以2-氯苯甲酸、5,5′-二甲基-2,2′-聯吡啶構筑的雙核鋱配合物的晶體結構和熒光性質
馬德運郭海福覃亮路寬潘勇
(肇慶學院化學化工學院,肇慶526061)
以2-氯苯甲酸、5,5′-二甲基-2,2′-聯吡啶構筑的雙核鋱配合物的晶體結構和熒光性質
馬德運*郭海福覃亮路寬潘勇
(肇慶學院化學化工學院,肇慶526061)
水熱條件下采用Tb(NO3)3·6H2O,2-氯苯甲酸和5,5′-二甲基-2,2-聯吡啶作為反應物合成出一個雙核鋱金屬配合物Tb2(2-cb)4(dmpy)2(NO3)2(2-cb=2-氯苯甲酸,dmpy=5,5′-二甲基-2,2′-聯吡啶)(1),并分別用元素分析,紅外光譜圖,差熱分析和X-射線單晶衍射等表征了該結構。晶體結構分析結果表明:化合物1為雙核鋱(Ⅲ)配合物,通過分子間的C-H…O氫鍵作用,雙核分子進一步被連接成二維平面結構。熱穩定性表明化合物1能夠穩定到280℃;熒光分析表明常溫固態下配合物1發射綠色熒光,熒光壽命為431.8μs。
鋱(Ⅲ)配合物;晶體結構;2-氯苯甲酸;5,5′-二甲基-2,2-聯吡啶;熒光性質
0 Introduction
The study of luminescent lanthanide metal complexes has gained great recognition over the last decade due to their superior functional properties and variety of potential applications[1-7].Lanthanide ions, especially Eu(Ⅲ)and Tb(Ⅲ),are excellent luminescent centers and their luminescent properties depend on organic ligands.Compared to first-row transition metals,lanthanide ions have a larger coordination sphere and more flexible coordination geometry,which make it even more difficult to control the structures. Thus,to be able to rationally design and construct lanthanide coordination polymers with predictedgeometries is still a great challenge,as many factors can affect the overall structural formation.A variety of fascinating lanthanide complexes have been constructed by carboxylate ligands and/or N-containing ligands as the auxiliary ligands[8-12]because of their ability to possess unusual structures and their sensitivity to molecular environments.2-chlorobenzoate(2-cb)is interesting in the field of coordination complexes due to its strong and various coordination modes.For example,Li et al.[13-14]obtained two dinuclear lanthanide complexes based on 2-cb and 2,2-bipy ligands.Zhang also successfully synthesized one dinuclear lanthanum complex involving 2-cb and 1,10-phenanthroline ligands[15].As part of an on-going study related to lanthanide metal carboxylates,we report the preparation and structural characterization of a new dinuclear lanthanide complex,Tb2(2-cb)4(dmpy)2(NO3)2(1).
1 Experimental
1.1 M aterials and measurements
All chemicals purchased were of reagent grade and used without further purification.All syntheses were carried out in 23 mL Teflon-lined autoclaves under autogenous pressure.Elemental analyses(C,H and N)were performed on a Perkin-Elmer 240 CHN elemental analyzer.Infrared spectra were recorded (4 000~400 cm-1)as KBr disks on Bruker 1600 FTIR spectrometer.Luminescence spectra and lifetime for crystal solid samples were recorded at room temperature on an Edinburgh FLS920 phosphorimeter. Thermogravimetry analyses(TGA)were performed on an automatic simultaneous thermal analyzer(DTG-60, Shimadzu)under a flow of N2at a heating rate of 10℃·min-1between ambient temperature and 800℃.
1.2 Synthese of 1
A mixture of Tb(NO3)3·6H2O(0.226 g,0.5 mmol), 2-chlorobenzoic acid(0.08 g,0.5 mmol),5,5′-dimethyl-2,2′-bipyridine(0.092 g,0.5 mmol)and H2O(15 mL)was stirred for 30 min in air and then sealed in a 23 mL Teflon reactor and kept under autogenous pressure at 150℃for 72 h.The mixture was cooled to room temperature at a rate of 5℃·h-1and colorless block crystals were obtained in a yield of 42%based on the 2-cb.Anal.Calcd.for C52H40Cl4N6O14Tb2(%):C,43.56;H,2.80;N,5.86. Found(%):C,43.50;H,2.82;N,5.89.IR(KBr pellet)(cm-1):3 470(s),1 617(s),1 475(m),1 428(m), 1 499(s),1 384(vs),1 307(m),1 215(w),1 087(w),1 024(w),808(m),761(m),705(w),616(s).
