具有新穎拓撲結構的吡啶基羧酸配體釔配合物的合成,晶體結構和拓撲學分析
王 彥董彥杰周菊紅王素娜劉光祥陳友存
(1安慶師范學院化學化工學院,安徽省功能配合物重點實驗室,安慶 246011)
(2南京大學配位化學國家重點實驗室,南京 210093)
(3聊城大學化學化工學院,聊城 252059)
具有新穎拓撲結構的吡啶基羧酸配體釔配合物的合成,晶體結構和拓撲學分析
王 彥*,1,2董彥杰1周菊紅1王素娜2,3劉光祥1陳友存1
(1安慶師范學院化學化工學院,安徽省功能配合物重點實驗室,安慶 246011)
(2南京大學配位化學國家重點實驗室,南京 210093)
(3聊城大學化學化工學院,聊城 252059)
利用水熱合成制備了一個新的吡啶基多羧酸釔配合物YL(HL)·2H2O(1)(H2L=5-((吡啶基-3-亞甲基)氨基)間苯二甲酸)。利用元素分析及X-射線單晶衍射對其進行了表征。結構分析結果表明標題化合物1屬于三斜晶系,P1空間群,晶胞參數為a= 1.015 35(11)nm,b=1.083 33(12)nm,c=1.287 40(14)nm,α=70.9430(10)°,β=127.689(3)°,γ=84.0680(10)°,晶胞體積V=1.317 0(2) nm3,Z=2,Dc=1.680 g·cm-3,F(000)=680,μ=2.284 cm-1,R=0.056 2,wR=0.1600。在化合物1中,每個Y的配位環境為八配位的反四棱柱配位構型。該配合物的二維結構是少見的(42.6)(42.67.8)層狀拓撲。我們對配合物1的粉末衍射數據和熱重分析也進行的討論。
晶體結構;釔配合物;拓撲;羧酸配體
0 Introduction
During the past decades,rational design and construction of novel complexes with diverse structures,especially interesting topologies,and applications have attracted more and more attentions of chemists[1-3].Many research groups have devoted their efforts on the development,design,and synthesis of novel metal-organic frameworks(MOFs)and significant results have been achieved by using transition/lanthanide metal salts and corresponding organic ligands[4-6].On the other side,mimicking of naturalminerals topologies and constructions of novel complexes with new interesting topologies have attracted more and more attentions of chemists. Because of the limitations in the symmetry or steric hindrance associated with connected nodes,the use of five-,seven-,or eight-connected blocks are quite rare with comparing to the connectivity of three-,four-,or six-connected blocks[7-8].Up to now,although various complexes with fascinating structural diversities as well as their interesting properties have been synthesized,controllable synthesis of metal-organic frameworks(MOFs)with designed structure is still a great challenge[9-11].
We concentrate our efforts on design and construction of MOFs with interesting topologies and properties by using N-or O-containing ligands[12,13]. According to different coordination affinities of N,O atoms for transition and lanthanidemetals,the system composed of ligands containing both N,O as coordination donors,like the pyridyl-carboxylate ligands, are now often used to synthesize heterometallic d-f metal complexes[14,15].In order to further investigate the self-assemblies of pyridyl-carboxylate with transition/ lanthanide metal salts,we used pyridyl-containing carboxylate ligand,namely,5-((pyridin-3-ylmethyl) amino)isophthalic acid(H2L)as starting ligand.Due to the flexibility resulting from themethylene group and varied coordination modes of carboxylate groups, crystals with diverse structures,especially interesting topologies,can be easily formed.In this paper,we report a coordination framework with interesting new topology based on N,O-bifunctional pyridylcarboxylate ligand and yttrium metal salt,namely,YL (HL)·2H2O(1)(H2L=5-((pyridine-3-ylmethyl)amino) isophthalic acid),which may provide useful strategy for construction of new coordination frameworks.
