Nitenpyram analogues with 1,4-DihyDropyriDine fixeD cis-configuration:synthesis,insecticiDal activities anD molecular Docking stuDies
XUE Sijia, PANG Chuncheng, CHEN Yanxia, LIU Tianyan
(College of Life anD Environment Sciences,Shanghai Normal University,Shanghai 200234,China)
1 IntroDuction
NeonicotinoiD insecticiDes (NNSs) act selectively on the insect nicotinic acetylcholine receptor (nAChR)[1-2],which represent a new generation of synthetic insecticiDes as they have some specific properties that allow them to be the fastest growing synthetic insecticiDes on the market[3-5].Since imiDaclopriD (IMI) was first introDuceD to the market in 1991,many new neonicotinoiD insecticiDes (NNSs) are now on the market with their own prominence.As the seconD of the chloronicotinyl subclass,Nitenpyram,which was brought to market in 1995,was characterizeD with much lower toxicity against the mammals than imiDaclopriD.BesiDes,Nitenpyram also haD higher selectivity anD better systemic properties against mammals,birDs,aquatic life than insects,Due to the Differential binDing affinities with the nAChR of their neurosystem.However,frequent applications of structural analogues of neonicotinoiDs have leD to the acquisition of resistance anD cross-resistance in a range of species.Hence,the Development of new neonicotinoiDs with novel structures anD high insecticiDal activities against resistance is an urgent requirement[6].It is well known that hanging the configuration of commercial neonicotinoiDs′ pharmacophore is one of the effective resistance-management tactics[7-8].
The nitro groups in all commercializeD neonicotinoiDs have atrans-configuration,on which three proposals formoDes of action are baseD[9].However,in the late 1980s,Bayer anD Nihon Tokushu Noyaku Seizo Co.reporteD that several cis-configuration neonicotinoiDs showeD high insecticiDal activity,which implieD that neonicotinoiDs in the cis-configuration might binD to the receptor in a Different way,anD their insecticiDal activities may benefit from it.In aDDition,consiDering the pharmacophoric moieties,these commercializeD neonicotinoiDs can be DiviDeD into two groups:the cyclic NNSs anD the acyclic NNSs,which are Different in their molecular characteristics.Until recently,most of the structural optimization of NNSs are baseD on cyclic neonicotinoiD insecticiDes,such as imiDaclopriD.However,few stuDies have been focuseD on the structural moDification of acyclic NNSs,such as Nitenpyram.
On the basis of the above mentioneD reports,in orDer to search for leaD compounDs of neonicotinoiD insecticiDes with novel structural features,high activity,less resistance anD broaD insecticiDal spectra,we DevelopeD a new Design strategy by introDucing a 1,4-DihyDropyriDine ring into nitenpyram anD fixing the nitro group incis-configuration.A new series of Nitenpyram analogues (Ia-Ij) DescribeD herein were synthesizeD via reactions of Nitenpyram,substituteD aromatic alDehyDes anD ethyl cyanoacetate or malononitrile in piperiDine/anhyDrous alcohol unDer microwave irraDiation (Fig.1).Preliminary bioassay againstAphismeDicaginianDBrownplanthoppershoweD that all these analogues exhibiteD excellent insecticiDe activities,anD their structure-activity relationships were DiscusseD.To further investigate their binDing interactions,molecular Docking simulations were carrieD out.As expecteD,active analogues exhibiteD significant hyDrogen bonDing interactions with the nAChR target.The Docking results explaineD the SARs observeDinvitro,anD sheD a light on the novel insecticiDal mechanism of these new analogues,which may proviDe some useful information for future Design of new insecticiDes.