1.3 Crystal structure analysis
A single crystal with dimensions of 0.33 mm× 0.28 mm×0.22 mm wasmounted on a glass fiber for data collection which was performed on a Bruker Apex IICCD diffractometer operating at 50 kV and 30 mA using Mo Kαradiation(λ=0.071 073 nm)at room temperature.In the range of 2.00°<θ<25.2°,a total of 19923 reflections were collected,of which 4863 were unique(Rint=0.0268)and 3 993 observed ones(I>2σ(I)) were used in the succeeding structure calculations. Data collection and reduction were performed using the APEX II software[16].Multi-scan absorption corrections were applied for all the data sets using the SADABS[12]. The structurewas solved by directmethods and refined by fullmatrix least squares on F2using the SHELXTL program package[17].All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms attached to carbon were placed in geometrically idealized positions and refined using a ridingmodel.The final R=0.024 5 and wR=0.090 6(w= 1/[σ2(Fo2)+(0.046 7P)2+3.411 8P],where P=(Fo2+2Fc2)/3) for3993 observed reflectionswith I>2σ(I).S=1.124,(Δ/ σ)max=0.001,(Δρ)max=1 777 e·nm-3and(Δρ)min=-617 e· nm-3.Crystal parameters and details of the data collection and refinement are given in Table 1.Selected bond lengths and angles are given in Table 2.CCDC:865399.
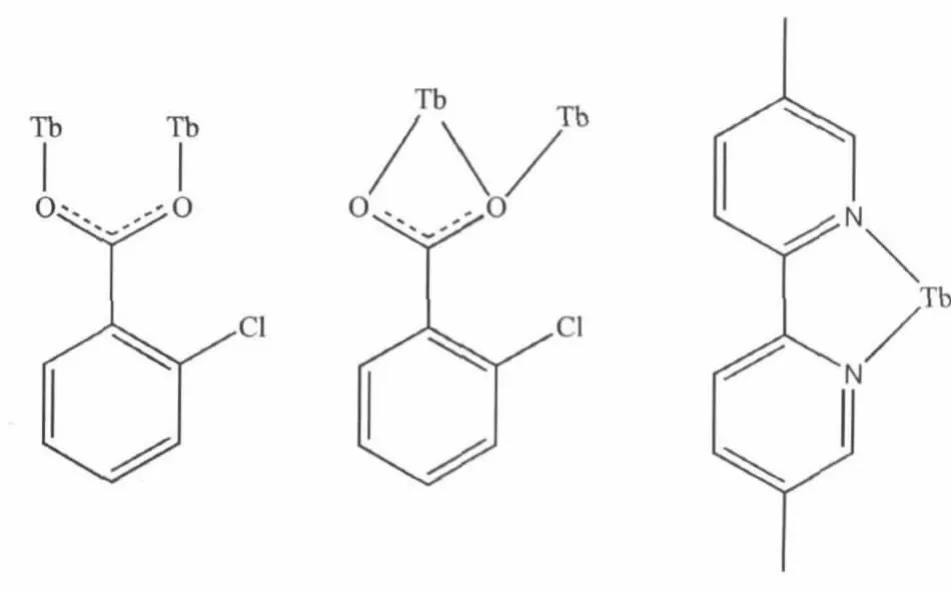
Scheme Coordinationmode of 2,4-dcp and dmpy ligands in the structures of 1
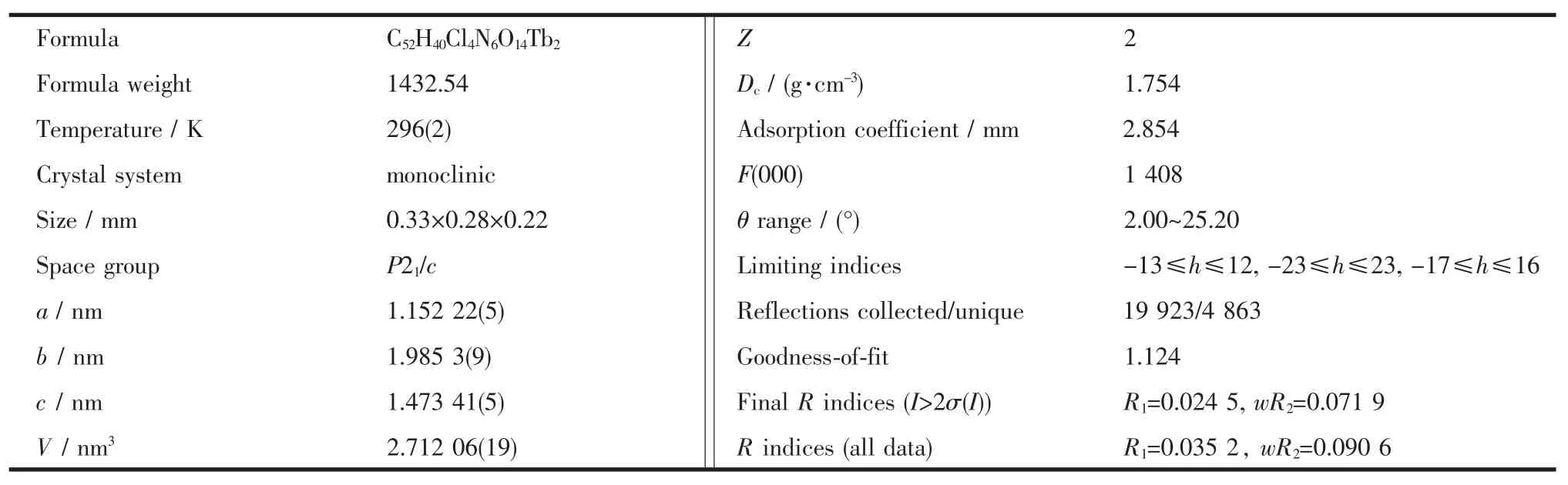
Table 1 Crystal data and structure refinement information for compound 1
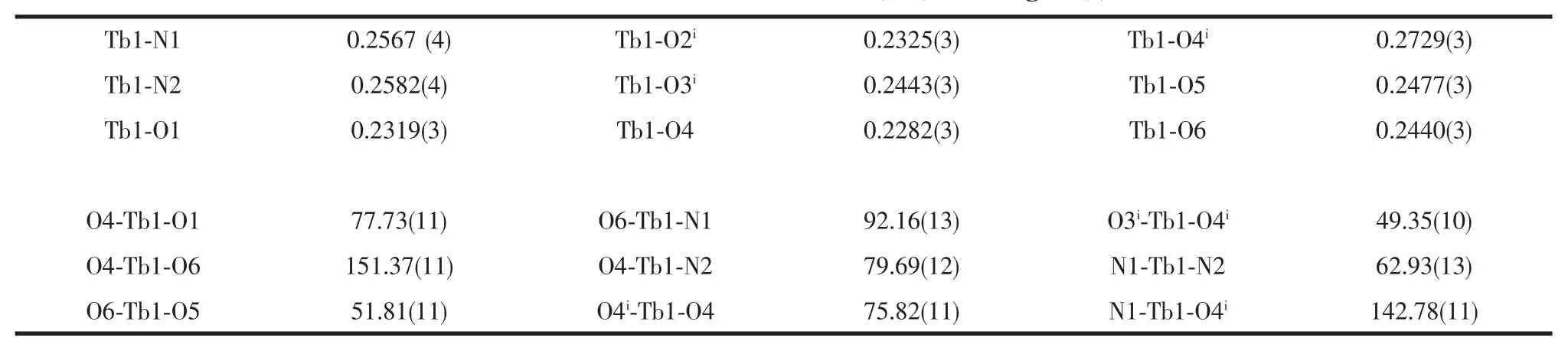
Table 2 Selected bond distances(nm)and angles(°)
2 Results and discussion
2.1 Structure description
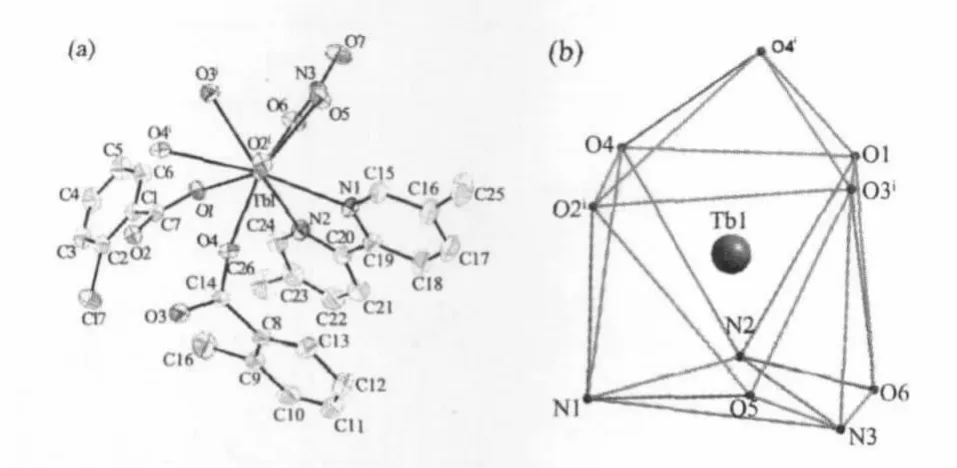
Fig.1(a)View of the asymmetric unitof 1.Non-H atoms are shown as 30%probability displacement ellipsoids;(b)Monocapped square antiprism (MSAP)geometry surronding a Tb(Ⅲ)atom of 1
Single-crystal X-ray diffraction analysis reveals that compound 1 is a centrosymmetric dinuclear structure and crystallizes in triclinic P21/c space group.Coordination environment of Tb(Ⅲ)atom is shown in Fig.1a.Each TbIIIion is nine-coordinated by five oxygen atoms from four 2-cb ligands,two