenylene-benzobisoxazole fibers induced by water vapor, hot water, and non-aqueous coagulation I molecular orientation along the fiber axis and fine structures[J].Journal of Macro-molecular Science Part B, 2015, 54(11): 1323-1340.
[20]TIKHONOV I V, TOKAREV A V, SHORIN S V, et al. Russian aramid fibres: Past-present-future[J]. Fibre Chemistry, 2013, 45(1): 1-8.
[21]王陽, 趙蕾, 姜波, 等. 一種基于第三單體的高性能有機纖維的制備與表征[J]. 化學與黏合, 2017, 39(1): 7-10.
WANG Yang, ZHAO Lei, JIANG Bo, et al. Preparation and characterization of a high-performance organic fiber based on the third comonomer[J]. Chemistry and Adhesion, 2017, 39(1): 7-10.
[22]田雪, 周承俊, 陳曉軍, 等. PBO-b-ABPBO多嵌段共聚物的制備及其性能[J]. 功能高分子學報, 2008, 21(2): 147-151.
TIAN Xue, ZHOU Chengjun, CHEN Xiaojun, et al. Preparation and properties of PBO-b-ABPBO block copolymer[J]. Journal of Functional Polymers, 2008, 21(2): 147-151.
[23]HAN G C, SATISH K. Making strong fibers[J]. Science, 2008, 319(5865): 908-909.
[24]WANG M, ZHANG S, DONG J, et al. A facile route to synthesize nanographene reinforced PBO composites fiber via in situ polymerization[J]. Polymers, 2016, 8(7): 251-261.
[25]WANG M, WANG C, SONG Y, et al. One-pot in situ polymerization of graphene oxide nanosheets and poly(p-phenylenebenzobisoxazole) with enhanced mechanical and thermal properties[J]. Composites Science amp; Technology, 2017, 141: 16-23.
[26]HU Z, SHAO Q, MOLONEY M G, et al. Nondestructive functionalization of graphene by surface-initiated atom transfer radical polymerization: An ideal nanofiller for poly(p-phenylene benzobisoxazole) fibers[J]. Macromolecules, 2017, 50(4): 1422-1429.
[27]LI X, HUANG L, LIU H, et al. Preparation of multiwall carbon nanotubes/poly(p-phenylene benzobisoxazole) nanocomposites and analysis of their physical properties[J]. Journal of Applied Polymer Science, 2006, 102(3): 2500-2508.
[28]ZHOU C, WANG S, ZHANG Y, et al. In situ preparation and continuous fiber spinning of poly(p-phenylene benzobisoxazole) composites with oligo-hydrox-yamide-functionalized multi-walled carbon nanotubes[J]. Polymer, 2008, 49(10): 2520-2530.
[29]LI J, CHEN X, LI X, et al. Synthesis, structure and properties of carbon nanotube/poly(p-phenylene benzo-bisoxazole) composite fibres[J]. Polymer International, 2010, 55(4): 456-465.
[30]CHARLES Y C, SANTHOSH U. The role of the fibrillar structures in the compressive behavior of rigid-rod poly-meric fibers[J]. Polymer Engineering amp; Science, 1993, 33(14): 907.
[31]DANG T D, WANG C S, CLICK W E, et al. Polybenzobisthiazoles with crosslinking sites for improved fibre axial compressive strength[J]. Polymer, 1997, 38(3): 621-629.
[32]SO Y H, BELL B, HEESCHEN J P, et al. Poly(p-Phenylenebenzobisoxazole) fiber with polyphenylene sulfide pendent groups[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1995, 33: 159-164.
[33]SO Y H, SEN A, KIM P, ET al. Molecular composite fibers from rigid rod polymers and thermoset resin matrixes[J]. Journal of Polymer Science A Polymer Chemistry, 1995, 33(17): 2893-2899.
[34]HARRIS W J, LYSENKO Z. Polybenzoxazoles having pendant methyl groups: US5151490[P]. 1992-09-29.
[35]毛婷婷, 陳漢庚, 徐繼偉, 等. 2,6-二羥基-3,5-二硝基甲苯的合成新工藝[J]. 精細化工, 2015, 32(12): 1431-1436.
