胰腺癌細胞株原鈣黏附蛋白8基因的甲基化狀態
呂順莉 高軍 杜奕奇 黃浩杰 王小瑋 金晶 龔燕芳 張玲 李兆申
·論著·
胰腺癌細胞株原鈣黏附蛋白8基因的甲基化狀態
呂順莉 高軍 杜奕奇 黃浩杰 王小瑋 金晶 龔燕芳 張玲 李兆申
目的分析原鈣黏附蛋白8(protocadherin 8,PCDH8)基因在胰腺癌細胞株的甲基化狀態。方法抽提6株胰腺癌細胞株PANC1、ASPC1、BxPC3、CFPAC、PaTu8988、SW1990和2例正常胰腺組織的總RNA,以甲基化特異性PCR(MSP)法檢測PCDH8甲基化情況。應用DNA甲基化轉移酶(DNMT)抑制劑5-氮雜-2′-脫氧胞苷(5-Aza-dC)處理6株胰腺癌細胞株,采用實時定量PCR法檢測處理前后細胞的PCDH8 mRNA表達。結果2例正常胰腺組織PCDH8基因未發生甲基化, PANC1、BxPC3、CFPAC胰腺癌細胞株 PCDH8基因部分甲基化,而PaTu8988、ASPC1、SW1990細胞完全甲基化。PCDH8 mRNA在PANC1、SW1990、PaTu8988胰腺癌細胞株中有表達,表達的相對值(RQ)分別為1.576±0.648、0.013±0.008、0.002±0.001;BxPC3、CFPAC、ASPC1細胞株無PCDH8 mRNA表達。5-Aza-dC處理后,胰腺癌細胞株PANC1、ASPC1、BxPC3、CFPAC、PaTu8988、SW1990均有PCDH8 mRNA表達,表達量較處理前明顯升高,相對表達量分別為7.463±2.628、10.696±1.539、7.852±2.762、421.815±1.493、118.595±4.089、6.690±1.884。結論PCDH8基因啟動子高甲基化是導致該基因在胰腺癌細胞株表達下降的主要原因之一。
胰腺腫瘤; 甲基化; 聚合酶鏈反應; 原鈣黏附蛋白8
DNA甲基化是在DNA甲基化轉移酶等作用下,給CpG二核苷酸胞嘧啶的第5位碳原子加上一個甲基基團,使非甲基化狀態的CpG位點變為甲基化狀態的CpG位點,阻礙了轉錄因子復合體與DNA的結合及延伸,導致基因轉錄受抑及表達沉默。DNA的異常甲基化在腫瘤的發生發展過程中起著重要作用,抑癌基因啟動子CpG島高甲基化可導致基因表達抑制,功能喪失[1-3]。原鈣黏附蛋白8(protocadherin 8,PCDH8)屬于原鈣黏附蛋白家族,其在胰腺癌發生、發展中的作用尚未見報道。為此,我們檢測胰腺癌細胞株PCDH8的表達及其甲基化狀態。
材料和方法
一、實驗材料
ASPC1、PANC1胰腺癌細胞株購于中國科學院細胞庫;BxPC3、CFPAC、SW1990胰腺癌細胞株購于美國ATCC公司;PaTu8988細胞株為德國Marburg Phillips大學Elsasser博士惠贈。2例正常胰腺組織取自上海長海醫院胰腺外科因胰腺囊腫行手術治療的患者,均獲得患者的知情同意。
二、甲基化特異性PCR
