團花樹皮的化學成分研究
徐曉俞,李尚真,宋啟示*
1中國科學院西雙版納熱帶植物園熱帶植物資源開放實驗室,昆明650223;2中國科學院研究生院,北京100049
團花樹皮的化學成分研究
徐曉俞1,2,李尚真1,宋啟示1*
1中國科學院西雙版納熱帶植物園熱帶植物資源開放實驗室,昆明650223;2中國科學院研究生院,北京100049
采用硅膠、MCI和Sephadex LH-20層析方法對團花樹皮的化學成分進行分離純化,運用現代波譜技術鑒定了10個化合物:4-carboxy-3-hydroxy-5-methylphenyl 3-methoxy-4-hydroxy-5-methylbenzoate(1),谷甾醇-3-O-(6'-O-棕櫚酰基)-β-D-葡萄糖苷(2),喹諾酸-3-O-α-L-鼠李糖苷(3),clethric acid(4),常春藤苷元(5),鉤藤苷元C (6),morolic acid(7),咖啡酸甲酯(8),卡丹賓(9)和3α-二氫卡丹賓(10)。其中化合物1為一個新的酚性成分,化合物2~8首次從該屬植物中分離得到。
樹皮;化學成分;4-carboxy-3-hydroxy-5-methylphenyl 3-methoxy-4-hydroxy-5-methylbenzoate
團花(Anthocephalus chinensis(Lam.)Rich.ex Walp.)別名黃梁木,為茜草科團花屬植物。團花是常綠或落葉大喬木,是一種速生樹種,生長非常迅速,10年左右即可成胸徑40~50 cm的大徑級木材,在1972年第七屆世界林業會上,被稱為“奇跡樹”[1]。團花樹皮在印度古醫“阿優吠陀(Ayurvedo)”經中用于治療蛇咬傷、解熱退燒、發燒、貧血、霍亂等多種疾病[2]。經現代印度植物學家整理和研究,印度的傳統醫藥及民間藥中團花屬植物可用于醫學美容和治療損容性皮膚病[3]。現在,隨著對團花研究的不斷深入,國內外學者報道了團花很多方面的生物活性,如抗氧化,降血糖和降血脂等多種活性[4]。國內外學者對團花的化學成分進行了大量研究,從團花的樹皮、枝葉、根、種子等部分分離到50多種化合物,主要為三萜及其皂苷類、環烯醚萜類及生物堿類等成分[2,5-14]。
本文對團花樹皮的化學成分進行了研究,分離鑒定了10種化合物,其中5種為三萜類成分,2種為生物堿類成分。分別為:4-carboxy-3-hydroxy-5-methylphenyl 3-methoxy-4-hydroxy-5-methylbenzoate (1),谷甾醇-3-O-(6'-O-棕櫚酰基)-β-D-葡萄糖苷(2),喹諾酸-3-O-α-L-鼠李糖苷(3),clethric acid (4),常春藤苷元(5),鉤藤苷元C(6),morolic acid (7),咖啡酸甲酯(8),卡丹賓(9)和3α-二氫卡丹賓(10)。其中化合物1為一個新的酚性成分,化合物2~8首次從該屬植物中分離得到。
1 儀器與材料
熔點在XTRC-1顯微熔點儀上測定;核磁共振譜用Bruker AV-400、DRX-500及AvanceⅢ-600超導核磁共振儀測定,內標為TMS;EI-MS用Waters AutoSpec Premier P776雙聚焦三扇型磁質譜儀測定,ESI-MS用API QSTAR Pulsar液相四極桿飛行時間質譜儀測定。柱層析硅膠(200~300目)、薄層層析硅膠板(50 mm×100 mm)均為青島海洋化工廠生產;反相柱色譜填料使用MCI;凝膠材料使用Sephadex LH-20。
團花樹皮,2006年7月采集于西雙版納熱帶植物園,由中國科學院西雙版納熱帶植物園宋啟示研究員鑒定為茜草科植物團花Anthocephalus chinensis L.的干燥樹皮,憑證標本存放于中國科學院西雙版納熱帶植物園。
2 提取與分離
將團花樹皮曬干,稱重得250 kg,粉碎。用90%甲醇浸提,方法如下:加熱回流提取3次,每次12 h,將所得提取液合并、過濾、減壓蒸干,得甲醇浸膏共30 kg,本實驗取用10 kg。將甲醇浸膏用水攪拌均勻后依次用石油醚、氯仿、正丁醇萃取,每種溶劑各萃取4次,分別得到石油醚部分130 g,氯仿部分334 g,正丁醇部分3143 g。將氯仿部分經硅膠柱(200~300目,3kg)層析,以氯仿-甲醇(v/v,9∶1;8∶2;7∶3;6∶4;5∶5)梯度洗脫得到五個組份(Fr.1~Fr.5)。