一個基于雙(2-苯并咪唑基)剛性配體Zn(Ⅱ)配合物:合成、晶體結(jié)構(gòu)及光致發(fā)光性質(zhì)
鄧月義吳鴻志金燕玲劉法謙
(1橡塑材料與工程教育部重點實驗室,青島科技大學高分子科學與工程學院,青島 266042)
(2青島科技大學外國語學院,青島 266042)
一個基于雙(2-苯并咪唑基)剛性配體Zn(Ⅱ)配合物:合成、晶體結(jié)構(gòu)及光致發(fā)光性質(zhì)
鄧月義*,1吳鴻志2金燕玲1劉法謙1
(1橡塑材料與工程教育部重點實驗室,青島科技大學高分子科學與工程學院,青島 266042)
(2青島科技大學外國語學院,青島 266042)
合成了一個配合物[Zn(OBimB)Cl2]·DMF·H2O(OBimB為1,2-雙(2-苯并咪唑基)苯),并用X射線單晶衍射儀測定了其晶體結(jié)構(gòu)。晶體屬單斜晶系,P21/c空間群,晶胞參數(shù)為:a=1.251 22(9)nm,b=1.039 80(7)nm,c=1.827 94(11)nm,β=95.508(2)°,V= 2.367 2(3)nm3,Z=4,Dc=1.509 g·cm-3。最后精修結(jié)果為:R1=0.043 0,wR2=0.093 9。在配合物結(jié)構(gòu)中每個Zn(Ⅱ)原子分別與2個來自V構(gòu)型OBimB配體的N原子、2個氯離子進行配位,形成了1個扭曲的四面體結(jié)構(gòu)。配體和配合物的光致發(fā)光測試顯示光發(fā)射屬于配體的分子內(nèi)熒光。
鋅(Ⅱ)配合物;雙苯并咪唑;晶體結(jié)構(gòu);光致發(fā)光
0 Introduction
The coordination chemistry of bis(2-benzimidazole)compounds have attracted much attention in recent years,such as the usefulness as geometrically constraining ligands[1],supramolecularaggregatesformed with transition metal ions[2-3].Themetal complexes of bis(2-benzimidazole)compounds also reveal potentialapplications as new functional materials,such as optics[4-6],polymerization catalysis[7-8],magnetism[1,9], andmodels for biological systems[10-11].
The spacer X of bis(2-benzimidazole)can be flexible or rigid.In recent literature,a large number of coordination compounds with flexible bis(2-benzimdazolyl)alkanes ligands have been synthesized. Owing to the length of the bridging alkane chain,the anion and the solution,mononuclear[12-14],binuclear[2,15-17]andmultinuclear[3-4]complexes are formed.In contrast to flexible ligands,the studies about such complexes with bis(2-benzimidazolyl)ligands of rigid spacer are as yet very limited[1,18-19].In this paper,we reported the synthesis,crystal structure,and photoluminescent property of a new Zinc(Ⅱ)complexes with 1,2-bis(2-benzimidazolyl)benzene(OBimB),[Zn(OBimB)Cl2]· DMF·H2O.
1 Experimental
1.1 Materials and measurement
All the chemical reagents for synthesizing the ligand OBimB and the title complex were purchased commercially and used without further purification. Elemental analyses(C,H and N)were carried out on a Perkin-Elmer 1400C analyzer.IR spectra were recorded in the range of 400~4000 cm-1on a Nicolet 170SX spectrometer with pressed KBr pellets.UV-Vis spetra were taken on a Perkin-Elmer UV-Vis spectrometer.Luminescence spectra were measured on a Hitachi F-4600 fluorescence spectrophotometer.
1.2 Preparation of the comp lexes
The bidentate ligand 1,2-bis(2-benzimidazolyl) benzene(OBimB)was synthesized by condensing odiaminobenzene with o-phthalic acid according to the method of the literature[19]with minor revisions.odiaminobenzene(4.7 g,44mmol)and o-phthalic acid (3.3 g,20 mmol)were finely ground together and heated to 230℃in 40 mL polyphosphoric acid and stirred for 4 h.The reaction mixture was poured into ice water with vigorous stirring after the mixture had cooled down to100℃.The precipitatewas then filtered off and stirred in a saturated sodium bicarbonate solution for 12 h.The raw solid was recrystallized from hotmethanol to give light yellow product(Yield, 38%).IR(KBr):3 049(w),2 878(w),1 620(w),1 534 (w),1 441(s),1 317(m),1 280(m),1 007(m),764(s).