以酚氯仿法抽提2例正常胰腺組織和6株胰腺癌細胞株的DNA,分光光度計(NanoDrop ND-1000,美國)測定DNA純度和濃度。參照亞硫酸鹽處理試劑盒說明書(QIAgen)對抽提的DNA進行亞硫酸鹽處理。通過methyl-primer express1.0軟件設計引物序列。甲基化引物(M)序列已申請專利(專利號為201010125668.0),產物大小為125 bp;非甲基化引物(U)上游5′-GAGGTGGTGTAGTTTTTGTAAGAGAT-3′,下游5′-ACACTCTTTACAAACCCTATACAAAA-3′,產物大小為125 bp,均由上海英駿生物有限公司合成。采用甲基化特異性PCR(MSP)法檢測甲基化狀態。PCR反應條件:95℃ 3 min;95℃ 30 s,61.3℃(M引物)或57.3℃(U引物) 30 s,72℃ 30 s,共35循環;72℃ 10 min。PCR產物經瓊脂糖凝膠電泳,復日FR-980生物電泳圖像分析系統攝影。
三、實時定量PCR
所有胰腺癌細胞株常規培養,待細胞生長至60%時更換含5-氮雜-2′-脫氧胞苷(5-Aza-dC)的培養液繼續培養48 h。采用Trizol試劑(Invitrogen)提取總RNA。分光光度計法測定RNA純度和濃度。取5 μg總RNA在20 μl反應體系逆轉錄合成cDNA。取1 μl cDNA行實時定量PCR反應。PCDH8上游引物5′-ATGAGTCCTGTGAGGCGTTG-3′,下游引物5′-GCTTGTGTCACCCGATACTTT-3′,產物大小為186 bp;內參GAPDH上游引物5′-GCACCGTCAAGGCTGAGAAC-3′,下游引物5′-ATGGTGGTGAAGACGCCAGT-3′,產物大小為142 bp。均由上海英駿生物有限公司合成。PCR反應條件:95℃ 10 s,95℃ 5 s,60℃ 34 s, 40循環。利用Real-Time PCR儀自帶軟件獲得Ct值,以2例正常胰腺組織作為對照。△△Ct=△Ct待測樣品-△Ct正常胰腺。△Ct待測樣品=Ct待測樣品-Ct內參,△Ct正常胰腺=Ct正常胰腺-Ct內參,表達量RQ=2-△△C。實驗重復3次,取均值。
結 果
一、胰腺癌細胞株PCDH8基因的甲基化狀態
2例正常胰腺組織PCDH8未發生甲基化, PANC1、BxPC3、CFPAC細胞 PCDH8基因部分甲基化,PaTu8988、ASPC1、SW1990細胞PCDH8基因完全甲基化(圖1)。
二、5-Aza-dC處理后胰腺癌細胞株PCDH8 mRNA的表達變化
5-Aza-dC 處理前PANC1、SW1990、PaTu8988細胞有PCDH8 mRNA表達,表達量分別為1.576±0.648、0.013±0.008、0.002±0.001;BxPC3、CFPAC、ASPC1細胞株無PCDH8 mRNA表達。5-Aza-dC處理后,胰腺癌細胞株PANC1、ASPC1、BxPC3、CFPAC、PaTu8988、SW1990均有PCDH8 mRNA表達,表達量分別為7.463±2.628、10.696±1.539、7.852±2.762、421.815±1.493、118.595±4.089、6.690±1.884(圖2),均較處理前明顯升高。
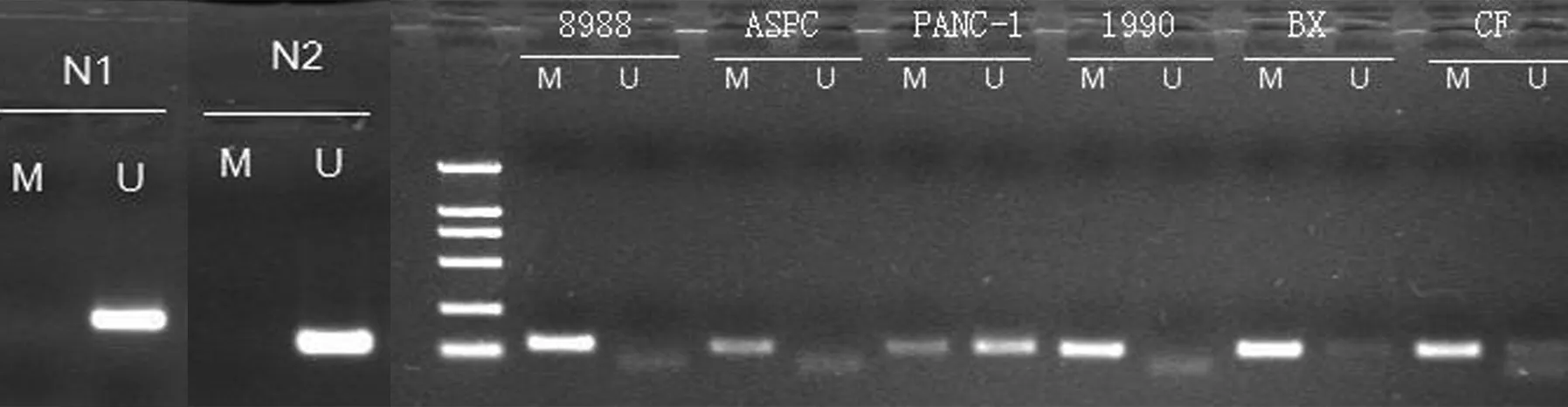