Fr.1再上硅膠柱,以氯仿-甲醇(v/v,98∶2)梯度洗脫得到Fr.1.1,Fr.1.1再上硅膠柱,以氯仿-甲醇梯度洗脫,經Sephadex LH-20(氯仿∶甲醇1∶1)純化,得到化合物7(138.8 mg),8(118.4 mg);以氯仿-甲醇(v/v,95∶5)梯度洗脫得到Fr.1.2,Fr.1.2再上硅膠柱,以氯仿-甲醇梯度洗脫,經Sephadex LH-20(氯仿∶甲醇=1∶1)純化,得到化合物4(608.6 mg),5(1710.3 mg),6(329.8 mg);以氯仿-甲醇(v/ v,92∶8)洗脫得到Fr.1.3,Fr.1.3再上硅膠柱,以氯仿-甲醇梯度洗脫,經Sephadex LH-20(氯仿∶甲醇= 1∶1)純化,得到化合物2(142.1 mg),3(403.1 mg)。Fr.2再上硅膠柱,以氯仿-甲醇(v/v,85∶15)梯度洗脫得到Fr.2.1,Fr.2.1再上硅膠柱,以氯仿-甲醇梯度洗脫,經Sephadex LH-20(氯仿∶甲醇1∶1)純化,得到化合物1(89.9 mg);以氯仿-甲醇(v/v,80∶20)洗脫得到Fr.2.2,Fr.2.2再上硅膠柱,以氯仿-甲醇梯度洗脫,經Sephadex LH-20(氯仿∶甲醇1∶1)純化,得到化合物9(740.7 mg),10(142.1 mg)。
3 結構鑒定
化合物1 無色針狀晶體(甲醇),ESI-MS:m/z 331[M-H]-,HRESI-MS:m/z 331.0814[M-H]-(C17H15O7,calc.331.0817)。計算其不飽和度為10。

表1 化合物1的1H和13C NMR數據(CD3OD,400 MHz)Table 1 1H and13C NMR data of 1 in CD3OD(400 MHz)
1H NMR低場區4個信號δH6.30、6.35、6.44和6.50,以及13C NMR低場區的14個信號δC97.8、108.3、110.2、114.9、116.0、116.7、139.9、144.4、154.3、160.5、161.6、164.6、168.3和175.9,提示分子中可能含有苯環(見表1)。根據HSQC譜,1H NMR譜中的δH3.81(3H,s)、2.32(3H,s)和2.61 (3H,s)分別對應于13C NMR譜中的δC56.4(q)、20.0(q)和23.6(q),為1個甲氧基和2個甲基信號。結合DEPT譜,13C NMR譜中的δC168.3(s),175.9(s)為兩個羧基碳信號,其中δC168.3(s)的羧基被酯化;δC154.3、160.5、161.6和164.6為4個與含氧基團相連的季碳;分子中還有4個季碳信號δC139.9、144.4、114.9和116.7。根據DEPT、1H和13C NMR譜數據,結合分子不飽和度,可知化合物1應含有兩個四取代的苯環。HMBC譜中顯示δH2.32與δC139.9(s)、110.2(d)相關,δH2.61與δC116.0(d)、116.7(s)和144.4(s)相關,推斷兩個甲基分別連接在δC139.9(s)和144.4(s)上;δH3.81與δC160.5(s)的相關,提示甲氧基連在δC160.5(s)上;δH6.30和6.35分別與δC114.9(s)和168.3(s)相關,提示該羰基連接在δC114.9(s)上,而另一羰基δC175.9(s)則連接在δC116.7(s)上。綜上所述,推斷出化合物1的結構如圖1所示,命名為 4-carboxy-3-hydroxy-5-methylphenyl 3-methoxy-4-hydroxy-5-methylbenzoate。
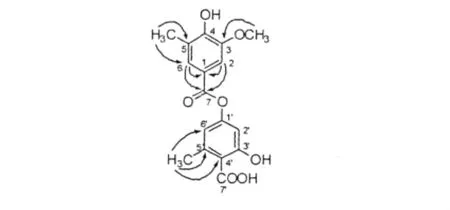
圖1 化合物1的主要HMBC相關Fig.1 Key HMBC correlations of 1
化合物2 白色粉末(甲醇),mp.151~154℃,分子式為 C51H90O7。EI-MS:m/z 414[M-CH3(CH2)14COOC6H10O4]+,396[M-CH3(CH2)14COOC6H11O5]+,256[C16H32O2]+,239[C15H31CO]+,185,129,73。碎片峰中的系列峰與棕櫚酸EI-MS圖譜一致,顯示含有棕櫚酸。1H NMR (C5D5N,400 MHz)δ:5.49(1H,br s,H-6),4.95 (1H,d,J=10.4 Hz,H-1'),4.48(1H,br s,H-6'a),4.02(1H,br s,H-6'b),3.85(1H,m,H-3),2.59 (1H,t,J=11.6 Hz,H-2''),1.24~1.27(br s,(CH2)n),1.00(3H,s,H-19),0.98(3H,d,J=6.4 Hz,H-21),0.86(12H,br s,(CH3)4),0.65(3H,s,H-18);13C NMR(C5D5N,100 MHz)δ:37.7(t,C-1),28.6(t,C-2),80.7(d,C-3),39.5(t,C-4),141.1(s,C-5),121.8(d,C-6),32.1(t,C-7),32.1(d,C-8),50.4(d,C-9),36.9(s,C-10),21.4(t,C-11),39.5 (t,C-12),42.5(s,C-13),56.9(d,C-14),24.6(t,C-15),29.6(t,C-16),56.4(d,C-17),12.0(q,C-18),19.5(q,C-19),36.5(d,C-20),19.1(q,C-21),34.5 (t,C-22),26.4(t,C-23),46.1(d,C-24),29.6(d,C-25),20.0(q,C-26),19.5(q,C-27),23.4(t,C-28),12.2(q,C-29),173.6(s,C-1''),34.5(t,C-2''),25.4(t,C-3''),29.5~30.1(t,C-4''~C-13''),32.2 (t,C-14''),23.0(t,C-15''),14.3(q,C-16''),3-OGlu:100.8(d,C-1'),78.0(d,C-2'),79.8(d,C-3'),71.6(d,C-4'),74.8(d,C-5'),64.6(t,C-6')。根據以上數據鑒定化合物2為谷甾醇-3-O-(6'-O-棕櫚酰基)-β-D-葡萄糖苷[15]。
化合物3 白色粉末(甲醇),mp.260~263℃,分子式為C36H56O9。1H NMR(CD3OD,500 MHz)δ: 5.61(1H,br s,H-12),4.71(1H,br s,H-1'),3.81 (1H,m,H-2'),3.72(1H,m,H-4'),3.69(1H,m,H-3'),3.64(1H,m,H-5'),3.05(1H,dd,J=11.3,4.5 Hz,H-3),2.24(1H,d,J=9.2 Hz,H-18),1.23(3H,d,J=6.3 Hz,H-6'),0.97(3H,s),0.91(3H,d,J= 6.0 Hz,H-29),0.91(3H,s),0.90(3H,s),0.89 (3H,s),0.78(3H,d,J=5.8 Hz,H-30);13C NMR (CD3OD,125 MHz)δ:39.8(t,C-1),26.6(t,C-2),90.3(d,C-3),37.8(s,C-4),56.6(d,C-5),19.4(t,C-6),37.9(t,C-7),39.9(s,C-8),48.0(d,C-9),39.9(s,C-10),23.8(t,C-11),130.4(d,C-12),133.8(s,C-13),57.2(s,C-14),26.4(t,C-15),25.7 (t,C-16),48.5(s,C-17),55.5(d,C-18),38.3(d,C-19),40.3(d,C-20),31.2(t,C-21),37.6(t,C-22),28.7(q,C-23),18.1(q,C-24),16.9(q,C-25),19.1(q,C-26),179.0(s,C-27),181.5(s,C-28),17.8(q,C-29),21.5(q,C-30),3-O-Rha:104.3(d,C-1'),72.5(d,C-2'),72.4(d,C-3'),74.1(d,C-4'),69.9(d,C-5'),17.0(q,C-6')。以上數據與文獻報道[16]一致,故化合物3鑒定為喹諾酸-3-O-α-L-鼠李糖苷。
化合物4 無色針狀結晶(甲醇),mp.284~287℃,分子式為C30H48O6。1H NMR(C5D5N,400 MHz)δ:5.60(1H,m,H-12),4.38(1H,m,H-3),4.86(1H,d,J=10.8 Hz,H-23a),4.64(1H,d,J= 11.28 Hz,H-24),4.23(1H,d,J=10.8 Hz,H-23b),3.96(1H,d,J=11.28 Hz,H-24),3.04(1H,s,H-18),1.77(3H,s,H-26),1.42(3H,s,H-27),1.10 (3H,s,H-25),1.09(3H,s,H-29),0.99(3H,s,H-30);13C NMR(C5D5N,100 MHz)δ:38.9(t,C-1),28.2(t,C-2),74.4(d,C-3),47.0(s,C-4),48.0(d,C-5),19.4(t,C-6),33.7(t,C-7),40.4(s,C-8),48.3(d,C-9),37.0(s,C-10),24.3(t,C-11),128.0 (d,C-12),140.0(s,C-13),42.1(s,C-14),29.3(t,C-15),26.4(t,C-16),48.6(s,C-17),54.6(d,C-18),72.7(d,C-19),42.4(d,C-20),27.0(t,C-21),38.5(t,C-22),63.5(t,C-23),63.3(t,C-24),16.0 (q,C-25),17.1(q,C-26),24.7(q,C-27),180.7(s,C-28),27.1(q,C-29),16.8(q,C-30)。以上數據與文獻報道[17]基本一致,故化合物4鑒定為clethric acid。