MAO Tingting, CHEN Hangeng, XU Jiwei, et al. Novel synthesis of 2,6-dihydroxy-3,5-dinitrotoluene[J]. Fine Chemicals, 2015, 32(12): 1431-1436.
[36]DEAN D R, HUSBAND D M, DOTRONG M, et al. Multidimensional benzobisoxazole rigid-rod polymers. II. Processing, characterization, and morphology[J]. Journal of Polymer Science Part A Polymer Chemistry, 1997, 35(16): 3457-3466.
[37]JIANG J M, ZHU H J, LI G, et al. Poly(p-phenylene benzoxazole) fiber chemically modified by the incorpora-tion of sulfonate groups[J]. Journal of Applied Polymer Science, 2008, 109(5): 3133-3139.
[38]金俊弘, 羅開清, 江建明, 等. 離子基團對PBO纖維的表面性能及其界面粘結性能的影響[J]. 復合材料學報, 2006, 23(6):69-74.
JIN Junhong, LUO Kaiqing, JIANG Jianming, et al. Effect of ionic groups on the surface and the interfacial adhesion properties of poly(p-phenylene benzoxazole) (PBO) fiber[J]. Acta Materiae Compositae Sinica, 2006, 23(6):69-74.
[39]ZHANG T, HU D, JIN J, et al. Improvement of surface wettability and interfacial adhesion ability of poly(p-phenylene benzobisoxazole) (PBO) fiber by incorporation of 2,5-dihydroxyterephthalic acid (DHTA)[J]. European Polymer Journal, 2009, 45(1): 302-307.
[40]YALVAC S, JAKUBOWSKI J J, SO Y H, et al., Improved interfacial adhesion via chemical coupling of cis-polybenzobisoxazole fibre-polymer systems[J]. Polymer, 1996, 37(20): 4657-4659.
[41]WALSH P, HU X, CUNNIFF P, et al. Environmental effects on poly-p-phenylenebenzobisoxazole fibers.I. Mecha-nisms of degradation[J] Journal of Applied Poly-mer Science, 2010, 102(4): 3517-3525.
[42]SO Y H, MARTIN S J, BELL B, et al. Importance of π-stacking in photoreactivity of aryl benzobisoxazole and aryl benzobisthiazole compounds[J]. Macromolecules, 2003, 36(13): 4699-4708.
[43]SAID M A, DINGWALL B, GUPTA A, et al. Investigation of ultra violet (UV) resistance for high strength fibers[J]. Advances in Space Research, 2006, 37(11): 2052-2058.
[44]宋波, 傅倩, 劉小云, 等. PBO纖維的紫外光老化及防老化研究[J]. 固體火箭技術, 2011, 34(3): 378-383.
SONG Bo, FU Qian, LIU Xiaoyun, et al. Study on the photolysis and stabilization of PBO fiber[J]. Journal of Solid Rocket Technology, 2011, 34(3): 378-383.
[45]張利, 蔡小川, 許漢, 等. 基于PBO共聚物改性纖維的制備及其耐紫外光老化性能的研究[J]. 山東化工, 2015, 44(19): 10-14.
ZHANG Li, CAI Xiaochuan, XU Han, et al. Preparation of modified fiber based on PBO copolymer and study of its anti-ultraviolet ageing performance[J]. Shandong Che-mical Industry, 2015, 44(19): 10-14.
[46]ZHANG T, JIN J, YANG S, et al. UV accelerated aging and aging resistance of dihydroxy poly(p-phenylene ben-zobisoxazole) fibers[J]. Polymers for Advanced Tech-nologies, 2011, 22(5): 743-747.
[47]JANG Y W, MIN B G, YOON K H. Enhancement in compressive strength and UV ageing-resistance of poly(p-phenylene benzobisoxazole) nanocomposite fiber contai-ning modified polyhedral oligomeric silsesquioxane[J]. Fibers and Polymers, 2017, 18(3): 575-581.
[48]LI Z F, LU F, LU S, et al. Fabrication of uvioresistant poly(p-phenylene benzobisoxazole) fibers based on hydrogen bond[J]. Journal of Applied Polymer Science, 2020,137(9): 48432-48443.