[Zn(OBimB)Cl2]·DMF·H2O:ZnCl2·2H2O(0.027 g,0.2 mmol)and OBimB(0.06 g,0.2 mmol)were added to ethanol(10 mL)and acetonitrile(10 mL), and the mixture was stirred and refluxed for half an hour.Then DMF(2 mL)was added.The resulting colorless solution was filtered and the filtrate was allowed to stay at ambient temperature for a period of about 2 d,to give colorless block crystals(yield:38%) suitable for structural determinations.Anal.Calcd.(%) for C23H23Cl2N5O2Zn:C 51.37,H 4.31,N 13.02;found (%):C 51.40,H 4.33,N 13.05.
2.3 X-ray crystallography
Block shaped crystals of the title complex was mounted on a Bruker SMART 1000 CCD area detector X-ray single crystal diffractometer with graphitemonochromated Mo Kαradiation(λ=0.071 073 nm) and aφ-ωscanning mode at 293(2)K.Intensities were corrected for Lorentz and polarization effects and empirical absorption.The structures were solved by directmethods via SHELXS 97 program[20]and refined by full-matrix least squares on F2via SHELXL 97 program[21].The correct positions for themetal atoms were deduced from E-map.Subsequent least-squares refinement and difference fourier calculations revealed the positions of the remaining non-hydrogen atoms. Non-hydrogen atoms were refined with independent anisotropic