小葉錦雞兒花提取物和不同極性黃酮組分的抗氧化活性
牛 宇,張麗珍,牛 偉,陳旺暉,鄧 奇,柴 磊,蒙秋霞
1山西省農業科學院農業資源與經濟研究所;2山西大學生命科學學院; 3山西省農業科學院;4山西省農業科學院農業環境與資源研究所,太原030006
Introduction
Also known as Xiao-ye-jin-que-hua,Caragana microphylla Lam.is a deciduous shrub belonging to the genus Caragana of the Fabaceae family.The species are mainly distributed in the prairie and desert regions in northern and southern-east China,forming large areas of shrubby-grassland in grazing pastures.The shrub is branchy and flowery,with well-developed nodulated roots and high resistance against drought,cold,heat,salinity and alkalinity,and is therefore an important species for revegetation and biological construction in arid and semi-arid areas of China[1].At present,year-round industrial seedling rearing has been applied to C.microphylla and it has been widely grown in arid,semi-arid and saline-alkaline areas of northern Shanxi.With a large biomass,the shrub has become important livestock forage and nectariferous plant[2].The entire plant of C.microphylla is used as Ning-tiao in traditional Chinese medicine.Its flowers,which are called Ningtiao-hua in traditional Chinese medicine(TCM),can nourishes yin and blood,and tranquilize liver.They are consequently used for the treatment of hypertension,dizziness,shortness of breath,debility and fatigue in TCM,besides being consumed as vegetable by the local people[3].
A large body of literature shows that the generation and excessive accumulation of free radicals in human body are closely associated with the aging of organs and many health conditions such as cardio-vascular diseases and cancer,and the prevention of these diseases can be facilitated by enhancing the free radical-clearance or antioxidative ability in human body[4-6].There will be increased incidences and mortality of cancer and other geriatric diseases due to the accelerative aging progression worldwide.To prevent the occurrence of geriatric diseases,slow down the aging progression of human body,and relieve the burden of patients’families and the society,it is of great significance to search for new and effective free radical scavengers or antioxidants.Flavonoids are an important class of antioxidant,whose antioxidative activity has been proved by enormous scientific studies[7,8].Currently the antioxidative activity of flavonoids from the flowers of C.sinica and the aerial part of C.leucophloea and C.acanthophylla has been studied[9,10],whereas the antioxidative activity of other Caragana species,including C.microphylla,has not been reported.Our previous studies have showed that different parts of C.microphylla contain flavonoids,and a high content of flavonoids was found in the flowers[11].Therefore it is necessary to study the separation method and antioxidative activity of flavonoid-containing fractions flowers of C.microphylla in an attempt to separate highly antioxidant products from the plant and enhance its development as a medicinal plant.
It has been reported that the correlation between the polarity of the flavonoid fractions and their antioxidative activity varies with the botanical source of the fractions and the free radical generation system adopted in the experiment.There is a positive correlation between the polarity of the flavonoid fractions from corn silk and their antioxidative activity in peroxidation of polyunsaturated fatty acids(PUFA)induced by Fe2+,but there is no correlation between the polarity of the flavonoid fractions and their antioxidative activity in hydroxyl free radical generation system[12].For flavonoid fractions of Caulis polygoni Multiflori,no correlation was found between the polarity of the flavonoid fractions and their antioxidative activity[13].The antioxidative activity of flavonoids with different polarity from flowers of C.microphylla has not previously reported.To obtain flavonoids with high antioxidative activity from the flowers of C.microphylla,it is therefore necessary to determine the relationship between the polarity of the flavonoid fractions from the flowers and their antioxidative activity.In this work,crude flavonoid extract of C.microphylla flowers was subjected to liquid-liquid partitioning with stepwise gradient of solvents after ultrasound crude extraction,the total flavonoid content and various antioxidative activities of the crude extract and fractions with different polarity were determined for the first time,and the correlations between the antioxidative activities and the polarity of the fractions were analyzed.The study will provide experimental evidence in search of safe and high-efficacy plant-derived antioxidants from C.microphylla flowers.
