微波輔助水熱法制備花狀氧化銅
曹霄峰 張 雷 李兆乾 陳學太
微波輔助水熱法制備花狀氧化銅
曹霄峰 張 雷 李兆乾 陳學太*
(南京大學配位化學研究所,配位化學國家重點實驗室,南京微結構國家實驗室,南京大學化學化工學院,南京 210093)
以硝酸銅為銅源,氨水為堿源,通過微波輔助水熱法制備了花狀CuO微納米材料。制備過程中沒有使用表面活性劑或離子液體等軟模板劑。利用X-射線衍射、電子能譜、場發射掃描電鏡以及高分辨透射電鏡表征了產物的物相和形貌。對照實驗顯示堿源對產物形貌有著很大的影響。通過時間演化實驗研究了花狀CuO的生長過程。結果顯示反應過程經歷了Cu2(OH)3NO3和Cu(OH)2兩種中間產物。
氧化銅,花狀,微波水熱法
Copper oxide (CuO),a typical narrow p-type semiconductor with a band gap of 1.2 eV[1-2],has attracted a great deal of attention because of its potential technological applications in heterogeneous catalysts,gas sensors,lithium ion electrode materials,solar cells,photocatalysts,and so on[3-5].Up to now,CuO nanomaterials with different morphologies have been prepared by several methods,including thermal oxidation, electrochemical method, sonochemical method,hydrothermal/solvothermal method,etc[6-10].
Recently,microwave has been widely used in organic synthesis and nanomaterials preparation.Compared to the traditionalheating,microwave heating is simple, rapid, uniform, efficient,economical,and environmentally friendly.Sofar,several studies have been performed on thepreparation of CuO nanomaterials via the microwaveassisted solution-phase process.Severalkindsof morphologies such as nanoparticles, nanoleaves,nanosheets,nanowhiskers,nanorods,chrysanthemumlike,flower-like,and sea urchin-like have been prepared by microwave-assisted solution-phase process[11-15].The surfactants or the ionic liquids were usually used to prepare the flower-like CuO hierarchicalnano-/microstructures[16-19].The use of soft template usually pollutesthesample and the post-processing is tedious.In the present paper,we report the preparation of flowerlike CuO nanomaterials via a 1 min microwave-assisted hydrothermal process without the use of any surfactants or ionic liquids.The effect of the alkali source on the morphologies has been studied.The crystal growth process of as-prepared CuO is suggested.
高管技術專長對創新投入的影響研究....................................................................................................................................劉力鋼 孫 亞(1)
1 Experimental
Allreagentswere purchased from Shanghai Chemical Company and used without further purification. All samples were prepared in a microwave system(2.45 GHz,Discover S-Class,CEM)equipped with in situ magnetic stirring.The exposure time and temperature were programmed. The automatic temperature-control system allowed continuous monitoring and control of the internal temperature of the reaction system (1℃).The preset profile (desired time and temperature)was followed automatically by continuously adjusting the applied microwave power.
In a typical preparation procedure,Cu(NO3)2·6H2O(1.0 mmol,AR)was dissolved in H2O(9.5 mL).Then 0.5 mL ammonia solution (30%)was added dropwise to the above solution under continuous stirring.The color of the solution gradually changed from light blue to dark blue.The reaction solution was stirred at room temperature for 10 minutes and then transferred to a 35 mL round-bottom flask.After treating the mixture at 150℃for 1 min under microwave irradiation, it was cooled to room temperature rapidly by compressed air.The product wascollected,washed with deionized waterand absolute ethanol,and dried in air at 60℃for 6 h.Other experiments were also conducted under different conditions such as using different alkali sources (NaOH,urea)while the otherreaction conditions were kept the same.