1 Experimental
1.1 General
All chemicals and solvents in this work were commercially obtained and used as received without further purification.The concerning ligand 5-((pyridine-3-ylmethyl)amino)isophthalic acid was synthesized according to the reported literatures[12-13]. C,H and N analyses were made on Elementar Vario EL-Ⅲelemental analyzer.Infrared(IR)spectrum was recorded on Avatar 360 FT-IR spectrophotometer by using KBr disc.Powder X-ray diffraction patterns were recorded on a RigakuD/max-RA rotating anode X-ray diffractometer with graphite monochromatic Cu Kα(λ=0.154 2 nm)radiation at room temperature. Thermogravimetric analysiswas performed on a simultaneous NETZSCH STA 409 PC LUXX thermal analyzer.Powder samples were loaded into alumina pans and heated under flowing N2at a heating rate of 10℃·min-1.
1.2 Structure determ inations
A suitable single crystal of 1 with dimensions of 0.15 mm×0.10 mm×0.10 mm was selected for data collection at 293 K,using a Bruker Smart ApexⅡCCD equipped with a Mo Kαradiation(λ=0.071 073 nm).The structures were solved by direct methods using SHELXTL and refined by full-matrix leastsquares methods anisotropically for non-hydrogen atoms[16].The hydrogen atoms except for those ofwater molecules were generated geometrically.Calculations were performed on a personal computer with the SHELXTL program package[16].Details of the crystal parameters,data collection,and refinement are summarized in Table 1.Selected bond lengths and angles of complexes 1 with their estimated standard deviations are listed in Table 2 and hydrogen bonding distances are given in Table 3.
CCDC:864386.
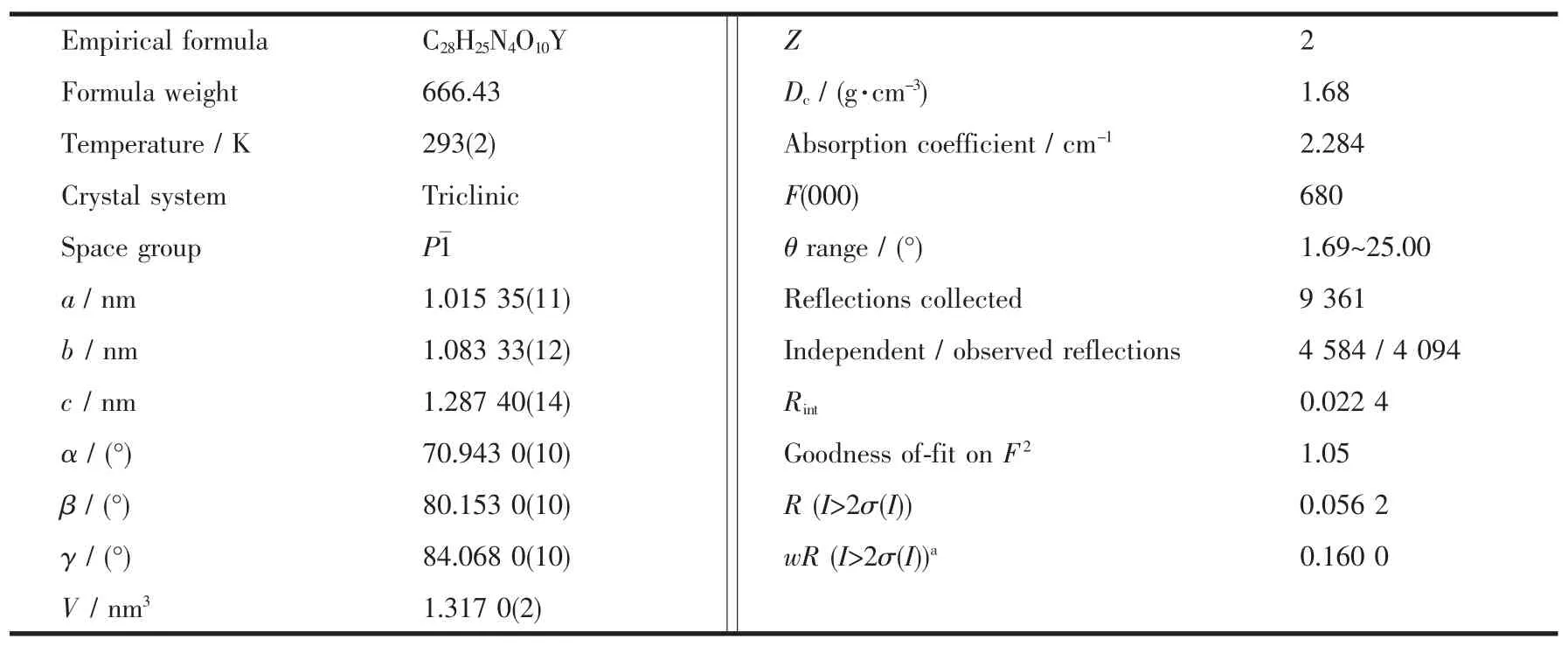