Material and Methods
Material and reagents
The fresh flowers of Caragana microphylla Lam.were collected in May 2010 from the experimental field of Institute of Agricultural Environment and Resources,Shanxi Academy of Agricultural Sciences,Taiyuan.1,1-Diphenyl-2-picrylhydrazyl(DPPH·)Free Radical Kit,Total Antioxidative Capacity(T-AOC)Kit,Generation and Detection of Scavenging Activity of Hydroxyl Free Radical Kit and Generation and Detection of Scavenging Activity of Superoxide Anion Radical Kit were purchased from Jiancheng Biological Research Center,Nanjing.Rutin,methanol,n-hexane,ethanol,dichloromethane,ethyl acetate and acetic acid were domestic analytical-grade products.
Methods
Ultrasound extraction and liquid-liquid partitioning of flavonoids from C.microphylla
The flowers of C.microphylla were washed,dried at 50℃,pulverized and sieved through a 40-mesh screen.The sieved powder(1.0 kg)was subjected to ultrasound extraction with n-hexane at 50℃(30 min×2).The n-hexane preparations were combined,filtered,evaporated in vacuo and dried to yield hexane fraction.The residue was dried at 40℃ to remove residual nhexane,and was subjected to ultrasound extraction with 95%(v/v)ethanol(30 min×2)and 60%(v/v) ethanol(30 min×2)at 50℃.The ethanolic preparations were combined,filtered,evaporated in vacuo and dried to yield crude extract.The crude extract was resuspended in distilled water and fractioned with dichloromethane and ethyl acetate respectively.The resulted fractions and residual water phase were evaporated in vacuo and dried to yield dichloromethane,ethyl acetate and water fractions.The crude extracts and the fractions were kept in freezer at-20℃ for further tests.Before testing,0.05 g extract or fraction of C.microphylla and 1 mL methanol were transferred in a centrifuge tube.Ultrasound was applied to make a 0.05 g/mL solution.The solution was centrifuged at 5000 rpm for 5 min at 4℃.The supernatant was used for the assays.
Determination of total flavonoid content
The concentration of flavonoids in samples was determined with sodium nitrite-aluminum nitrate colorimetric method.The rutin standard curve was prepared as described in literature[12].The sample of crude extract or fractions was dissolved in appropriated solvents to prepare a 100 mg/mL solution.An appropriated volume of solution was added into 6 10-mL tubes,respectively.Solution in Tube 0 was made up precisely to 10 mL with 60% ethanol and no colorimetric solution was added.For tubes 1 to 5,each tube was added with 0.5 mL 5%NaNO3solution,shook and sit for 6 min,then added with 0.5 mL 5%Al(NO3)3solution,shook and sit for another 6 min.Each tube was subsequently added with 4 mL 4%NaOH solution,shook and made up precisely to 10 mL with 60%ethanol,shook and sit for 5-15 min before subjected to colorimetric test.The absorbance of the 5 replicates was measured at 506 nm using Tube 0 as blank.The content of flavonoid was calculated according to linear regression equation of the rutin standard curve.
Determination of diphenylpicrylhy-drazyl radical(DPPH·)scavenging activity
Preparation of DPPH solution:As described in the instructions of the DPPH Free Radical Kit,0.0197 g reagent was dissolved in precisely 250 mL methanol to make a 2×10-4moL·L-1DPPH standard solution according to the instructions of the kit.Light was avoided during the testing process.
Determination of sample solutions:Sample solutions were diluted serially in 10-mL centrifuge tubes to make two identical groups of sample solutions with the indicated concentrations in triplicate.Each tube of the sample solutions was added with 2 mL methanol.Each tube of one group was then added with 2 mL DPPH standard solution(whose absorbance was recorded as AS),whereas each tube of another group was added with 2 mL methanol(whose absorbance was recorded as Ar).A separate centrifuge tube was added with 4 mL methanol(Tblank),and another centrifuge tube was added with a mixed solution of 2 mL methanol and 2 mL DPPH standard solution(whose absorbance was recorded as A0).The tubes with DPPH solution were wrapped with aluminum foil to avoid the interference of light.All tubes were sit for 30 min for reaction before their absorbance was measured at 517 nm using Tblankto zero set the spectrophotometer.The scavenging rate of samples was calculated with the following formula:

Determination of total antioxidative capacity
The solutions of reagents were prepared according to the instructions of the Total Antioxidative Capacity(TAOC)Kit.The sample and reagent solutions were added into test tubes in triplicate in an order shown in Table 1.The solutions in all test tubes were well mixed and sit for 10 min before their absorbance(OD)was measured at 520 nm using distilled water to zero set the

Table 1 Addition of sample and reagent solutions in determination of T-AOC
spectrophotometer.T-AOC was represented in units (U).One unit represents the increase of 0.01 in absorbance caused by 1 mg·mL-1of sample inthe reaction system per minute at 37 oC.The T-AOC of samples was calculated with the following formula:
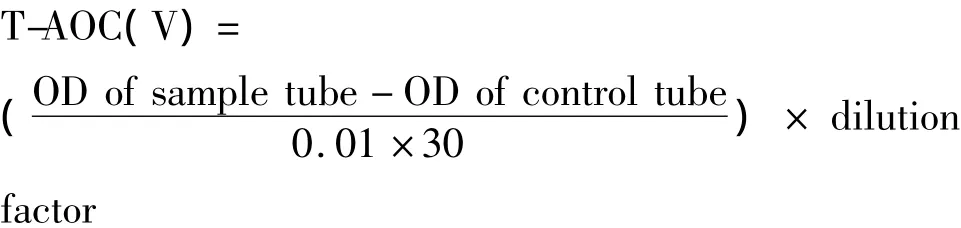
Determination of hydroxyl free radical scavenging activity The solutions of reagents were prepared according to the instructions of the Generation and Detection of Scavenging Activity of Hydroxyl Free Radical Kit.The sample and reagent solutions were added into 5-mL test tubes in triplicate in an order shown in Table 2.Solution in each tube were mixed and reacted at 37 oC in water bath for exactly 1 min before the addition of 2 mL color developing reagent to stop the reaction.The tubes were mixed and sit for 20 min at room temperature before their absorbance(OD)was measured at 550 nm using distilled water to zero set the spectrophotometer.The scavenging rate of hydroxyl free radical was calculated with the following formula:


Table 1 Addition of sample and reagent solutions in determination of scavenging activity of hydroxyl free radical
Determination of superoxide anion radical scavenging activity The solutions of reagents were prepared according to the instructions of the Generation and Detection of Scavenging Activity of Superoxide Anion Radical Kit.The sample and reagent solutions were added into 5 mL test tubes in triplicate in an order shown in Table 3.The tubes were mixed and sit for 10 min at room temperature before their absorbance(OD)was measured
at 550 nm using distilled water to zero set the spectrophotometer.The relative scavenging rate of superoxide anion radical of samples was calculated with the following formula:


Table 3 Addition of sample and reagent solutions in determination of scavenging activity of superoxide anion radical
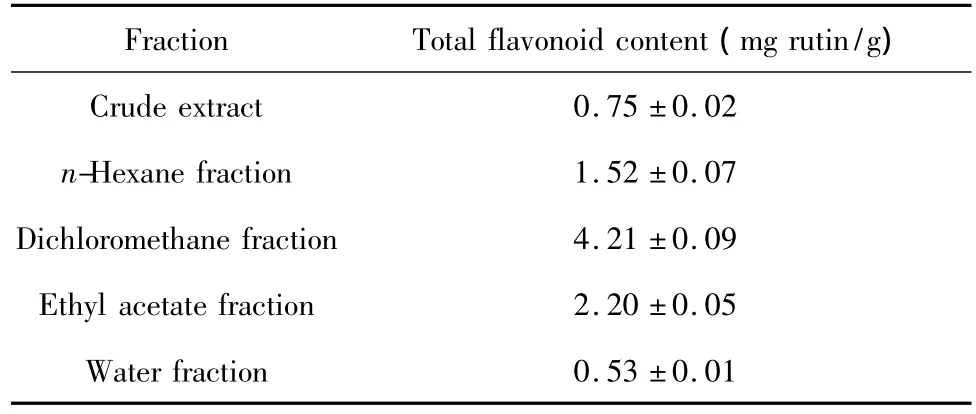
Table 4 Total flavonoid contents in crude extract and fractions of C.microphylla flowers with different polarity
Data analysis
Statistical analyses of all data were conducted with Excel 2007 and PASW Statistics 18.The concentrations at which flavonoid fractions scavenged 50%of DPPH· (EC50)and the P values of two-tailed Pearson correlation between the variables were calculated in PASW Statistics 18.
Results and Discussion
Results
Contents of total flavonoid in fractions of C.microphylla flowers
The linear regression equation of rutin standard curve was obtained as y=16.139x-0.032(R2=0.9948).Here,y was the absorbance of rutin standard solution;x was the concentration of rutin standard solution.The contents of flavonoids in fractions of C.microphylla flowers with different polarity were summarized in Table 4.Dichloromethane fraction contained the highest content of flavonoids,followed by Ethyl acetate fraction and n-hexane fraction.Water did not dissolve high concentration of flavonoids and the total flavonoid content in water fraction was lower than that of crude extract.
Scavenging activity of DPPH·free radical
The scavenging activities of flavonoids in crude extract and fractions of C.microphylla flowers with different polarity were illustrated in Fig.1.The concentrations at which thefractions scavenged 50% of DPPH· (EC50)were listed in Table 5.As shown in Figure 1,ethyl acetate and dichloromethane fractions showed the highest scavenging activity of DPPH·,followed by crude extract and water fraction.n-Hexane fraction had the lowest DPPH·scavenging capacity.These suggest that the flavonoid fractions of C.microphylla flowers with moderate polarity have higher DPPH·scavenging than the fractions with extremely high or low polarities.Here,the polarity index of solvents n-hexane,dichloromethane,ethyl acetate and water was designated as 0.0,3.1,4.4,9.0,respectively[14],and a two-tailed Pearson’s correlation analysis was performed between the polarity index and the EC50values of the DPPH· scavenging activity of the four fractions.The results showed that no significant correlation was found between the polarity index and the EC50s of the fractions (P=0.733).These results indicate that fractions with moderate polarity appeared to have high scavenging activity of DPPH·,but the DPPH·scavenging activity is not significantly related to the polarity of the fractions.