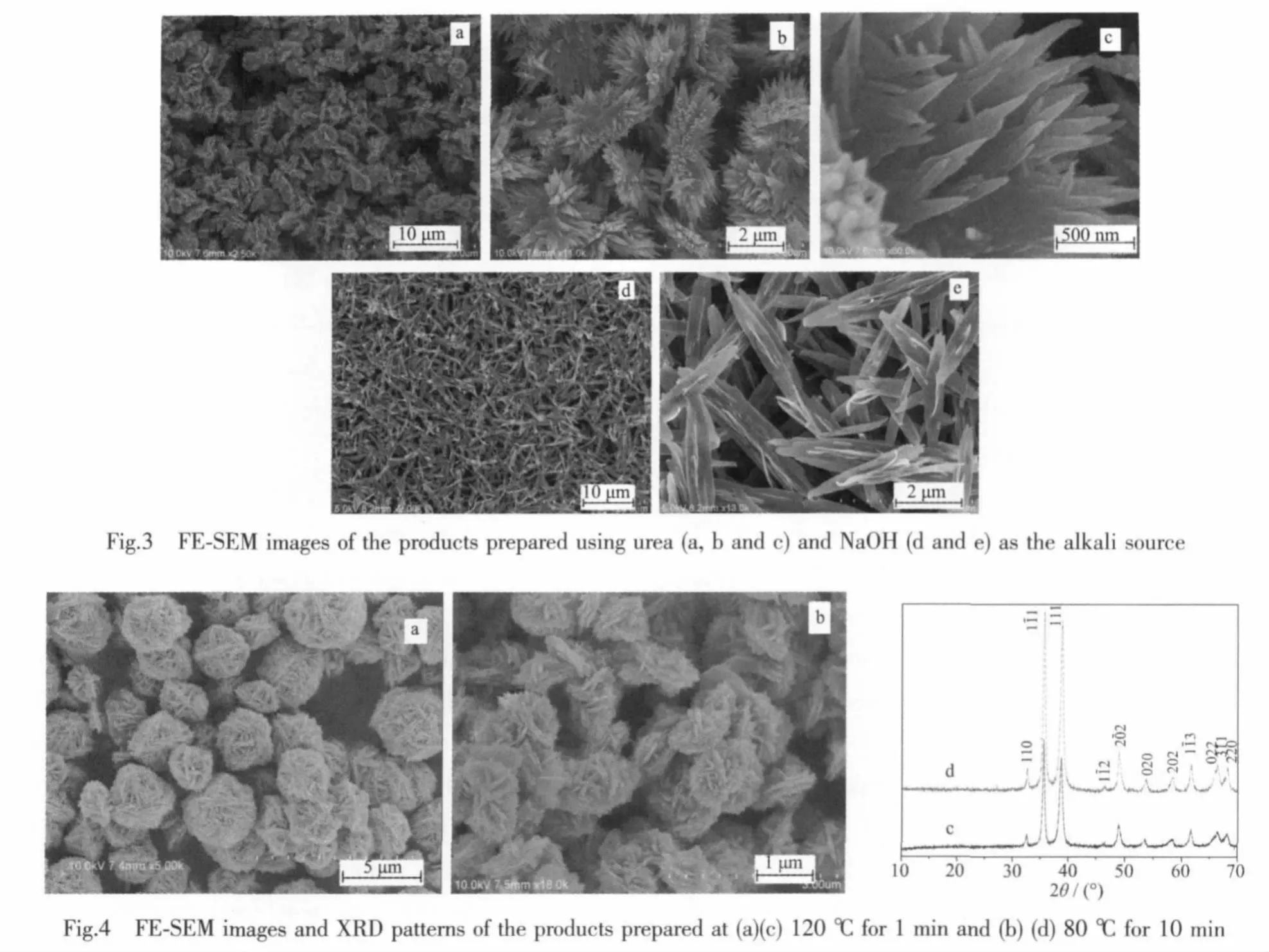
The alkali source was an important influence factor on the morphologies[20]. Fig.3 shows the morphologies of the products prepared using urea and NaOH as the alkali source.Fig.3a and b show that the product is platy flower-like particles with diameters ranging from 2.5 to 3.5 μm.Fig.3c is the magnified FE-SEM image of the platy flower-like particles,exhibiting that the assembly units are the same with those of the product prepared in the typical procedure.Fig.3d and e are the FE-SEM images of the CuO nanoparticles prepared using NaOH as the alkali source.The images show that the product is leaf-like nanosheets with widths in the range of 200~650 nm and lengths in the range of 3~4 μm.The smaller nanosheets with ca.20 nm thickness are perpendicular to the leaf-like nanosheets.No selfassembled nanostructures are obtained.The possible reason could be that the NaOH has the stronger basicity.