oxygen atoms from one nitrate ligand and two nitrogen atoms from one dmpy ligand,adopting a distorted monocapped square antiprism(MSAP)geometry(Fig. 1b)with Tb-O,Tb-N bond lengths and O-Tb-O,O-Tb-N bond angles ranging from 0.2282(3)to 0.2729(3) nm and 49.35(10)to 151.37(11)°,respectively,all of which are within the range of those reported in other nine-coordinated Tb(Ⅲ)compounds with oxygen and nitrogen donating ligand[14,18-19].Differing from[Tb(2-cb)2·2,2-bipy]2(a)[14]and La2(phen)2(2-cb)6(b)[15],whose 2-cb ligands exhibited three coordination modes,2-cb coordinates to Tb(Ⅲ)in twomodes(scheme):bridging bidentate in the syn-syn configuration(Tb1-O1-C7 137.90(2)°and Tb1i-O2-C7 137.00(3)°)and chelatingbridging tridentate.The Tb…Tb separation of0.396 3(2) nm is shorter than that of a(La…La 0.419 nm)and b (Tb…Tb 0.3991 nm),indicative of no metal-metal interaction.The centrosymmetric dinuclear molecules are further connected into a layer through intermolecular C-H…O hydrogen bonding interactions (Fig.2).Moreover,intramolecular C-H…O hydrogen bonds are also observed.
