不等彎孢菌HS-FG-257中一個新的苯甲酸類化合物
龐艷偉,王繼棟,向文勝,劉乾峰,王相晶*
1東北農(nóng)業(yè)大學(xué)生命科學(xué)與生物技術(shù)中心,黑龍江150030;2浙江海正藥業(yè)股份有限公司,浙江318000
Introduction
Fungi have been considered to be a rich source of natural products with interesting biological activities and a high level of biodiversity.It is well known that about 38%biologically active compounds originated from microorganisms are produced by fungi,and some of them are the most important products of the pharmaceutical industry,such as penicillins,cephalosporins,mevastatin and lovastatin[1,2].The species of Fungi Imperfeci are most abundant in the soil of which dematiaceous Hyphomycetes account for about or more than 50 percent of the fungal population.As one class of widely distributed dematiaceous hyphomycetes,Curvularia spp have attracted much attention for their ability to produce a variety of natural products such as modiolide and pyrone derivatives[3],11-(-methoxycurvularin and(S)-5-ethyl-8,8-dimethylnonanal[4],10-membered lactones[5]and melanin pigment[6]in addition to the dyestuff intermediate,cynodontin[7,8].In the effort to search for more pharmaceutically active metabolites from the genus of Curvularia,a chemical investigation was undertaken.In our primary work,we found that the crude extract from Curvularia inaequalis strain HS-FG-257 exhibited cytotoxicity against certain tumor cell lines.Subsequent isolation resulted in radicinin(4)as the major active component.To further investigate minor amount of constituents in this strain,the detailed fractionation of the crude extract led to the isolation of a new benzoic acid derivative,4-hydroxy-3-(3-methyoxy-3-methylbutyl)-benzoic acid(1),along with two known benzoic acid variants,4-hydroxy-3-(3-hy-droxy-3-methylbutyl)-benzoic acid(2)and 4-hydroxy-3-prenylbenzoic acid(3).
Experimental
General
1H and13C NMR spectra were recorded on a Bruker DRX-400(400 MHz for1H and 100 MHz for13C) spectrometer with tetramethylsilane(TMS)as internal standard;1H and13C NMR assignments were supported by1H-1H COSY,HMQC and HMBC experiments;The electrospray ionization mass spectrometry(ESIMS) and HR-ESI-MS spectra were taken on a Q-TOF Micro LC-MS-MS mass spectrometer;The UV spectra were obtained on a Varian CARY 300 BIO spectrophotometer;IR spectra were measured with a Nicolet Magna FT-IR 750 spectrometer(νmaxin cm-1);Melting point was measured using a Fisher-Johns micro-melting point apparatus;The analytic(Zorbax SB-C18,5 μm,250× 4.6 mm i.d)and semi-preparative(Zorbax SB-C18,5 μm,250×9.4 mm i.d)RP-HPLC were conducted on an Agilent 1100 series;Commercial silica gel(Qing Dao Hai Yang Chemical Group Co.,100~200 mesh) and sephadex LH-20 gel were used for column chromatography;Spots were detected on TLC under UV.
Fungal material
The producing strain HS-FG-257 was isolated from a soil sample collected from woodland of Heilongjiang Province,China.It was provided and identified as Curvularia inaequalis Shear by professor Tianyu Zhang at Shandong Agricultural University,China.The strain HS-FG-257 has been deposited in the Pharmaceutical Research Culture Collection,Zhejiang Hisun Group Co.,Ltd.
Fermentation,extraction and isolation
The strain was grown and maintainned on PDA slant and incubated for 6-7 days at 24oC.The stock culture was transferred into 1-L Erlenmeyer flasks containing 250 mL of the seed medium and incubated at 24°C for 24 h,shaken at 150 r.p.m.Then,1-L of the culture was transferred into a 50-L fermentor containing 30-L of the producing medium consisting of peptone 0.5%,soluble starch 0.5%,yeast extract 0.2%,NaCl 0.4%,KH2PO40.1%,MgSO4·7H2O 0.05%,CaCO30.2%(pH 6.2-6.4).The fermentation was carried out at 24°C for 7 days stirred at 100 r min-1with an aeration rate of 900 L of air per hour.The final 30 L of broth from 50 L fermentator was filtered and the resulting cake was washed with water(3 L)and subsequently extracted with MeOH(3 L).The supernate and the wash water were subjected to a Diaion HP-20 resin column eluting with 95% EtOH(5 L).The MeOH extract and the EtOH eluents were evaporated under reduced pressure to approximately 1 L at 50°C and the resulting concentrate was extracted three times using an equal volume of EtOAc.The combined EtOAc phase was concentrated under reduced pressure to yield a mixture(15 g).