農業機械的保養要嚴格按照使用說明書及當地農機管理部門規定的內容進行。機車的高級保養應在機務管理人員指導下在室內進行。燃油動力機械要做到四小漏 (小漏油、小漏水、小漏氣、小漏電)、五凈(油、水、氣、機器、工具)、六封閉(柴油箱口、汽油箱口、機油加注口、機油檢視口、汽化器、磁電機)、一完好(技術狀態完好);配套農具要實行常年修理,做到三靈活(操作、轉動、升降靈活)、五不(不曠、不鈍、不變形、不銹蝕、小不件)、一完好(技術狀態完好)。
The products were characterized by X-ray powder diffraction (XRD)with a Bruker D8 Advance Powder X-ray Diffractometer, equipped with graphite monochromatized Cu Kα radiation (λ=0.154 06 nm),employing a scanning rate of 4°·min-1,in the 2θ range from 10°to 70°.The operation voltage and current were maintained at 40 kV and 30 mA,respectively.FE-SEM images and EDS were measured on a Hitachi S-4800 field emission scanning electron microanalyser employing an operating voltage of 5 kV or 25 kV.TEM imageswere obtained on a JEM-200CX transmission electronic microscope,employing an accelerating voltage of 200 kV.
2 Results and discussion
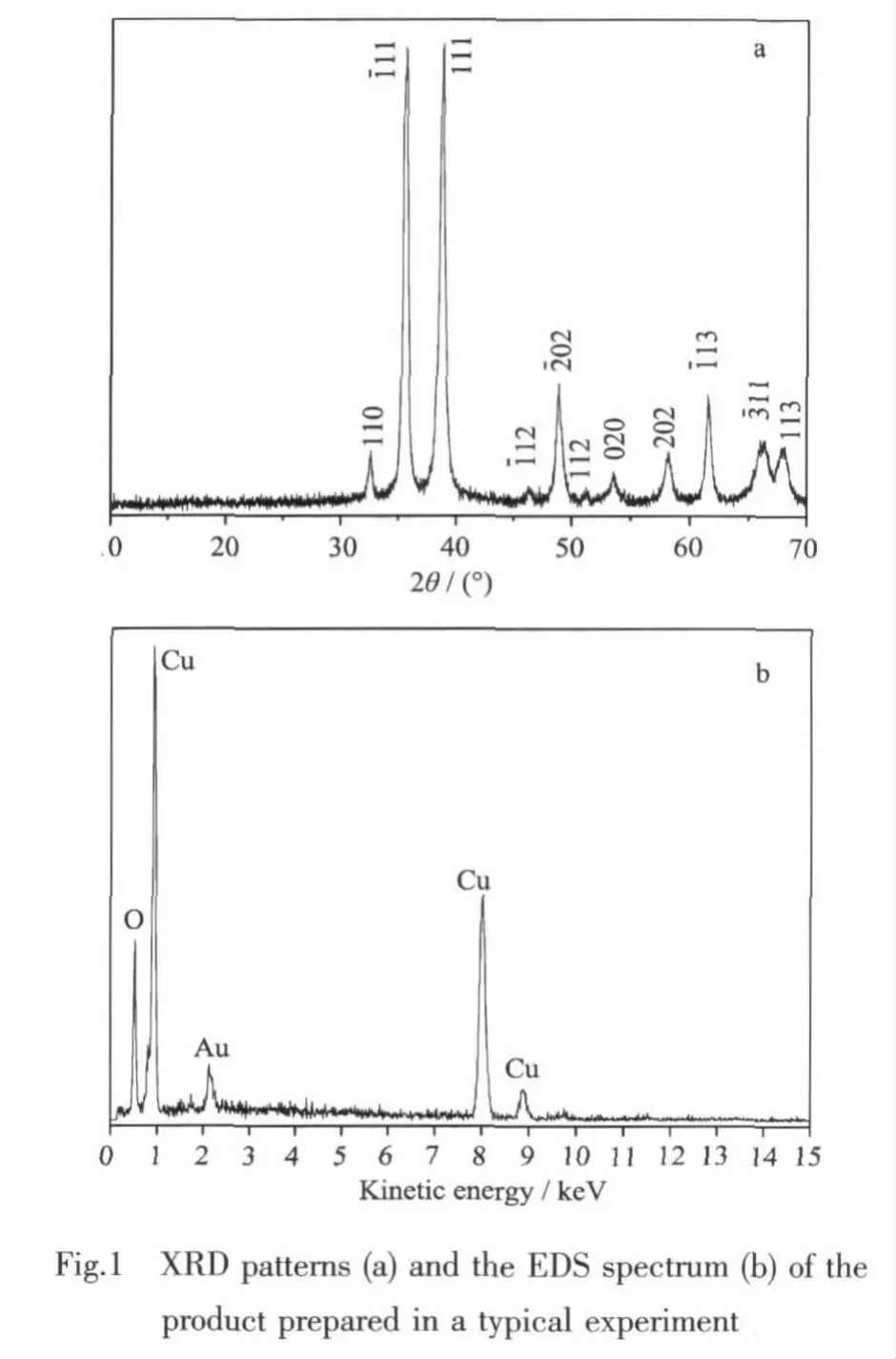
Fig.2a is the low magnified FE-SEM image of the sample obtained in the typical procedure,indicating that the product is flower-like particles with diameters ranging from 2 to 3 μm.The magnified FE-SEM images (Fig.2b and 2c)show the CuO flower is constructed by the triangular