化合物5 無色針狀晶體(甲醇),mp.332~334℃,分子式為 C30H48O4。1H NMR(C5D5N,500 MHz)δ:5.48(1H,br s,H-12),4.22(1H,d,J=10.8 Hz,H-3),4.19(1H,d,J=10.4 Hz,H-23a),3.71 (1H,d,J=10.4 Hz,H-23b),3.29(1H,dd,J= 13.8,3.9 Hz,H-5),1.22(3H,s,H-24),1.04(3H,s,H-25),1.03(3H,s,H-26),0.99(3H,s,H-27),0.96 (3H,s,H-29),0.92(3H,s,H-30);13C NMR (C5D5N,125 MHz)δ:38.8(t,C-1),27.7(t,C-2),73.5(d,C-3),42.9(s,C-4),48.7(d,C-5),18.6(t,C-6),33.0(t,C-7),39.8(s,C-8),48.2(d,C-9),37.3(s,C-10),23.9(t,C-11),122.6(d,C-12),144.9(s,C-13),42.2(s,C-14),28.4(t,C-15),23.7 (t,C-16),46.7(s,C-17),42.0(d,C-18),46.5(t,C-19),31.0(s,C-20),34.2(t,C-21),33.2(t,C-22),68.0(t,C-23),13.2(q,C-24),16.0(q,C-25),17.5 (q,C-26),26.2(q,C-27),180.2(s,C-28),33.3(q,C-29),23.8(q,C-30)。以上數據與文獻報道[18]一致,故化合物5鑒定為常春藤苷元。
化合物6 白色顆粒晶體(氯仿-甲醇),mp.315~316℃,分子式為C30H50O5。1H NMR(C5D5N,500 MHz)δ:5.58(1H,br s,H-12),5.05(1H,br s,H-6),4.39(1H,d,J=10.4 Hz,H-23a),4.27(1H,dd,J=11.5,4.1 Hz,H-3),4.03(1H,d,J=10.4 Hz,H-23b),3.59(1H,br s,H-5),3.33(1H,dd,J=13.9,4.1 Hz,H-18),1.71(3H,s,H-24),1.66(3H,s,H-25),1.62(3H,s,H-26),1.25(3H,s,H-27),0.99 (3H,s,H-30),0.92(3H,s,H-29);13C NMR (C5D5N,125 MHz)δ:41.1(t,C-1),28.1(t,C-2),73.3(d,C-3),44.1(s,C-4),49.3(d,C-5),67.6(t,C-6),41.1(t,C-7),39.2(s,C-8),48.8(d,C-9),37.0(s,C-10),23.8(t,C-11),123.0(d,C-12),144.3(s,C-13),42.7(s,C-14),28.4(t,C-15),24.0 (t,C-16),46.7(s,C-17),42.1(d,C-18),46.5(t,C-19),31.0(s,C-20),34.3(t,C-21),33.3(t,C-22),67.1(t,C-23),14.8(q,C-24),17.5(q,C-25),18.7 (q,C-26),26.3(q,C-27),180.3(s,C-28),33.3(q,C-29),23.8(q,C-30)。以上數據與文獻報道[19]一致,故化合物6鑒定為鉤藤苷元C。
化合物7 白色針狀晶體(丙酮),mp.269~271℃,分子式為 C30H48O3。1H NMR(C5D5N,600 MHz)δ:5.32(1H,s,H-19),5.23(1H,br s,OH), 3.62(1H,br s,H-5),3.48(1H,dd,J=9.6,6.3 Hz,H-3),0.82~2.72(23H,m),1.23(3H,s),1.15 (3H,s),1.08(3H,s),1.05(3H,s),1.03(3H,s),0.96(3H,s),0.84(3H,s);13C NMR(C5D5N,150 MHz)δ:40.0(t,C-1),27.0(t,C-2),78.5(d,C-3),39.8(s,C-4),56.5(d,C-5),19.2(t,C-6),35.5(t,C-7),41.5(s,C-8),52.0(d,C-9),38.0(s,C-10),21.8(t,C-11),28.8(t,C-12),42.2(d,C-13),43.5 (s,C-14),30.5(t,C-15),34.8(t,C-16),49.1(s,C-17),139.5(s,C-18),132.5(d,C-19),32.9(s,C-20),34.7(t,C-21),34.6(t,C-22),29.1(q,C-23),16.9(q,C-24),16.8(q,C-25),17.4(q,C-26),15.9 (q,C-27),179.7(s,C-28),31.3(q,C-29),29.8(q,C-30)。以上數據與文獻報道[20]一致,故化合物7鑒定為morolic acid。