圖1MSP法檢測正常胰腺組織(N1,N2)和胰腺癌細胞株中PCDH8基因的甲基化狀態
討 論
原鈣黏附蛋白為膜結合蛋白,屬鈣黏蛋白大家族中具有特殊功能的新一類分支家族。該家族除具有黏附功能外,其胞內區參與細胞骨架形成和胞內信號轉導,調控細胞的生長和分化[4-5]。依據在基因組的分布和種系發生學等情況主要分為成簇和非成簇原鈣黏附蛋白,其中非成簇原鈣黏附蛋白又包含Delta型和其他類型家族,Delta型家族又分Delta1和Delta2兩亞型,兩者區別在于胞內區包含的保守基序。研究發現,多數原鈣黏附蛋白家族均顯示為抑癌基因的特性[6-14]:在腫瘤組織中高甲基化、雜合性缺失、突變和低表達,相鄰正常組織低甲基化和高表達,轉染野生型可導致細胞生長抑制、遷移抑制等抑制腫瘤惡性表型的功能[6, 10-12]。
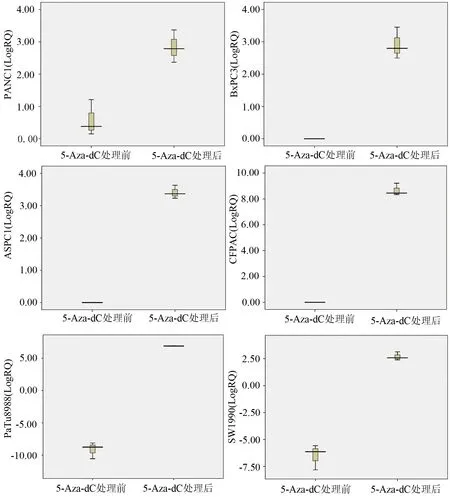
圖25-Aza-dC處理前后胰腺癌細胞株PCDH8 mRNA的表達量(LogRQ)
本實驗結果顯示,PCDH8基因在6株胰腺癌細胞株均存在不同程度的甲基化,其中PANC1、BxPC3、CFPAC細胞部分甲基化,而PaTu8988、ASPC1、SW1990細胞完全甲基化;2例正常胰腺組織PCDH8未發生甲基化。PANC1、SW1990、PaTu8988細胞有PCDH8 mRNA表達,而BxPC3、CFPAC、ASPC1細胞株無PCDH8 mRNA表達。胰腺癌細胞株PCDH8甲基化狀況和mRNA表達的結果不一致,提示PCDH8基因在胰腺癌中低表達的原因不只是高甲基化導致,還存在其他原因共同參與,可能包括雜合性缺失、突變等原因。
目前已有針對DNA甲基化轉移酶(DNMT)的抑制劑,5-Aza-dC是其中去甲基化作用最強的一種,屬于非特異性的去甲基化藥物[15],本實驗應用該抑制劑對6株胰腺癌細胞株進行干預,檢測處理前后mRNA表達水平,結果顯示處理后PCDH8的mRNA表達量增加,證實了PCDH8基因的高甲基化是導致低表達的一個重要因素,但不是唯一的因素。
[1] Egger G,Liang G,Aparicio A,et al.Epigenetics in human disease and prospects for epigenetic therapy.Nature,2004,429:457-463.
[2] Issa JP.CpG island methylator phenotype in cancer.Nat Rev Cancer,2004,4:988-993.
[3] Laird PW.The power and the promise of DNA methylation markers.Nat Rev Cancer,2003,3:253-266.
[4] Redies C,Vanhalst K, Roy F.delta-Protocadherins: unique structures and functions.Cell Mol Life Sci,2005,62:2840-2852.