[49]WANG Q W, YOON K H, MIN B G. Chemical and physical modification of poly(p-phenylene benzobisoxazole) polymers for improving properties of the PBO fibers.I. Ultraviolet-ageing resistance of PBO fibers with naphtha-lene moiety in polymer chain[J]. Fibers and Polymers, 2015, 16(1): 1-7.
[50]LI J, WANG W, ZHAO L, et al. In situ synthesis of PBO-α-(amino phthalocyanine copper) composite fiber with excellent UV-resistance and tensile strength[J]. Journal of Applied Polymer Science, 2018, 135(48): 46870-46880.
Research progress on copolymerization modification of poly(p-phenylene benzobisoxazole) fibers
ZHANG" Dianbo1," BAI" Jinwang1," ZHONG" Weihua1," LIANG" Chen1," ZHANG" Junxian2
(1.Institute 53, China North Industries Group, Jinan 250031, China;
2.Analysis and Testing Center, Shenzhen Technology University, Shenzhen 518118, China)
Abstract:
The PBO fiber is currently one of the high-performance organic fibers with the best comprehensive performance, featuring high strength, modulus, flame retardancy, and heat resistance. It is dubbed the \"super fiber of the twenty-first century.\" The strength of PBO fibers reaches 5.8 GPa, and their modulus can reach 280 GPa. Their limiting oxygen index (LOI) is 68, and the thermal decomposition temperature is 650 ℃. Due to these excellent properties, PBO fibers can be applied in aerospace, weaponary and naval vessels, building reinforcement, high-temperature filtration, special protection and other fields.However, PBO fibers have performance shortcomings such as poor UV-aging resistance and weak interfacial adhesion, which limit its further application and development. They can not meet the stringent criteria of aerospace, weaponary, and other fields. Therefore, it is urgent to modify PBO fibers to improve their performance. This paper summarized the research progress of copolymerization modification technology of PBO fibers at home and abroad in recent years. It can be divided into four aspects based on the modification objectives: improving tensile mechanical properties, enhancing compressive strength, improving interfacial adhesion performance, and improving UV-aging resistance. The mechanical characteristics of PBO fibers can be further enhanced by copolymerizing PBO molecular chains with functionalized carbon nanotubes or graphene; although PBO fibers have a high tensile strength and modulus, they have a weak transverse compressive strength. By introducing chemical cross-links into the PBO macromolecular chain, their compressive strength can be significantly improved. However, PBO fibers have a smooth surface and do not contain polar groups, resulting in a weaker ability to bond with resin matrices. Adding carboxyl or hydroxyl groups to PBO molecular chains can effectively enhance the composite ability of PBO fibers with resins; the anti-UV aging performance of PBO fibers is relatively weak. Introducing intermolecular hydrogen bonds or fused ring structures into PBO molecular chains can help improve the anti-UV aging performance of PBO fibers.In summary, PBO fibers possess excellent physicochemical properties but also have certain performance defects. The in-situ copolymerization modification technique, designed from the perspective of chemical structural, can fundamentally address the performance defects of PBO fibers and has high practical value. Nonetheless, the preparation process of PBO fibers involves liquid crystals spinning. If the addition of a third monomer disrupts the liquid crystal behavior of the spinning solution, the spinnability may be diminished. Co-polymerization modification may also break the structural symmetry and sequence consistency of PBO macromolecules, which could change the regularity of the PBO fibers' ultimate condensed state structure and lessen their mechanical qualities. Therefore, copolymerization modified PBO fibers should completely evaluate the impact of the third monomer on spinning performance and fiber condensed structure. However, it can not be denied that PBO fibers remain one of the organic fibers with the best comprehensive properties at the moment. Introducing new monomer structures into PBO fibers and developing new benzoxazole-based high-performance fibers would allow them to broaden their application value and play a larger role in military and civilian industries.
Keywords:
PBO fiber; copolymerization modification; mechanical properties; interfacial bonding performance; UV-aging resistance
基金項目:國家重點研發計劃項目(2022YFB3707900)
第一作者:張殿波(1986—),男,山東煙臺人,副研究員,博士,主要從事高性能纖維及其改性技術方面的研究。