The mixture was subjected to a Sephadex LH-20 gel column eluted with CHCl3-MeOH(1∶1,v/v)and detected by TLC to give five fractions(Fr.1 to Fr.5) .The Fr.5 was subjected to a silica gel column and eluted with a stepwise gradient of CHCl3-MeOH(100∶0-50∶50,v/v)to obtain four fractions(Fr.5-1,F(xiàn)r.5-2,F(xiàn)r.5-3 and Fr.5-4)based on the TLC profiles.The Fr.5-3 was further subjected to a Sephadex LH-20 column and eluted with CHCl3-MeOH(1∶1,v/v).During this step,three fractions were obtained(Fr.5-3-1,F(xiàn)r.5-3-2 and Fr.5-3-3).Fr.5-3-3 was isolated by semi-preparative HPLC eluting with CH3CN-H2O(40∶60,v/v)to obtain compounds 1(tR12.9 min,1.7 mg)and 2(tR7.9 min,3.2 mg).The Fr.5-2 was further purified by semi-preparative HPLC using CH3CNH2O mixture(50∶50,v/v)to obtain compound 3(tR12.6 min,167 mg).The Fr.4 was chromatographed on a silica gel and successively eluted with a stepwise gradient of CHCl3-MeOH(100∶0-70∶30,v/v)to obtain three fractions Fr.4-1 to Fr.4-3 based on the TLC profiles.Fr.4-1 was fractionated by semi-preparative HPLC eluting with CH3CN/H2O(40∶60,v/v)to yield compound 4(tR9.9 min,553 mg).
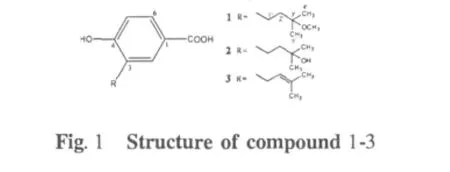

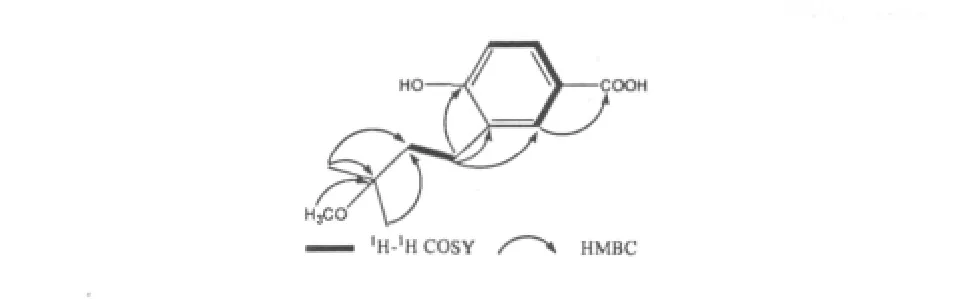
Fig.3 Key1H-1H COSY and HMBC correlations of 1
Identification
4-Hydroxy-3-(3-methyoxy-3-methylbutyl)-benzoic acid(1)
White powder;mp 159-160℃;UV(EtOH)λmaxnm 255(log ε 3.72),208(log ε 4.11);IR(KBr):vmax3421,2973,1683,1607,1540,1456,1385,1275, 1064,778 cm-1;1H and13C NMR data see Table 1;ESIMS m/z 237[M-H]-;HRESIMS m/z 261.1104[M +Na]+(calcd for C13H18O4Na,261.1097).
4-Hydroxy-3-(3-hydroxy-3-methylbutyl)-benzoic acid(2)
White powder;1H and13C NMR data see Table 1;ESIMS m/z 223[M-H]-.
4-Hydroxy-3-prenylbenzoic acid(3)
White powder;1H and13C NMR data see Table 1;ESIMS m/z 205[M-H]-.
Radicinin(4)
White powder;1H NMR(400 MHz,DMSO-d6):δ 6.73 (1H,m,H-10),6.32(1H,s,H-8),6.27(1H,dd,J =15.6,1.6 Hz,H-9),5.87(1H,d,J=5.2 Hz,3-OH),4.55(1H,m,H-2),3.97(1H,dd,J=10.8,5.2 Hz,H-3),1.91(3H,dd,J=7.0,1.6 Hz,H3-11),1.45(3H,d,J=6.3 Hz,H3-12);ESIMS m/z[M+H]+237.