化合物8 無色顆粒晶體(甲醇),mp.161~163℃,分子式為 C10H10O4。1H NMR(CD3OD,500 MHz)δ:7.56(1H,d,J=15.9 Hz,H-7),7.03(1H,d,J=1.8 Hz,H-2),6.93(1H,dd,J=8.2,1.8 Hz,H-6),6.78(1H,d,J=8.2 Hz,H-5),6.28(1H,d,J =15.9 Hz,H-8),3.74(3H,s,OCH3);13C NMR (CD3OD,125 MHz)δ:127.6(s,C-1),114.8(d,C-2),146.8(s,C-3),149.6(s,C-4),115.1(d,C-5),122.9(d,C-6),146.9(d,C-7),116.5(d,C-8),169.8(s,C-9),52.0(q,OCH3)。以上數據與文獻[21]基本一致,故鑒定化合物8為咖啡酸甲酯。
化合物9 白色片狀結晶(甲醇),mp.215~217℃,分子式為C27H32N2O10。1H NMR(C5D5N,400 MHz)δ:7.69(1H,d,J=7.6 Hz,H-12),7.64(1H,s,H-17),7.55(1H,d,J=8.0 Hz,H-9),7.50(1H,dd,J=7.6,7.3 Hz,H-11),7.24(1H,dd,J=8.0,7.3 Hz,H-10),5.76(1H,d,J=9.2 Hz,H-21),5.08~5.17(4H,m,H-1'~4'),4.43(1H,m,H-19),3.57 (3H,s,OCH3),2.09(2H,m,H-14),1.77(1H,m,H-20);13C NMR(C5D5N,100 MHz)δ:134.2(s,C-2),91.6(s,C-3),52.5(t,C-5),22.3(t,C-6),110.4(s,C-7),126.5(s,C-8),119.4(d,C-9),119.4(d,C-10),122.4(d,C-11),112.2(d,C-12),137.9(s,C-13),25.9(d,C-15),110.7(s,C-16),152.9(d,C-17),59.0(t,C-18),73.4(d,C-19),97.8(d,C-21),167.1(s,C=O),51.1(q,OCH3),21-O-Glu:102.7 (d,C-1'),74.9(d,C-2'),78.5(d,C-3'),71.9(d,C-4'),78.5(d,C-5'),63.1(t,C-6')。以上數據與文獻[22]一致,故鑒定化合物9為卡丹賓。
化合物10 無定形粉末(甲醇),mp.179~184℃,分子式為 C27H34N2O10。1H NMR(CD3OD,500 MHz)δ:7.54(1H,s,H-17),7.38(1H,d,J=7.8 Hz,H-9),7.30(1H,d,J=8.0 Hz,H-12),7.05(1H,t,J=7.5 Hz,H-11),6.98(1H,t,J=7.5 Hz,H-10),5.54(1H,d,J=9.0 Hz,H-21),4.80(1H,d,J=7.8 Hz,H-1'),4.35(1H,m,H-3),3.98(1H,d,J=12.5 Hz,H-19),3.83(1H,d,J=12.2 Hz,1.8 Hz,H-6'),3.79(3H,s,OCH3),3.65(1H,dd,J=12.3,7.0 Hz,H-6'),3.64(1H,br d,J=6.0 Hz,H-5a),3.40(1H,dd,J=8.9,8.9 Hz,H-3'),3.34(1H,ddd,J=8.0,8.0,2.2 Hz,H-5'),3.30(1H,dd,J=8.9,8.0 Hz,H-4'),3.29(1H,dd,J=9.0,7.8 Hz,H-2'),3.17(1H,m,H-18a),3.14(1H,m,H-18b),3.08(2H,m,H-5b,6a),2.76(1H,br s,H-6b),2.73(1H,br s,H-15),2.42(1H,br d,J=14.5 Hz,H-14a),2.05(1H,m,H-20),1.87(1H,m,H-14b);13C