displacement parameters.H atoms were positioned geometrically(C-H=0.093 nm)and allowed to ride on their parent atomswith Uiso(H)=1.2 times Ueq(C).Crystalographic data for the complexes are listed in Table 1.
CCDC:861572.
2 Results and discussion
2.1 Description of the crystal structure
The ORTEP diagram of[Zn(OBimB)Cl2]of the title complex with the atomic numbering scheme isshown in Fig.1,respectively,showing 50%probability displacement ellipsoids.The selected bond lengths and bond angles are listed in Table 2.The center Zn atom in the title complex is coordinated by two N atoms from a OBimBmolecule and two Cl-anions in a distorted tetrahedralgeometry.The bond angles around Zn atom are in the range of 96.86(9)°~114.59(7)°.The Zn-N distances are almost similar(Zn-N(1)0.202 0(2) nm and Zn-N(3)0.200 3(2)nm.The Zn-Cl bond lengthsare0.22402(8)and 0.22255(9)nm,respectively. The OBimB ligand affords two coordination sites and chelates a Zn atom to form a seven-member ring, resulting in the construction of a mononuclear complex.The overall structure of the ligand can be described as twisted V-shaped conformation,so that the two coordinated N-atoms of the benzimidazoles are more exposed for metal binding.The dihedral angles of the benzimidazolyl ring