[5] Yang X,Chen MW,Terry S,et al.A human-and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells.Cancer Res,2005,65:5263-5271.
[6] Yu J,Cheng YY,Tao Q,et al.Methylation of protocadherin 10,a novel tumor suppressor,is associated with poor prognosis in patients with gastric cancer.Gastroenterology,2009,136:640-651.
[7] Waha A,Guntner S,Huang TH,et al.Epigenetic silencing of the protocadherin family member PCDH-gamma-A11 in astrocytomas.Neoplasia,2005,7:193-199.
[8] Willecke M,Hamaratoglu F,Kango-Singh M,et al.The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size.Curr Biol,2006,16:2090-2100.
[9] Imoto I,Izumi H,Yokoi S,et al.Frequent silencing of the candidate tumor suppressor PCDH20 by epigenetic mechanism in non-small-cell lung cancers.Cancer Res,2006,66:4617-4626.
[10] Ying J,Gao Z,Li H,et al.Frequent epigenetic silencing of protocadherin 10 by methylation in multiple haematologic malignancies.Br J Haematol,2007,136:829-832.
[11] Ying J,Li H,Seng TJ,et al.Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal,esophageal and multiple other carcinomas with frequent methylation.Oncogene,2006,25:1070-1080.
[12] Yu JS,Koujak S,Nagase S,et al.PCDH8,the human homolog of PAPC,is a candidate tumor suppressor of breast cancer.Oncogene,2008,27:4657-4665.
[13] Okazaki N,Takahashi N,Kojima S,et al.Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation.Carcinogenesis,2002,23:1139-1148.
[14] Kawaguchi M,Toyama T,Kaneko R,et al.Relationship between DNA methylation states and transcription of individual isoforms encoded by the protocadherin-alpha gene cluster.J Biol Chem,2008,283:12064-12075.
[15] Bender CM,Pao MM,Jones PA.Inhibition of DNA methylation by 5-aza-2′-deoxycytidine suppresses the growth of human tumor cell lines.Cancer Res,1998,58:95-101.
2009-10-23)
(本文編輯:呂芳萍)
MethylationofPCDH8inpancreaticcarcinomacelllines
LVShun-li,GAOJun,DUYi-qi,HUANGHao-jie,WANGXiao-wei,JINJing,GONGYan-fang,ZHANGLing,LIZhao-shen.
DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
Correspondingauthor:LIZhao-shen,Email:zhsli@81890.net
ObjectiveTo investigate the methylation status of PCDH8 gene in pancreatic carcinoma.MethodsMethylation of PCDH8 gene in 2 samples of normal pancreatic tissues and 6 pancreatic carcinoma cell lines (PANC1, ASPC1, BxPC3, CFPAC, PaTu8988 and SW1990) was detected by the methylation-specific PCR (MSP) method. The expression of PCDH8 mRNA was detected with 5-Aza-2-deoxycytidine (5-Aza-dC) treatment, a kind of DNA methyltransferase (DNMT) inhibitor in 6 pancreatic carcinoma cell lines by real-time-PCR.ResultsThe methylation of PCDH8 gene was not detected in normal tissues, while it was partially methylated in PANC1, BxPC3, CFPAC and it was totally methylated in PaTu8988, ASPC1, SW1990. PCDH8 mRNA was expressed in PANC1, SW1990, PaTu8988 and the relative quantities of mRNA expression (RQ) were 1.576±0.648, 0.013±0.008, 0.002±0.001; PCDH8 mRNA was not expressed in BxPC3, CFPAC, ASPC1. After 5-Aza-dC treatment, PCDH8 mRNA was expressed in PANC1, ASPC1, BxPC3, CFPAC, PaTu8988, SW1990 and the relative quantities of mRNA expression all significantly increased, and they were 7.463±2.628, 10.696±1.539, 7.852±2.762, 421.815±1.493, 118.595±4.089, 6.690±1.884.ConclusionsThe methylation of PCDH8 gene may be the major mechanism of down-regulated expression of PCDH8 gene in pancreatic carcinoma.
Pancreatic neoplasms; Methylation; Polymerase chain reaction; Protocadherin 8
10.3760/cma.j.issn.1674-1935.2010.03.014
200433 上海,第二軍醫大學長海醫院消化內科
李兆申,Email:zhsli@81890.net