NMR(CD3OD,125 MHz)δ:138.2(s,C-2),64.8(s,C-3),56.2(t,C-5),22.7(t,C-6),108.4(s,C-7),128.0(s,C-8),118.8(d,C-9),120.1(d,C-10),122.5(d,C-11),112.3(d,C-12),138.2(s,C-13),36.3(t,C-14),34.0(d,C-15),111.0(s,C-16),153.7(d,C-17),59.5(t,C-18),66.2(d,C-19),44.2(d,C-20),97.8 (d,C-21),169.3(C=O),52.2(OCH3),21-O-Glu: 101.2(d,C-1'),74.5(d,C-2'),78.3(d,C-3'),71.0 (d,C-4'),77.9(d,C-5'),62.3(t,C-6')。以上數據與文獻[23]基本一致,故鑒定化合物10為3α-二氫卡丹賓。
致謝:所有光譜數據均由中國科學院昆明植物研究所植物化學與西部植物資源持續利用國家重點實驗室分析測試中心測定。
1 Zhu GL(朱桂蘭).Fast-growing tree special:Anthocephalus chinensis.Forest Invent Plan(云南林業調查規劃),1993,(4):58-59.
2 Wei H(韋宏).Iridoids from the Bark of Anthocephalus chinensis(A.cadamba).Guangxi Sci(廣西科學),1999,6 (2):111-114.
3 Jin JN(金久寧),Wu JL(吳菊蘭),Xie X(謝秀),et al.Study of traditional medicinal drugs cosmetic herbs.Chin J Aesthetic Med(中國美容醫學),2001,10(1):16-19.
4 Kumar V,Khanna AK,Khan MM,et al.Hypoglycemic,lipid lowering and antioxidant activities in root extract of Anthocephalus indicus in alloxan induced diabetic rats.Indian J Clin Biochem,2009,24:65-69.
5 Kitagawa I,Wei H,Nagao S,et al.Indonesian medicinal plants.14.Characterization of 3'-O-caffeoylsweroside,a new secoiridoid glucoside,and kelampayosides A and B,two new phenolic apioglucosides,from the bark of Anthocephalus chinensis(Rubiaceae).Chem Pharm Bull,1996,44:1162-1167.
6 Handa SS,Gupta SK,Vasisht K,et al.Quinoline alkaloids from Anthocephalus chinensis.Planta Med,1984,50:358.
7 Brown RT,Fraser SB.Anthocephalus alkaloids-cadambine and 3alpha-dihydrocadambine.Tetrahedron Lett,1974,15: 1957-1959.
8 Brown RT,Fraser SB,Banerji J.Anthocephalus alkaloidsisodihydrocadambine.Tetrahedron Lett,1974,15:3335-3338.
9 Brown RT,Chapple CL.Anthocephalus alkaloids-3beta-dihydrocadambine and 3beta-isodihydrocadambine.Tetrahedron Lett,1976,17:2723-2724.
10 Brown RT,Chapple CL.Anthocephalus alkaloids-cadamine and isocadamine.Tetrahedron Lett,1976,17:1629-1630.
11 Zhou H,He HP,Kong NC,et al.Indole alkaloids from the leaves of Anthocephalus chinensis.Helv Chim Acta,2008,91: 2148-2152.