2.2 IR spectra and TGA
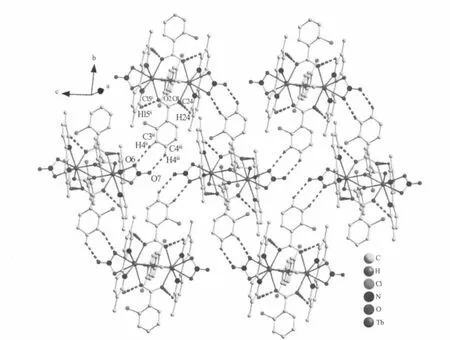
Fig.2 View of the 2D layer structure of 1 formed by C-H…O hydrogen bonds interactions
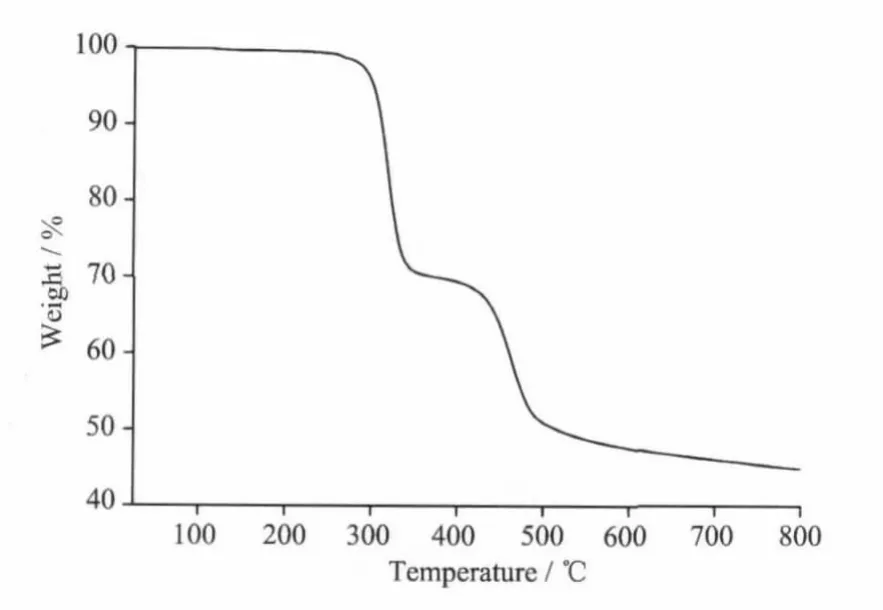
Fig.3 TGA trace of compound 1
The IR spectra of compound 1 shows broad℃·min-1.The TGA curve is depicted in Fig.3,which shows that 1 has thermal stability as no strictly clean weight loss step occurs below 280℃.The weight-loss step occurred above 280℃which corresponds to the decomposition of framework structure. bands in the 3470 cm-1,whichmay be assigned to the ν(O-H)stretching vibrations of the free watermolecules(impurity).The features at 1 617 and 1 475,1 428 cm-1are associated with the asymmetric(COO)and symmetric(COO)stretching vibrations.TheΔν(νas(COO-)-νs(COO-))values are 142 and 189 cm-1(between 105 and 200 cm-1),indicating the coordination of 2-cb with Tb(Ⅲ)in bridging bidentate mode[20],which iswell consistentwith X-ray diffraction structural analysis.
Thermal gravimetric analyses(TGA)were carried out to examine the thermal stability of 1.The samples were heated up in flowing N2with a heating rate of 10
2.3 Lum inescent properties
Solid-state excitation and emission luminescence spectra of 1 at room temperature is shown in Fig.4. The excitation spectrum for 1 showed a wide excitation band centered at 339 nm.The emission spectrum of 1 revealed the characteristic emission of Tb(III)with intense green-emission,attributed to5D4→7FJ(J=6,5,4,and 3)centered at 490,543,584,and620 nm,respectively.5D0→7F5is the strongest emission,indicating efficient ligand-to-metal energy transfer(LMCT)[21-22].The luminescent lifetimes of solid 1 using an Edinburgh FLS920 phosphorimeter with 450W xenon lamp as excitation source show lifetimes for 1 of 431.8μs(Fig.5),which could be used for light-emitting luminescentmaterials.