sheets.In the HRTEM image (Fig.2d)taken from the edge of the individual microstructure,the (110)planes are marked,with the spacing of the adjacent lattice planes of 0.278 nm,which is very closetothetheoreticalvalueof 0.275 nm.
Fig.1a shows the XRD pattern of the product prepared in the typical procedure.All diffraction peaks could be indexed to the monoclinic phase of CuO by comparison with the data from PDF card No.65-2309.No peaks of any other phases or impurities are detected.Fig.1b is the EDS spectrum of a typical sample.The peaks of Cu and O are found.No impurities peaks are detected except Au,which is due to the gold plating.
馬國平巡視著全連,語重心長地說:“是我這個當連長的,讓大家提出自己的愿望。四班長敢于提出自己的要求,是誠實的表現。再說,愛,也是人的本性,風華正茂的青年,愛慕美女也是應該的。特別是在生死未卜的戰場,表白自己的愛戀,是對腳下熱土的依戀、是對生命的眷戀。”
The temperature does not greatly influence the morphology of the product.When the reaction is carried out at 120℃for 1 min,the reaction rate is still fast and the product is flower-like CuO particles(Fig.4a and c).With the further decrease of the reaction temperature to 80℃,the product is also the flower-like particles(Fig.4b and d).But the formation is slow compared with that obtained at 150 and 120℃.