planes(comprising imidazole ring atoms N1,C1,C6,N2,N7 is designed as plane 1 and N3,C14,C15,N4,C20,as plane 2) with the central phenyl ring plane(comprising atoms C8-C13)are 47.34°and 38.09°,respectively.The dihedral angle between the planes(plane 1 and 2)of the benzimidazolyl group in the ligand is 57.41°
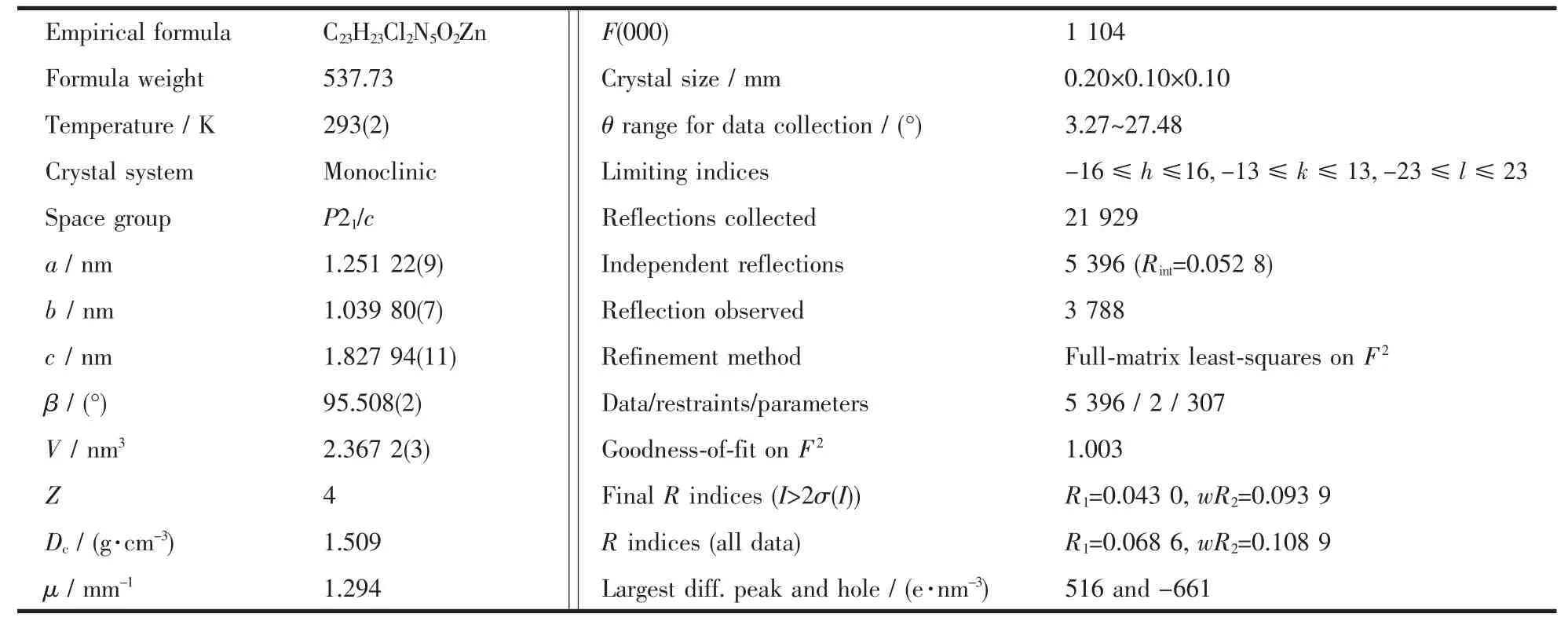
Table 1 Crystals and structures refinement data for the title comp lex
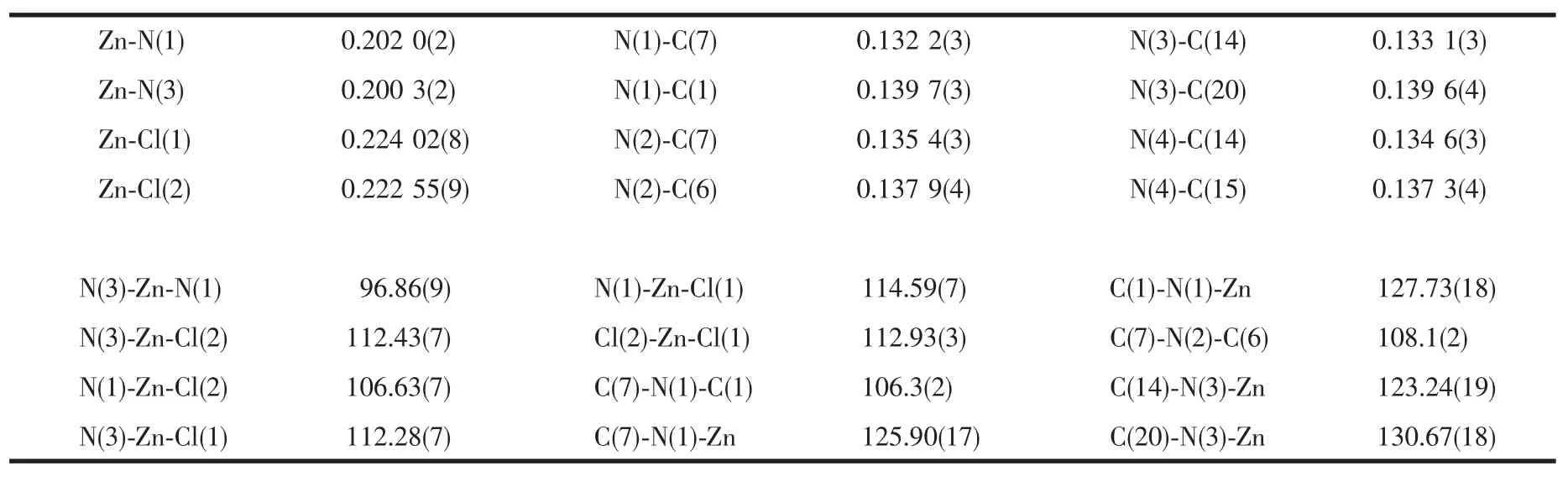
Table 2 Selected bond lengths(nm)and bond angles(°)for the complex
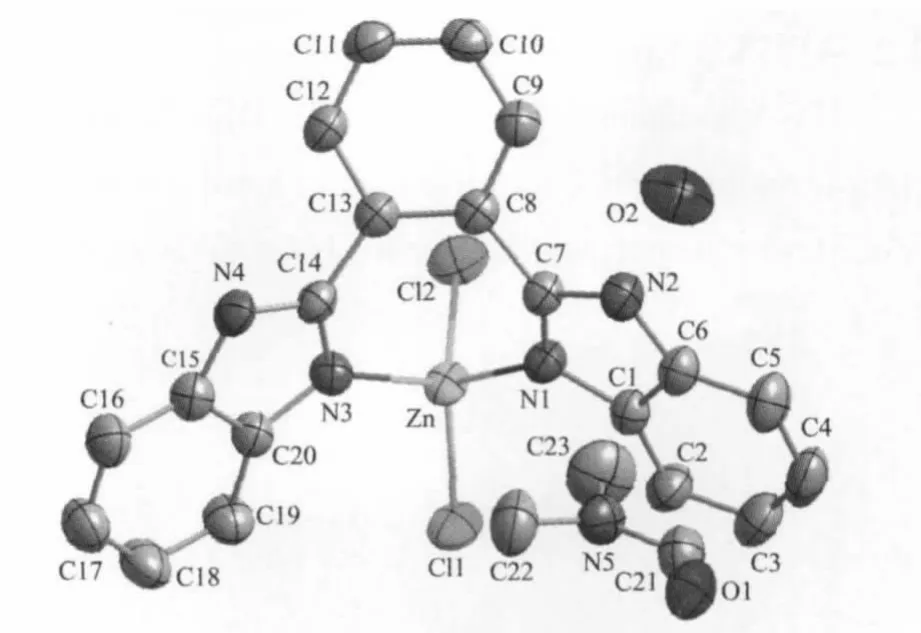
Fig.1 Structure of the title complex at50%probability displacement ellipsoids with atomic numbering scheme
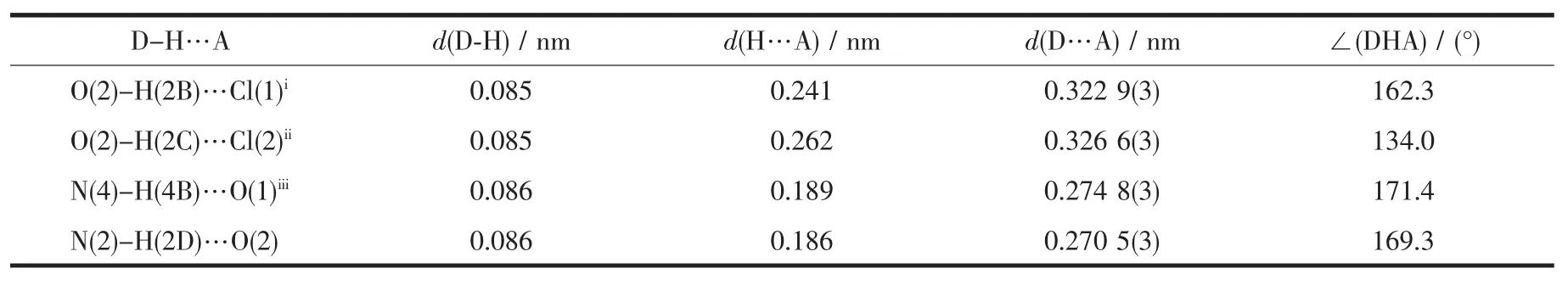
Table 3 Hydrogen bonds of the complex
In the complex,an amine NH group of the OBimB ligand is involved in hydrogen-bonding with H2Omolecule(N2-O2 0.270 5(3)nm),and therealsoexit weak hydrogen bonds between the H2O molecule and Cl-anion(O2-Cl1i0.322 9(3)nm;O2-Cl2ii0.326 63 nm; symmetry codes:ix,y-1,z;ii-x,-y+1,-z).These hydrogen bonds link the complex units into a onedimensional(1D)chain(Fig.2).The other amine NH group of the OBimB ligand is involved in hydrogenbonding with DMFmolecule(N4-O1iii0.274 8(3)nm, symmetry code:x,-y+1/2,z-1/2).