12 Liu LL,Di YT,Zhang QA,et al.Aminocadambines A and B,two novel indole alkaloids from Neolamarckia cadamba.Tetrahedron Lett,2010,51:5670-5673.
13 Sahu NP,Koike K,Jia ZH.Structures of two novel isomeric triterpenoid saponins from Anthocephalus cadamba.Magn Reson Chem,1999,37:837-842.
14 Sahu NP,Koike K,Jia ZH,et al.Triterpene glycosides from the bark of Anthocephalus cadamba.J Chem Res-S,2000,1: 22-23.
15 Dong X(董學),Wang GR(王國榮),Yao QQ(姚慶強).Chemical constituentsofSparganium stoleniferum.Acta Pharm Sin(藥學學報),2008,43:63-66.
16 Kang WY(康文藝);Shi YY(石淵淵);Hao XJ(郝小江).Quinovic acid triterpenoid saponins from bark of Mitragyna rotundifolia.China J Chin Mat Med(中國中藥雜志),2007,32:2015-2017.
17 Takahashi K,Takani M.Studies on constituents of medicinalplants.21.constituents of leaves of clethra-barbinervis sieb et zucc.2.and c-13 nuclear magnetic-resonance spectra of 19alpha-hydroxyurs-12-en-28-oic acid type of triterpenoids.Chem Pharm Bull,1978,26:2689-2693.
18 Zhang ZP(張中朋),Yang ZL(楊中林),Tang DF(唐登峰),et al.Isolation and structure identification of chemical constituents from Stachys Geobombycis.Chin Tradit Patent Med(中成藥),2004,26:1051-1053.
19 Yang CJ(楊成金),Zhang J(張峻),Wu DG(吳大剛).Triterpenoids from Uncaria rhynchophylla.Acta Bot Yunnan(云南植物研究),1995,17:209-214.
20 Zhang P,Hao J,Liu J,et al.Efficient synthesis of morolic acid and related triterpenes starting from betulin.Tetrahedron Lett,2009,65:4304-4309.
21 Zhao XH(趙曉宏),Chen DH(陳迪華),Si JY(斯建勇),et al.Studies on the phenolic acid constituents from chinese medicine“Sheng-Ma”,rhizome of Cimicifuga foetida L..Acta Pharm Sinica(藥學學報),2002,37:535-538.
22 Handa SS,Borris RP,Cordell GA,et al.NMR spectral-analysis of cadambine from Anthocephalus-chinensis.J Nat Prod,1983,46:325-330.
23 Endo K,Oshima Y,Kikuchi H,et al.Validity of the oriental medicines.50.Hypotensive principles of Uncaria hooks.Planta Med,1983,49:188-190.
Chemical Constituents of the Bark of Anthocephalus chinensis
XU Xiao-yu1,2,LI Shang-zhen1,SONG Qi-shi1*1Laboratory of Tropical Plant Resource Sciences,Xishuangbanna Tropical Botanical Garden,Chinese Academy of Sciences,Kunming 650223,China;2Graduate School of Chinese Academy of Sciences,Beijing 100049,China
Ten compounds were isolated and purified from the bark of Anthocephalus chinensis by column chromatography on silica gel,MCI and Sephadex LH-20,and their structures were elucidated as 4-carboxy-3-hydroxy-5-methylphenyl 3-methoxy-4-hydroxy-5-methylbenzoate(1),sitosterol-3-O-(6'-O-palmityl)-β-D-glucoside(2),quinovic acid-3-O-α-L-rhamnopyranoside(3),clethric acid(4),hederagenin(5),uncargenin C(6),morolic acid(7),caffeic acid methyl ester(8),cadambine(9),and 3α-dihydrocadambine(10)by modern spectroscopic methods.Compound 1 was a new phenolic compound,and compounds 2-8 were obtained from Anthocephalus plants for the first time.
Anthocephalus chinensis;bark;chemical constituent;4-carboxy-3-hydroxy-5-methylphenyl 3-methoxy-4-hydroxy-5-methylbenzoate
1001-6880(2011)03-0393-06
2010-12-22 接受日期:2011-04-29
中國科學院知識創新工程重要方向項目子課題(KSCX2-YW-R-132;KSCX2-EW-R-15),國家863項目子課題(2007AA021504)
*通訊作者 E-mail:songqs@xtbg.ac.cn
R284.2;Q946.91
A