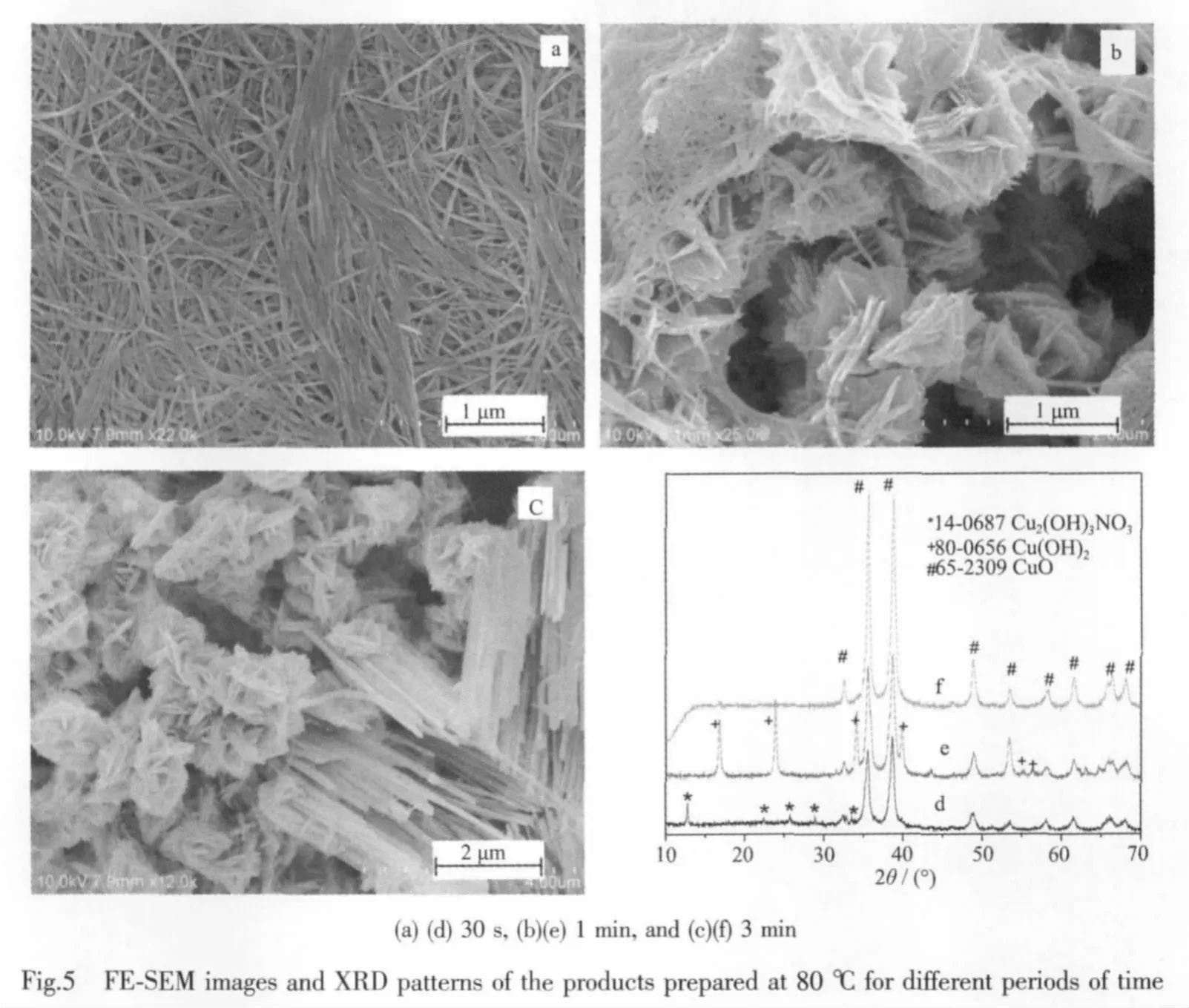
In order to study the crystal growth process,the time-dependent controlled experiments were performed.As the high reaction temperature would result in the fast formation of CuO,the crystal growth process could not be detected at 150℃in the typical procedure.In controlled experiments,lower reaction temperature (80℃)was adopted to decrease the reaction rate to investigate the crystal growth behaviors.When the reaction time was 30s at 80 ℃,the product was nanowires with 25~40 nm in width and several microns in length (Fig.5a).The XRD pattern(Fig.5d)shows that the product is a mixture of Cu2(OH)3NO3(monoclinic phase,PDF No.14-0687)and CuO(monoclinic phase,PDF No.65-2309).When the reaction time is extended to 1 min,some flowerlike particles appear and the major product is still nanowires(Fig.5b).Correspondingly,the phase of the product is changed (Fig.5e).The monoclinic phase of Cu2(OH)3NO3disappears and the product is a mixture of Cu(OH)2(orthorhombic phase,PDF No.80-0656)and CuO (monoclinic phase,PDF No.65-2309).Further extending the reaction time to 3 min,the nanowires disappear.The flower-like particles and nanowires bundles coexist in the product(Fig.5c).The product is CuO entirely and all of the impurities disappear(Fig.5f).When the reaction time is 10 min,the product is entirely the flower-like particles and the phase of monoclinic CuO had been maintained(Fig.4b and 4d).The experimental phenomenon shows that a typical Oswald ripening process is involved in the process from 3 min to 10 min.The possible reactions could be described as follows:
產生這種問題的原因除了對適用繼續盤問制度的錯誤理解和認識外,更多的原因是對相對人權益保護的意識不夠,流浪者、乞討者在沒有擾亂社會治安秩序的情況下,公安機關對其應當進行幫助,或及時與其他行政主管機關聯系,對相對人予以救助或對其合法權益予以保護。再有,對公安機關錯誤適用繼續盤問制度的監督檢查不夠。如果監督檢查到位,對流浪者、乞討者的所有處置措施都能夠納入到監督檢查的范圍內,這樣也會在某種程度上制約對繼續盤問范圍適用的擴大化。
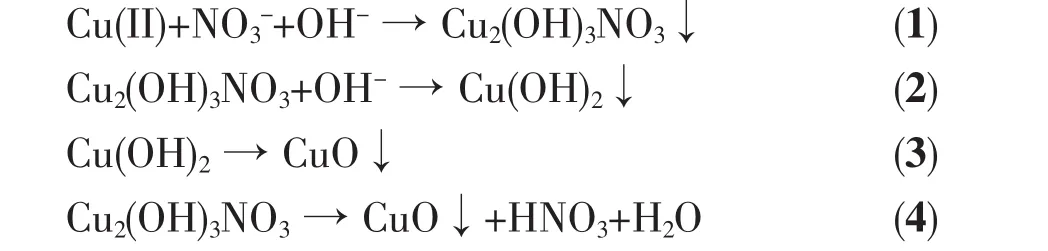
Firstly,Cu2+cation can coordinate with ammonia to form the[Cu(NH3)4]2+complex ions[21].Subsequently,[Cu(NH3)4]2+complex ions can react with OH-and NO3-to form the intermediate Cu2(OH)3NO3(eq.1).The Cu2(OH)3NO3has the botallackite-type layer structures[22].Two Cu2+cations have the different coordination and chemicalenvironments.In the alkaline condition,Cu2(OH)3NO3can react with OH-to form 1D Cu(OH)2(eq.2)[23].Thirdly,Cu(OH)2can decompose to produce CuO under the microwaveassisted hydrothermal condition (eq.3).Simultaneously,the Cu2(OH)3NO3might decompose to produce CuO directly(eq.4)[24].
3 Conclusions
In summary, we have prepared flower-like CuO via a simple microwave-assisted hydrothermal process.The results show that the alkali source could significantly influence the morphologies of the products.When ammonia or urea was used as the alkali source,the assembled flower-like nanostructures were obtained.When NaOH is used the products are leaf-like nanosheets.The formation process of flowerlike CuO shows that intermediates of Cu2(OH)3NO3and Cu(OH)2are involved in the reaction.