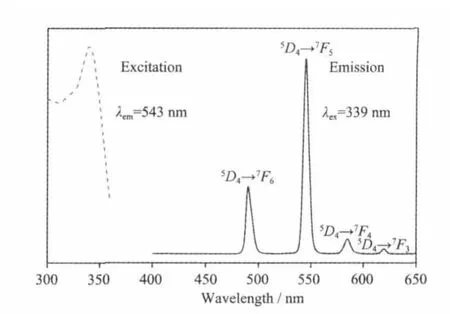
Fig.4 Excitation and emission spectra for 1 at room temperature
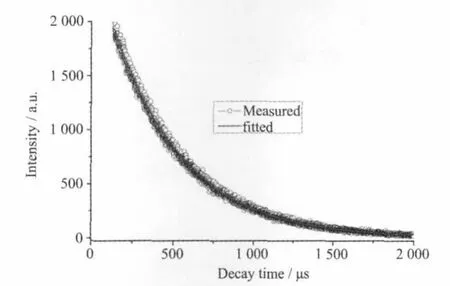
Fig.5 Luminescent lifetime of 1 in solid state
[1]Ma D,Wang W,Li Y,et al.CrystEngComm,2010,12: 4372-4377
[2]Tsukube H,Juanes S.Chem.Rev.,2002,102:2389-2403
[3]Bünzli JCG.Chem.Rev.,2010,110:2729-2755
[4]Smith P H,Brainard JR,Morris D E,et al.J.Am.Chem. Soc.,1989,111:7437-7443
[5]LiX,Sun H L,Wu XS,etal.Inorg.Chem.,2010,49:1865-1871
[6]Allendorf M D,Bauer C A,Bhakta R K,et al.Chem.Soc. Rev.,2009,38:1330-1352
[7]Chen B,Wang L,Zapata F,et al.J.Am.Chem.Soc.,2008, 130:6718-6719
[8]Liu JQ.J.Coord.Chem.,2011,64:1503-1512
[9]Ma D,Li Y,Li Z.Chem.Commun.,2011,47:7377-7379
[10]Lee J Y,Olson D H,Pan L,et al.Adv.Funct.Mater., 2007,17:1255-1262
[11]Deacon GB,Forsyth CM,Behrsing T,etal.Chem.Commun., 2002:2820-2821
[12]Seward C,Hu N X,Wang S.Dalton Trans.,2001:134-137
[13]Li X,Zhang T T,Ju Y L,et al.J.Coord.Chem.,2007,60: 2121-2132
[14]Li X,Ju Y L,Li Y Q.J.Coord.Chem.,2008,61:692-704
[15]Zhang B S,Z.Kristallogr.New Cryst.Struct.,2006,221:191-194
[16]Bruker.APEXII software,Version 6.3.1,Bruker AXS Inc, Madison,Wisconsin,USA,2004.
[17]Sheldrick GM.Acta Cryst.,2008,A64:112-122
[18]Wang Y,Zheng X,Zhuang W,et al.Eur.J.Inorg.Chem., 2003:3572-3582
[19]Yan B,Song Y S,Chen Z X.J.Mol.Struct.,2004,694:115-120
[20]Zheng Y,Xu D M,Liu S X.Inorg.Chim.Acta,1999,294: 163-169
[21]Yamada T,Shinoda S,Sugimoto H,et al.Inorg.Chem., 2003,42:7932-7937
[22]Petoud S,Cohen SM,Bunzli JC G,et al.J.Am.Chem. Soc.,2003,125:13324-13325
A Dinuclear Terbium(Ⅲ)Com plex Based on 2-Chlorobenzoate and 5,5′-Dimethyl-2,2′-bipyridine Ligands:Crystal Structure and Lum inescence
MA De-Yun*GUO Hai-Fu QIN Liang LU Kuan PAN Yong
(School of Chemistry and Chemical Engineering,Zhaoqing University,Zhaoqing,Guangdong 526061,China)
The reaction of 2-chlorobenzoic acid,5,5′-dimethyl-2,2′-bipyridine and Tb(NO3)3·6H2O under hydrothermal condition gave one new dinuclear terbium(Ⅲ)complex,Tb2(2-cb)4(dmpy)2(NO3)2(1)(2-cb=2-chlorobenzoate,dmpy=5,5′-dimethyl-2,2′-bipyridine),which has been characterized by elemental analysis,IR, TGA and single-crystal X-ray diffraction.Complex 1 is a dinuclear terbium(Ⅲ)complex,which is further extended to a 2D layered network by C-H…O hydrogen bonding interactions.The results of thermal analysis indicate that complex 1 is quite stable up to 280℃.1 emits the intensely green characteristic luminescence of Tb3+ion at room temperature,with lifetime of up to 431.8μs.CCDC:865399.
terbium complex;crystal structure;2-chlorobenzoate;5,5′-dimethyl-2,2′-bipyridine;luminescent
book=2012,ebook=65
O614.341
A
1001-4861(2012)11-2473-05
2012-02-13。收修改稿日期:2012-05-06。肇慶學院博士啟動基金資助項目。
*通訊聯系人。E-mail:mady@zqu.edu.cn