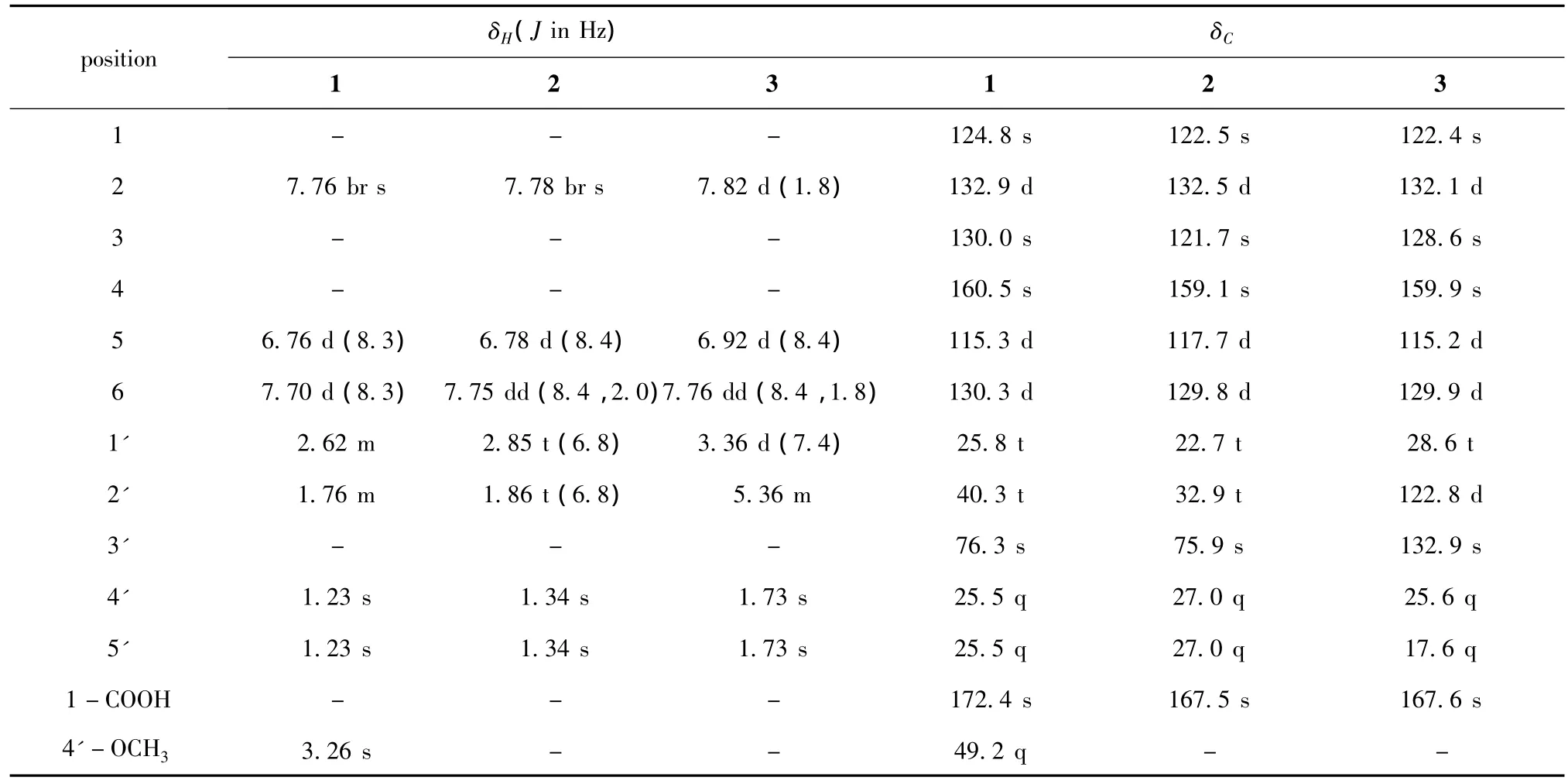
Table 1 1H and13C NMR data for compounds 1-3(1 in methanol-d4,2,3 in acetone-d6)
Results and Discussion
Compound 1 was isolated as white amorphous powder.The molecular formula of 1 was determined to be C13H18O4on the basis of the HRESIMS m/z 261.1104[M+Na]+(calcd for C13H18O4Na,261.1097)in conjunction with NMR data(Table 1).Its IR spectrum showed a broad absorption band for the hydroxy group (3421 cm-1).The1H NMR spectrum of 1 showed the signals of a trisubstituted benzene ring at δ 7.76(1H,br s),6.76(1H,d,J=8.3 Hz),7.70(1H,d,J= 8.3 Hz),two overlapped singlet methyls at δ 1.23 (6H,s),two methylene signals at δ 1.76(2H,m),2.62(2H,m)and a methoxy at δ 3.26(3H,s).Its13C NMR and DEPT data revealed a carboxy carbonyl carbon at 172.4(s),three aromatic methines at δ 132.9(d),130.3(d),115.3(d),three aromatic quaternary carbons(one oxygenated)at δ 124.8(s),130.0(s),160.5(s),an oxygenated aliphatic quaternary carbon at δ 76.3(s),2 aliphatic methylene carbons at δ 25.8(t),40.3(t),one methoxyl carbon at δ 49.2(q)and two methyls at δ 25.5(q).The observed crossing peak of H-1'(δ 2.62)and H-2'(δ 1.76)in the1H-1H COSY spectrum and the HMBC correlations(Figure 3)from H3-4'and H3-5'to C-3' (δ 70.6),from the methoxy proton signal at δH3.26 to C-3'indicated the presence of a 3-methoxy-3-methylbutyl moiety in 1.Considering the molecular formula of 1,a hydroxyl and a carboxy group were presented in 1 in addition to the trisubstituted benzene ring and the 3-methoxy-3-methylbutyl moieties.The HMBC correlations between H-1'and C-2,C-3,C-4,between H-2 and the carboxy group established the structure of 1 as shown in Figure 1.