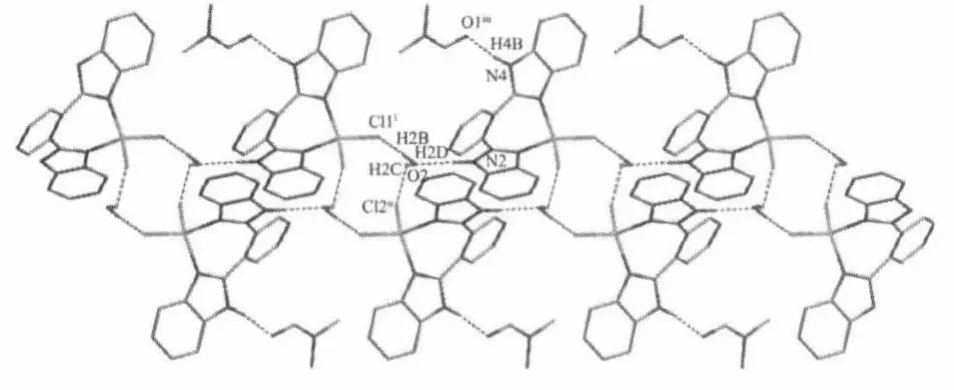
Symmetry code:ix,y-1,z;ii-x,-y+1,-z,iiix,-y+1/2,z-1/2Fig.2 1D chain structure formed by N-H…O andO-H…Cl hydrogen bonds interactions
2.2 UV-Vis Spectra
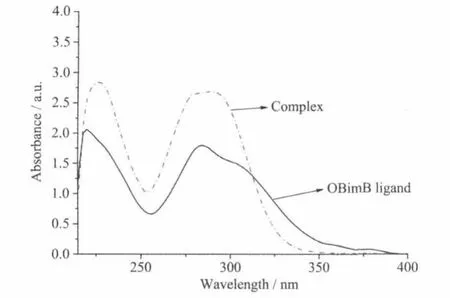
Fig.3 UV-Vis spectra of the complex and the OBimB ligand
UV-Vis spectra of the ligand ObimB and the complexes in ethanolsolution exhibitsimilar transitions (Fig.3),which suggests that theseabsorptionsare owned to the ligand.The two transitions can be assigned to intramolecular charge transfer transitions of the ligand and ascribed toπ→π*transitions in the ligand.The absorption peaks of the complexes(226 and 292 nm) are slightly red-shifted comparing to the ligand(222 and 284 nm),which shows that the transitions of the ligand are slightly disturbed by the coordination field of metal ion.The weak absorption band at 350~390 nm for the ligand is as a result of n→π*transitions in the ligands.The absorption of the complex at this band is almost disappear,which can be ascribe to the coordination of N atomswith Zn atom.
2.3 Photolum inescence
The emission spectra of the ligand and the complex were investigated in the solid state at room temperature.As shown in Fig.4,the ligand displays a broad emission band around 407.2 nm upon excitation at 270 nm.The complex exhibits an emission peak at 400.8 nm.The complex shows weak emission and the emission peak has a small blue-shift of 6.4 nm compared to that of the ligand,which can be as a result of the coordination of N atoms with Zn atom. Due to quite similar band profiles of the ligand and the complex,the emissions are tentatively assigned to the intraligand fluorescence[22].