Table 1 Crystallographic data for complex 1
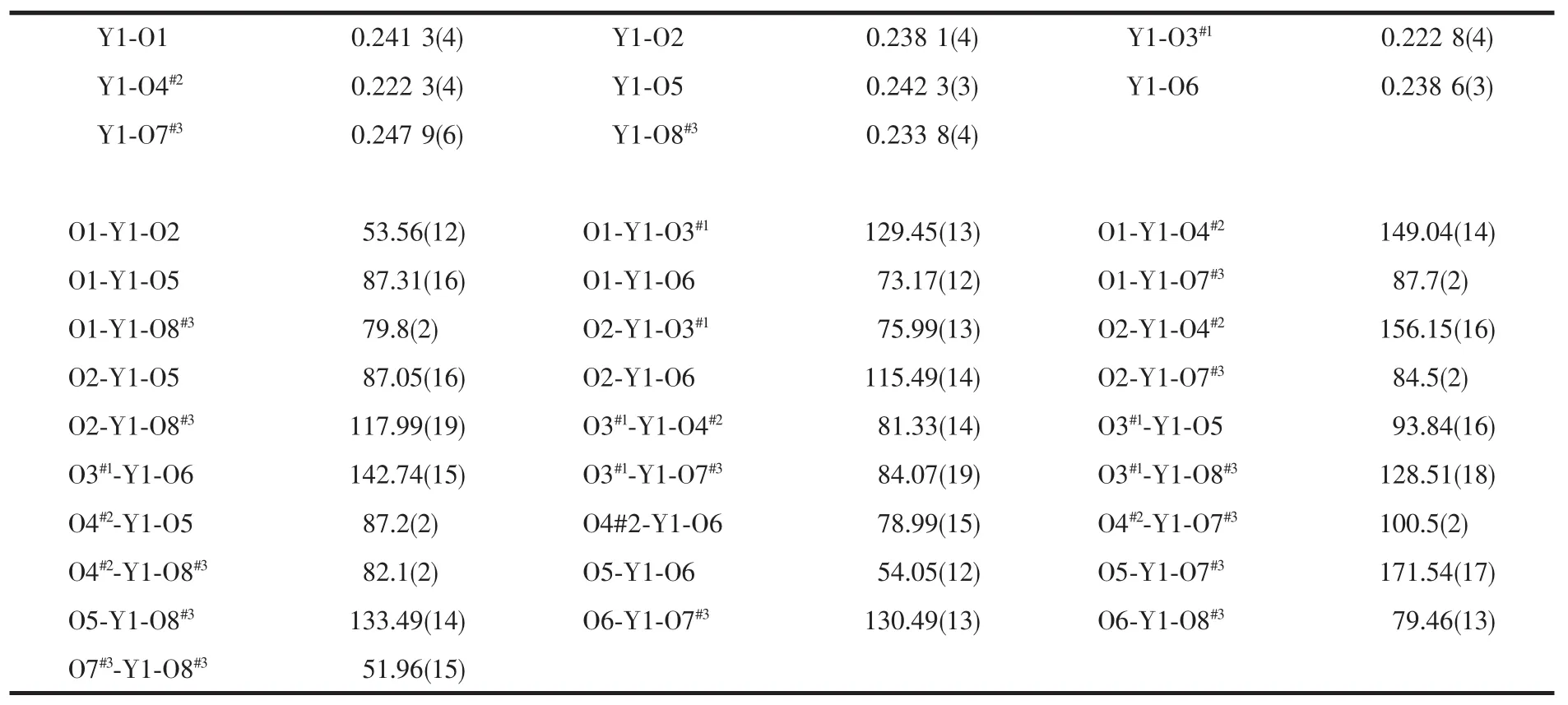
Table 2 Selected bond lengths(nm)and bond angles(°)for com plex 1
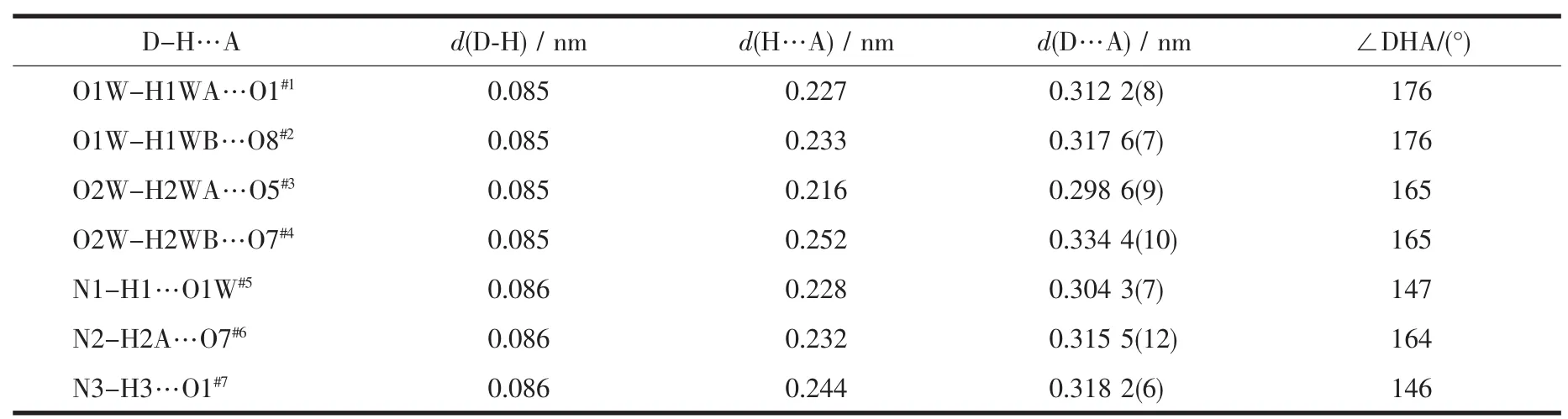
Table 3 Hydrogen bond lengths and bond angles
1.3 Synthesis of YL(HL)·2H2O(1)
To a suspension of H2L(40.8mg,0.15mmol)in 15 mL water was added Y(NO3)3·6H2O(38.3 mg,0.1 mmol)and LiOH(0.012 g,0.30 mmol).After being stirred for 30 min,the resulting solution was sealed into a bomb equipped with a Teflon liner and heatedat 100℃for 72 h.After slow cooling of the reaction mixture to room temperature,pale yellow crystals of 1 were obtained in ca.40%yield.Anal.Calcd.for compound 1,C28H25N4O10Y(%):C 50.46,H 3.79,N 8.41;found(%):C 50.74,H 3.62,N 8.35.FT-IR(KBr pellet,cm-1):3 450 br,1702m,1633m,1 606m,1 543 vs,1 428 s,1 410 s,1 386 vs,1 258 s,1 109w,1 044 w, 742m,624m.