Compound 2 was obtained as a white amorphous powder.Comparison of the1H and13C NMR data of 2 with those of 1,suggested that 2 was structurally similar to 1.The only difference between 2 and 1 was that the methoxy group of C-4'in 1 was replaced by a hydroxy group in 2.Thus,compound 2 was elucidated as 4-hydroxy-3-(3-hydroxy-3-methylbutyl)-benzoic acid[9].
Compounds 3 and 4 were readily identified as 4-hydroxy-3-prenylbenzoic acid and radicinin,respectively,by comparing the NMR spectral data with those reported in literatures[10,11].
1 Demain AL,Sanchez S.Microbial drug discovery:80 years of progress.J Antibiot,2009,62:5-16.
2 Cragg GM,Newman DJ.Biodiversity:a continuing source of novel drug leads.Pure Appl Chem,2005,77:7-24.
3 Trisuwan K,Rukachaisirikul V,Phongpaichit S,et al.Modiolide and pyrone derivatives from the sea fan-derived fungus Curvularia sp PSU-F22.Arch Pharm Res,2011,34: 709-714.
4 Busi S,Peddikotla P,Upadyayula SM,et al.Secondary metabolites of Curvularia oryzae MTCC 2605.Rec Nat Prod,2009,3:204-208.
5 Greve H,Schupp PJ,Eguereva E,et al.Ten-membered lactones from the marine-derived fungus Curvularia sp.J Nat Prod,2008,71:1651-1653.
6 Umnova EF,Vorobyova LI.The peculiarities of melanin genesis in fungus Curvularia lunata.Biol Nauki(Moscow),1992,3:122-126.
7 Hobson DK,Edwards RL,Wales DS.Cynodontin:a secondary metabolite and dyestuff intermediate.J Chem Technol Biotechnol,1997,70:343-348.
8 Hobson D,Wales DS.'Green'dyes.J Soc Dyers Colour,1998,114:42-44.
9 Dey SP,Dey DK.Acid-catalyzed rearrangements of allyl 4-hydroxybenzoate and 3-methyl-but-2-enyl-4-hydroxybenzoate.J Indian Chem Soc,2009,86:485-487.
10 Abraham WR,Arfmann HA.Hydroxy-(methylbutenynyl)-benzoic acid and derivatives from curvularia fallax.Phytochemistry,1990,29:2641-2644.
11 Ohms G,Porzel A,Zepter R,et al.2D-1H-NMR-spektren von radicinin.Z Chem,1986,26:402-403.