Fig.1 The DPPH·scavenging activity of extract and fractions of C.microphylla flowers

Table 5 EC50values of the DPPH· scavenging activity of flavonoid fractions of C.microphylla flowers with different polarity
Total antioxidative capacity
Antioxidants can reduced Fe3+to Fe2+,which reacts with phenanthroline compounds to form stable colored complex.Colorimetric method can therefore be used to determine the antioxidative activity of samples.A high U value of total antioxidative capacity(T-AOC)of a fraction here suggests a high reducing activity.As shown in Table 6,the order of T-AOC of flavonoid extract and fractions of C.microphylla flowers with different polarity was water fraction>dichloromethane fraction>crude extract> ethyl acetate fraction > nhexane fraction.Of the four fractions,there is a positive correlation between the T-AOC and their polarity(P= 0.020),i.e.the higher the polarity of a fraction,the stronger its T-AOC was.

Table 1 T-AOC of extract and flavonoid fractions of C.microphylla flowers
Hydroxyl free radical scavenging activity
Fenton reaction is a typical reaction for generation of hydroxyl free radicals.The quantity of H2O2is in direct proportion to the quantity of hydroxyl free radicals it generates.When electron acceptor and the color developing reagent are added,red substance will from in the solution,whose absorbance is in direct proportion to the quantity of hydroxyl free radicals.As summarized in Table 7,the highest scavenging activity of hydroxyl free radical was found in water fractions,followed by n-hexane fraction,crude extract and dichloromethane fractions.Opposite to the DPPH scavenging activity,ethyl acetated fraction had the lowest scavenging activity of hydroxyl free radical.The results showed that fractions with high or low polarity were of high scavenging activity of hydroxyl free radical,and fractions with moderate polarity displayed lower scavenging activity.However,no correlation was found between scavenging activity of hydroxyl free radical of the four fractions and their polarity(P=0.677).

Table 7 Hydroxyl free radical scavenging activity ofextract and flavonoid fractions of C.microphylla flowers
Superoxide anion radical scavenging activity
The reactions of the kit simulate the xanthine-oxidase system in organisms and generate superoxide anion radicals.When electronic transferor is added,a purple color is developed with Gress reagent,whose absorbance is in direct proportion to the quantity of superoxide anion radical.Due to the scavenging effect of superoxide anion radical by antioxidants in the sample,the absorbance of the sample tube is lower than that of the control tube.The clearing activity of superoxide anion radicals by sample under test can thus be calculated using Vc as a standard.The percentages of superoxide anion radical scavenging activity by 1 mg·mL-1tested samples relative to that of 0.15 mg/mL VCwere shown in Table 8.Water fraction showed the highest superoxide anion radical scavenging activity,followed by crude extract;both exhibited activity more than three time that of 0.15 mg/mLVC.Dichloromethane and n-hexane fractions had very low superoxide anion radical scavenging activity,which were less than 100% of that of 0.15 mg/mL VC.The results of Pearson’s correlation analy-sis showed that no correlation was found between scavenging activity of superoxide anion radical of the four fractions and their polarity(P=0.235).