2 Results and discussion
2.1 Structure description
Crystals of structure 1 form under hydrothermal condition as air-stable pale yellow blocks.The crystallographic analysis reveals that the compound crystallizes in triclinic system,space group P1.The asymmetric unit of 1 consists of one yttrium atom,two ligands,and two distinct water molecules(Fig.1).In complex 1,the Y(Ⅲ)ions are eight-coordinated by carboxylic O atoms from five different ligands with YO bond lengths varying from 0.222 3(4)to 0.247 9(4) nm,which is comparable to other reported Y-O distances[17].The O-Y-O coordination angles are in the range of 51.96(15)°to 171.54(17)°.Thus,the coordination environment around the Y(Ⅲ)ions can be regarded as distorted square-antiprismatic geometry. There are two kinds of ligands in the structure of 1: (1)One links three different Y(Ⅲ)ions(μ3-L),whose carboxylate groups adoptμ2-η1∶η1andμ1-η1∶η1coordination modes,respectively(e.g.the ligands containing O1,O2,O3 and O4),and the dihedral angle between the pyridine ring and benzene ring is 5.5°. (2)The other ligands(e.g.the ligands containing O6, O5,O7 and O8)serve asμ2-pillar linking two Y(Ⅲ)atoms,where the two carboxylate groups all adoptμ1-η1∶η1coordination mode,and the corresponding dihedral angle between pyridine and benzene is 80.6°.
In 1,with ignoring the coordination of theμ2-ligand,eachμ3-L connects three yttrium atoms and each Y(Ⅲ)links three differentμ3-L to form a one dimensional(1D)ladder-like structure with Y…Y distances of 0.557 and 0.822 nm,respectively(Fig. 2).The chain units were further connected byμ2-ligands to form a two-dimensional network(Fig.3), which stack along the ac plane to give the whole