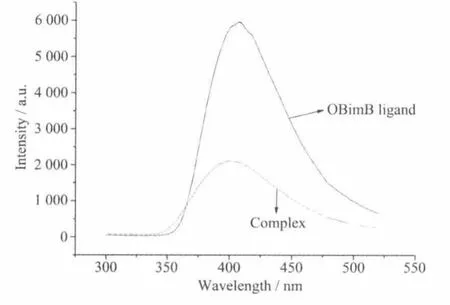
Fig.4 Emission spectra of the OBimB ligand and the complex in the solid state at room temperature
References:
[1]Stibrany R T,Lobanov M V,Schugar H J.Inorg.Chem., 2004,43:1472-1480
[2]Chen C L,Tan H Y,Yao JH,et al.Inorg.Chem.,2005,44: 8510-8520
[3]Meng F Y,Zhou Y L,Zou H H et al.J.Mol.Struct.,2009, 920:238-241
[4]Guo X F,Li Z F,Yue C,etal.Polyhedron,2010,29:384-390
[5]Obara S,Itabashi M,Okuda F,et al.Inorg.Chem.,2006, 45:8907-8921
[6]Wei L H,Babich JW,Ouellette W,et al.Inorg.Chem., 2006,45:3057-3066
[7]Stibrany R T,Schulz D N,Kacker S.Macromolecules,2003, 36:8584-8586
[8]Patil A O,Zushma S,Stibrany R T,et al.J.Polym.Sci.Part A Polym.Chem.,2003,41:2095-2106
[9]Galan-mascaros JR,Dunbar K R.Angew.Chem.Int.Ed., 2003,42:2289-2293
[10]Bouwman E,Driessen W L,Reedijk J.Coord.Chem.Rev., 1990,104:143-172
[11]Albada GA,van SmeetsW JJ,Spek A L.Inorg.Chim.Acta, 1999,288:220-225
[12]Albada G A,van Smeets W J J,Veldman N.Inorg.Chim. Acta,1999,290:105-111
[13]Albada G A,van Smeets W J J,Spek A L.Inorg.Chim. Acta,2000,299:35-40
[14]BernardinelliG,Kübel-Pollak A,Rüttimann S,etal.Chimia, 1992,46:155-158
[15]Bernardinelli G,Kübel-Pollak A,Rüttimann S,et al.Zeit. Krist.,1993,203:132-135
[16]Albada G A,van Lakin M T,Veldman N.Inorg.Chem., 1995,34:4910-4917
[17]Albada G A.Inorg.Chim.Acta,1997,259:27-30
[18]Stibrany R T,Potenza J A.Acta Cryst.,2008,C64:m213-216
[19]Carina R F,Williams A F,Bernardinelli G.Inorg.Chem., 2001,40:1826-1832
[20]Sheldrick GM.Acta Crystallogr.,Sect.A,1997,46:467-473 [21]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Refinement,University of G?ttingen,Germany, 1997.
[22]Seward C,Jia W L,Wang R Y,et al.Angew.Chem.Int. Ed.,2004,43:2933-2936
A Zinc(Ⅱ)Comp lex Based on a Rigid Ligand Containing Bis(2-benzim idazolyl)Groups: Synthesis,Crystal Structure,and Photolum inescent Property
DENG Yue-Yi*,1WU Hong-Zhi2JIN Yan-Ling1LIU Fa-Qian1
(1Key Laboratory of Rubber-plastics,Ministry of Education,College of Polymer Science and Engineering, Qingdao University of Science and Technology,Qingdao,Shandong 266042,China)
(2School of Foreign Languages,Qingdao University of Science and Technology,Qingdao,Shandong 266042,China)
A complexe[Zn(OBimB)Cl2]·DMF·H2O based on a rigid bis(benzimidazoles)ligand OBimB(OBimB= 1,2-bis(2-benzimidazolyl)benzene)has been synthesized and structurally characterized by X-ray single crystal diffractometry.The complex crystallizes in monoclinic system,P21/c space group with the cell parameters of a= 1.251 22(9)nm,b=1.039 80(7)nm,c=1.827 94(11)nm,β=95.508(2)°,V=2.367 2(3)nm3,Z=4,and Dc=1.509 g· cm-3,the final R1=0.043 0,wR2=0.093 9.In the structure of the complex the Zn atom is coordinated by two N atoms from a twisted V-shape conformational OBimB ligand and two Cl-anions in a distorted tetrahedral geometry.The photoluminescence measurements of the ligand and the complex show that the emissions are assigned to the intraligand fluorescence.CCDC:861572.
zinc(Ⅱ)complex;bis(benzimidazoles);crystal structure;photoluminescenc
book=2419,ebook=48
O614.24+1
A
1001-4861(2012)11-2463-05
2012-01-10。收修改稿日期:2012-05-16。
國家自然科學基金(No.20871072),山東省博士啟動基金(No.2007BS04023),青島科技大學博士啟動基金資助項目。
*通訊聯(lián)系人。E-mail:dengyyqust@hotmail.com