Table 8 Scavenging activity of superoxide anion radical by extract and flavonoid fractions of C.microphylla flowers
Discussion
In this work,the ethanolic crude extract and flavonoid fractions of C.microphylla flowers exhibited substantial antioxidative activity in various tests,suggesting that the flowers of the species can be a potential source for natural antioxidants.The antioxidative activity of flavonoid fractions of C.microphylla flowers varied considerably with their polarity.There was a significant positive Pearson’s correlation between T-AOC and the polarity of the flavonoid fractions(P<0.05).However,significant correlations were not found between scavenging activities of DPPH·,hydroxyl and superoxide anion radicals and the polarity of the flavonoid fractions (P>0.05).Moreover,the antioxidative activities of the same fraction varied in different antioxidative determination systems.Therefore,different fractions of C.microphylla can be used for different antioxidative purposes.Liquid-liquid extraction with stepwise gradient of solvents could help separate the flavonoid fractions with different polarity and antioxidative property from flowers of C.microphylla.Specifically,n-hexane fraction of high hydroxyl free radical scavenging activity was first extracted,followed by ethyl acetate and dichloromethane fractions of high DPPH· scavenging activity.The residual water fraction was of high T-AOC and scavenging activities of hydroxyl free radical and superoxide anion radical.This method can therefore serve as guidance on separation and application of natural antioxidants from C.microphylla flowers.
1 Niu XW.Peashrub research.Beijing:Science Press,2003.15-89.
2 Niu XW.Collected works on peashrub research.Taiyuan:Science and Technology Press of Shanxi Province,2003.145-149,171-172.
3 Meng QX,Niu Y,Niu XW,et al.Ethnobotany,phytochemistry and pharmacology of the genus Caragana used in traditional Chinese medicine.J Ethnopharm,2009,124:350-368.
4 Harman D.Role of free radicals in aging and disease.Ann.N Y Acad Sci.,1992,673:126-141.
5 Beck R,Dejeans N,Glorieux C,et al.Molecular chaperone Hsp90 as a target for oxidant-based anticancer therapies.Curr Med Chem,2011,18:2816-2825.
6 Sugamura K,Keaney JF Jr.Reactive oxygen species in cardiovascular disease.Free Radical Bio Med,2011,51:978-992.
7 Boudet AM.Evolution and current status of research in phenolic compounds.Phytochem,2007,68:2722-2735.
8 Ndhlala AR,Moyo M,Van Staden J.Natural antioxidants: fascinating or mythical biomolecules?Molecules,2010,15: 6905-6930.
9 Gao HF,Zheng BB,Wang JH,et al.Flavonol derivatives from Caragana leucophloea and their antimicrobial and antioxidant activities.Nat Prod Res Dev(天然產物研究與開發),2011,23:853-856.
10 Tai ZG,Cai L,Dai L,et al.Antioxidant activities of Caragana sinica flower extracts and their main chemical constituents.Molecules,2010,15,6722-6732.
11 Meng QX,Zhang LZ,Niu Y.Total Flavonoid Dynamics of Caragana microphylla Lam.Acta Agres Sin,2011,19:943-947.
12 Niu PF,Duan YF,Qiu NX,et al.Studies on anti-oxidative activity of different polarity flavonoids from corn Silk.Acta Agri.Boreali-occidentalis Sin(西北農業學報),2006,15:72-74.
13 Shi JY,Duan YF,Niu FG,et al.Study on anti-oxidative activity of different polarity flavonoids from extract of Caulis Poligoni multiflori.Sci.&Tech.Food Indust,2009,30:112-114.
14 Phenomenex Inc.Chromatography product guidance 2011.Torrance:Phenomenex Inc,2011.379.