three-dimensional framework.In addition,the adjacent pyridine rings of theμ2-andμ3-ligand on the same sheet as well as the pyridine rings and benzene rings of adjacent net are offset stacked with centroid…centroid distances of 0.349,0.360,0.351 nm and dihedral angles of 2.88°,5.94°,5.24°,respectively, indicating strongπ…πinteractions between these aromatic groups,which play important roles in the stabilization the 2D and 3D structure of 1(Fig.4). Although the N donors did not coordinate to anymetal ions in 1,probably due to the“hard-soft acid-base”theory,they are still important by forming hydrogen bonds to stabilize the whole framework of the title complex(Table 3).Meanwhile,the uncoordinated water molecules occupy the voids between the 2D layers and form O-H…O hydrogen bonds with corresponding carboxylate O atoms,which also further
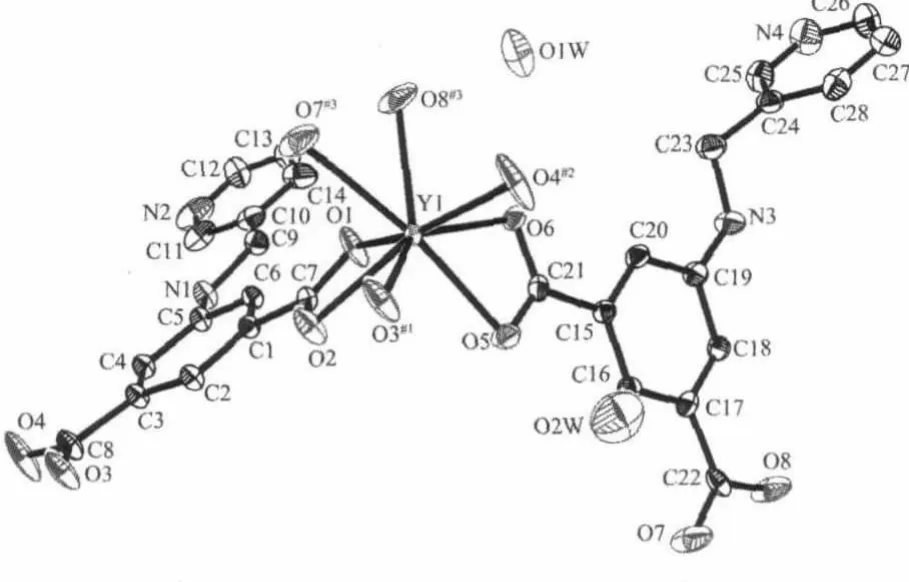
Fig.1 Asymmetric unitof complex 1,with additional atoms completing the coordination environments of yttrium atom
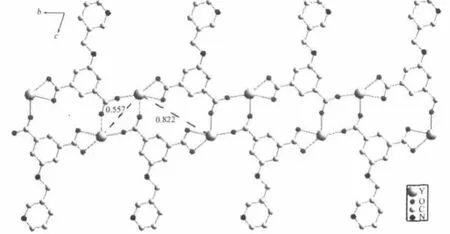
Fig.2 One-dimensional chain in 1 with Y…Y distances marked(nm)viewing along the a axis
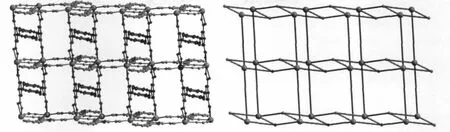
Fig.3(Left)Two dimensional net structure of 1;(right)schematic drawing of the 2D(42.6)(42.67.8)topological sheet in 1, where theμ3-ligand and Y(Ⅲ)atoms are represented by three-and five-spokes radiation from a solid point, respectively

Fig.4 Three-dimensional framework stacked by 2Dsheets in 1 viewing along the b axis,in which the hydrogen bonds andπ…πinteractions were alsomarked(nm)