[1]Wang H,Shen Q,Li X P,et al.Langmuir,2009,25:3152-3158
[2]Gao X P,Bao J L,Pan GL,et al.J.Phys.Chem.B,2004,108:5547-5551
[3]Reitz J B,Solomon E I.J.Am.Chem.Soc.,1998,120:11467-11478
[4]Hsieh C T,Chen J M,Lin H H,et al.Appl.Phys.Lett.,2003,83:3383-3385
[5]Hou H W,Xie Y,Li Q.Cryst.Growth Des.,2005,5:201-205
[6]Hsieh C T,Chen J M.Appl.Phys.Lett.,2003,82:3316-3318
[7]Ni Y H,Li H,Jin L N,et al.Cryst.Growth Des.,2009,9:3868-3873
[8]Jiang X C,Herricks T,Xia Y N,Nano Lett.,2002,2:1333-1338
[9]Cao M H,Hu C W,Wang Y H,et al.Chem.Commun.,2003:1884-1885
[10]Liu B,Zeng H C.J.Am.Chem.Soc.,2004,126:8124-8125
[11]Wang H,Xu J Z,Zhu J J,et al.J.Cryst.Growth,2002,244:88-94
[12]Liang Z H,Zhu Y J.Chem.Lett.,2004,33:1314-1315
[13]Liang Z H,Zhu Y J.Chem.Lett.2005,34:214-215
[14]Wang W W,Zhu Y J,Cheng G F,et al.Mater.Lett.,2006,60:609-612
[15]Volanti D P,Orlandi M O,Andres J,et al.CrystEngComm,2010,12:1696-1699
[16]Volanti D P,Keyson D,Cavalcante L S,et al.J.Alloys Compd.,2008,459:537-542
[17]Zhang M,Xu X D,Zhang M L.J.Dispersion Sci.Technol.,2008,29:508-513
[18]Xu X D,Zhang M,Feng J,et al.Mater.Lett.,2008,62:2787-2790
[19]Xia J X,Li H M,Luo Z J,et al.J.Phys.Chem.Solid.,2009,70:1461-1464
[20]Sun Q B,Zeng Y P,Zuo K H,et al.J.Cryst.Growth,2011,324:1-6
[21]Lu C H,Qi L M,Yang J H,et al.J.Phys.Chem.B,2004,108:17825-17831
[22]Ruiz E,Liunell M,Cano J,et al.J.Phys.Chem.B,2006,110:115-118
[23]Park S H,Kim H J.J.Am.Chem.Soc.,2004,126:14368-14369
[24]Zhang Z K,Guo D Z,Zhang G M.J.Colloid Interface Sci.,2011,357:95-100
Microwave-Assisted Hydrothermal Preparation of Flower-Like CuO
CAO Xiao-Feng ZHANG LeiLI Zhao-Qian CHEN Xue-Tai*
(Coordination Chemistry Institute,State Key Laboratory of Coordination Chemistry,Nanjing National Laboratory of Microstructures,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,China)
The flower-like CuO was prepared via a simple,rapid,microwave-assisted hydrothermal process,using Cu(NO3)2·6H2O as the copper source and ammonia solution as the alkali source.No soft template such as surfactant or ionic liquid was used in the preparation.The phases and morphologies of the as-prepared products were characterized by using powder X-ray diffraction (XRD),energy dispersive X-ray spectroscopy (EDS),field emission scanning electron microscopy(FE-SEM)and high resolution transmission electron microscopy(HRTEM).The controlled experiments showed that the morphologies were influenced greatly by the alkali source.The timedependent experiments were performed to study the crystal growth process.The results also showed that the intermediates of Cu2(OH)3NO3and Cu(OH)2were involved in the reaction.
CuO;flower-like;microwave-assisted hydrothermal
O614.12;O614.24+1
A
1001-4861(2012)11-2373-06
2012-03-03。收修改稿日期:2012-05-29。
國家自然科學基金(No.21071078)資助項目。。
*通訊聯系人。 E-mail:xtchen@netra.nju.edu.cn;Tel:+86-25-83597147;Fax:+86-25-83314502;會員登記號:S06N8228M1004。