Topological study performed using the software package TOPOS 4.0[19]reveal that this topology is an interesting two-nodal net.In 1,each yttrium(Ⅲ)connects five different ligands,while each ligand links three or twometal centers.Thus,theμ3-ligand and Y(Ⅲ)units can be defined as 3-and 5-connected nodes, respectively.In the simplification way,with theμ2-ligand simplified as a pillar,the 2D network of 1 can be described as a two nodal 3,5-c net of(42.6)(42.67.8) topology with“long”Schl覿fli symbol(4.4.63) (4.4.6.63.63.63.63.64.64.810),which is,to the best of our knowledge,a rare example of such topological 2D network.
2.2 XPRD and thermogravimetric analysis
The XPRD analysis of 1 has been performed to evaluate the purity of the as-synthesized sample of 1. As shown in Fig.5,the positions of diffraction peaksare consistent with the simulated ones,indicating the phase purity of the as-synthesized simple.
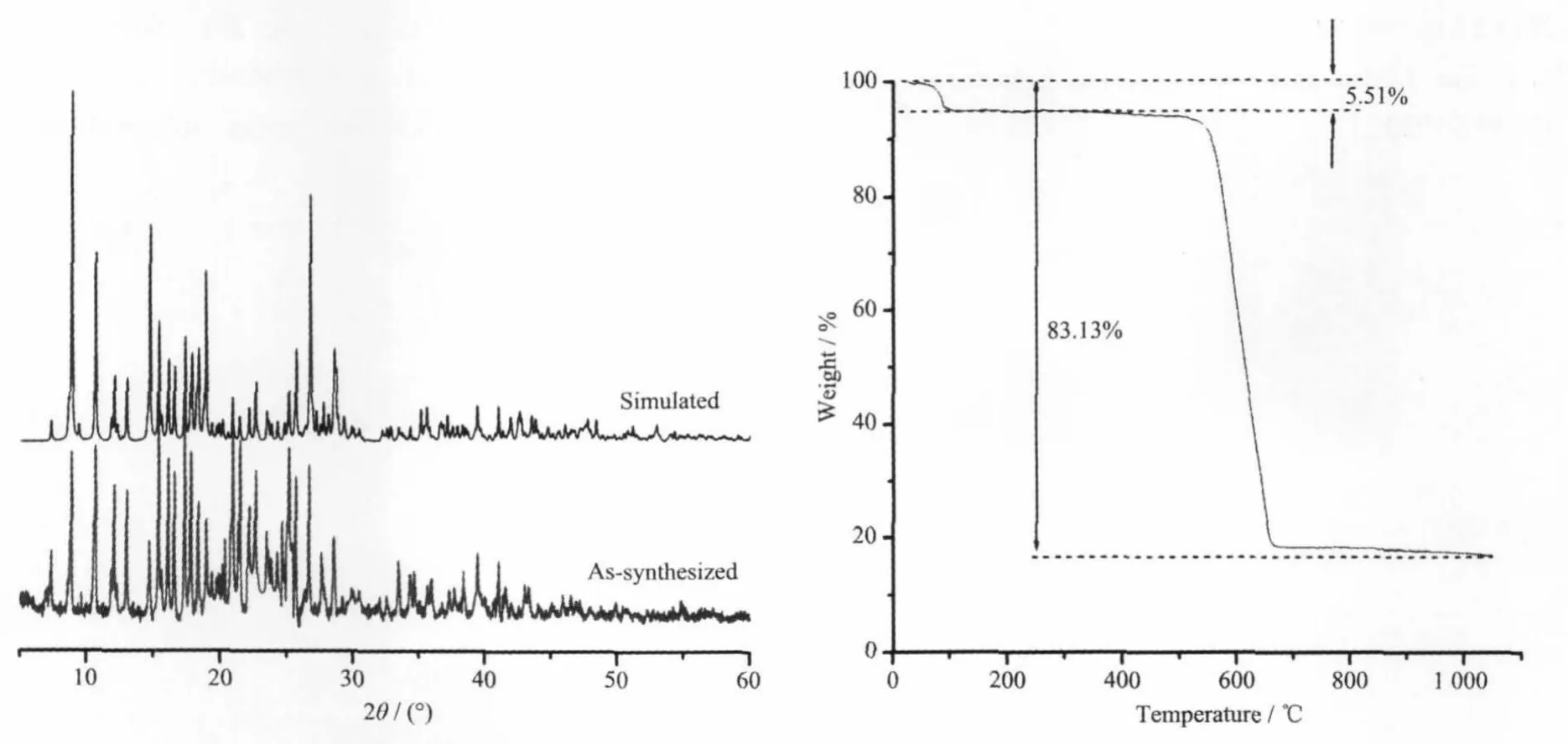
Fig.5(Left)XPRD pattern and(right)TGA curve of complex 1
The Thermogravimetric analysis of complex 1 wascarried outin the range20~1 100℃undernitrogen atmosphere.The TGA data show that the initial weight loss of 5.51%(Calcd.5.40%)occurred from 30 to 102℃corresponding to the loss of the coordinated water molecules and the second begins at 360℃where the decomposition of the residue starts with totalweight loss of 83.13%(Calcd.83.06%based on Y2O3).
3 Conclusions
In summary,by using pyridyl-carboxylate ligand and yttrium(Ⅲ)nitrate,we successfully synthesized a novel polymer exhibiting interesting new 2D sheet structure of(42.6)(42.67.8)topology,in which themetal centers acted as rare five-connecting blocks.The whole 3D framework of 1 was stabilized by abundant π…πand hydrogen bond interactions.Thiswork may offer a strategy for preparing such MOFs with new topologies.
[1]Su Z,Cai K,Fan J,et al.Cryst.Growth Des.,2010,12:100-108
[2]WANG Xiao(王瀟),HOU Xiang-Yang(侯向陽),FU Feng(付峰),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2010,27(1):174-178
[3]Su Z,Fan J,Okamura T,et al.Cryst.Growth Des.,2010, 10:1911-1922
[4]Zhang JP,Horike S,Kitagawa S,et al.Angew.Chem.Int. Ed.,2007,46:889-892
[5]NiW X,LiM,Zhou X P,etal.Chem.Commun.,2007:3479 -3481
[6]Yamaguchi T,Costes JP,Kishima Y,et al.Inorg.Chem., 2010,49:9125-9135
[7]Liu G X,Zhu K,Xu H M,et al.CrystEngComm,2010,12: 1175-1185
[8]Xu G C,Ding Y J,Okamura T,et al.Cryst.Growth Des., 2009,9:395-403
[9]Wang Y,Huang Y Q,Liu G X,et al.Chem.Eur.J.,2007, 12:7523-7531
[10]Bai Z S,Xu J,Okamura T,et al.Dalton Trans.,2009:2528-2539
[11]Na L Y,Hua R N,Zhang L Y,et al.J.Chem.Crystallogr., 2009,39:688-691
[12]WANG Yan(王彥),LIU Guang-Xiang(劉光祥).Chinese J. Inorg.Chem.(Wuji Huaxue Xuebao),2009,25:713-719
[13]WANG Yan(王彥),WANG Tao(王濤),LIU Guang-Xiang(劉光祥),etal.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2010,26:1467-1471
[14]Li Z Y,DaiW J,Wang N,et al.Cryst.Growth Des.,2010, 10:2746-2751
[15]Peng G,Qiu Y C,Liu H Z,et al.Cryst.Growth Des.,2010, 10:114-121
[16]XSCANS,Version 2.1,Siemens Analytical X-ray Instruments, Madison,WI,1994.SHELXTL,Version 5.0,Siemens Industrial Automation,Analytical Instruments,Madison,WI, 1995.
[17]Zhou JP,Chen M H,Zhang L Z,etal.J.Chem.Crystallogr., 2011,41:1820-1833
[18]HU Zong-Zhi(胡宗智),ZHAO Jun(趙君),KE Xi-Jun(柯希俊),etal.Chinese J.Inorg.Chem.,2011,27:184-188
[19]Blatov V A,Shevchenko A P.TOPOS,Version 4.0 Professional,Samara State University,Samara,Russia,2006.
An Yttrium(Ⅲ)Comp lex Based on Pyridyl-Carboxylate Ligand Exhibiting Distinctive New Topology
WANG Yan*,1,2DONG Yan-Jie1ZHOU Ju-Hong1WANG Su-Na2,3LIU Guang-Xiang1CHEN You-Cun1
(1School of Chemistry and Chemical Engineering,Anhui Key Laboratory of Functional Coordination Compounds,Anqing Teachers College,Anqing,Anhui246011,China)
(2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093,China)
(3School of Chemistry and Chemical Engineering,Liaocheng University,Liaocheng,Shandong 252059,China)
By using hydrothermal method,the reaction of 5-((pyridine-3-ylmethyl)amino)isophthalic acid(H2L) with yttrium nitrate led to the formation of a novel yttrium complex,namely,YL(HL)·2H2O(1),with interesting two-dimensional(2D)topology.The title complexwas characterized by elementalanalysisand IR spectroscopy and its structure was determined by single crystal X-ray structural analysis.The resultof structuralanalysis shows that the 1 crystallizes in triclinic,space group P1 with a=1.015 35(11)nm,b=1.083 33(12)nm,c=1.287 40(14)nm, α=70.9430(10)°,β=127.689(3)°,γ=84.0680(10)°,V=1.317 0(2)nm3,Z=2,Dc=1.680 g·cm-3,F(000)=680,μ=2.284 cm-1,R=0.056 2,wR=0.160 0.The Yatoms was eight-coordinated with carboxylate O atoms in squareantiprismatic geometry.In complex 1,the 2D layer comprised ofμ2/μ3-ligands and 5-connected Ycenters is rare example of 2-nodal net of(42.6)(42.67.8)topology.The XPRD and TGA analysis were also investigated. CCDC:864386.
crystal structure;Y(Ⅲ)complex;topochemistry;carboxylate ligand
book=2501,ebook=16
O614.32+2
A
1001-4861(2012)11-2407-06
2012-02-16。收修改稿日期:2012-06-26。
國家自然科學基金(No.20901004,20801025)資助項目。
*通訊聯系人。E-mail:njwangy@126.com