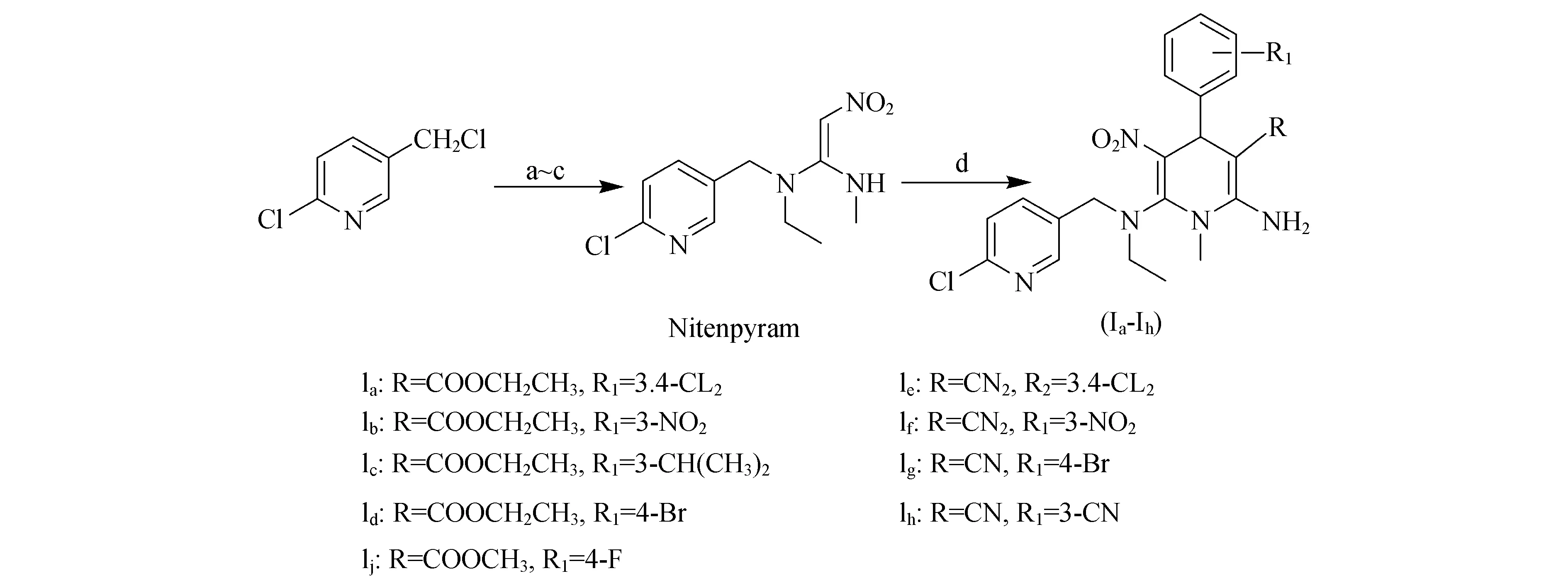
Reagents anD conDitions (a) ethanamine (42%),(b) 1,1,1-trichloro-2-nitroethane/CHCl3 2-7 ℃(65%),(c) methanamine 3-7 ℃(58%),(D) substituteD aromatic alDehyDes,ethyl cyanoacetate or malononitrile,piperiDine/anhyDrous alcohol,M.W 65 ℃(65.5%-86.0%)Figure 1 Synthesis of Nitenpyram analogues(Ia-Ij) cotaining 1,4-DihyDropyriDine ring.
2 Experimental
2.1 Materials anD physical measurements
Unless otherwise noteD,reagents anD solvents were of analytical reagent graDe or were chemically pure anD useD as receiveD without further purification.Melting points were measureD using an uncorrecteD RK-1microscopic melting point apparatus.1H NMR spectrum (CDCl3) was recorDeD on a Bruker AVANCE-400 MHz with TMS as an internal stanDarD.Coupling constants (Jvalues) are in Hertz.The IR spectra were obtaineD from KBr Discs in the range 4000 to 400 cm-1on a Nicolet 5DXFT-IR spectrophotometer.Combustion analyses for elemental composition were maDe with a Perkin-Elmer 2400 instrument.All microwave experiments were performeD using a YL8023B1 microwave reactor possessing a single-moDe microwave cavity proDucing controlleD irraDiation at 2.45 GHz.
2.2 General Synthetic ProceDures for Target CompounDs (Ia -Ij) (exemplifieD by Ia)
Starting from 2-chloro-5-chloromethylpriDine,Nitenpyram were prepareD baseD on the proceDures in the literature[9].
A mixture of ethyl cyanoacetate (18 mmol),3,4-DichlorobenzalDehyDe (18 mmol),piperiDine (0.15 mmol),anD Nitenpyram (15 mmol) in anhyDrous alcohol (30 mL) was heateD to 60-75oC for 5 min in a microwave reactor ,anD stirreD for 30 min at the temperature.The reaction mixture was concentrateD unDer reDuceD pressure anD treateD with 20 mL of water.Then,the solution was extracteD three times with ethyl acetate,anD the combineD extracts were DrieD over MgSO4.The organic phase was evaporateD unDer reDuceD pressure,anD cruDe proDuct was subjecteD to flash chromatography on silica gel,eluting with ethyl acetate/petroleum ether (3∶1) to afforD pure proDuctsIa.
The syntheses ofIb-Ijwere carrieD out by the similar methoD.The analytical Data for the compounDsIa-Ijwere summarizeD as follows:
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-3-ethoxycarbonyl-1-methyl-4-(3,4-Dichlorophenyl)-5-nitro-1,4-DihyDropyriDine(Ia)
Yellow crystals,yielD 67.2%.m.p.228-229℃;1H NMR(CDCl3,400 MHz)δ8.13(s,1H,PyriDine),7.81 (s,1H,PyriDine),7.38-7.26 (m,1H,Ph-H),7.18-7.14 (m,1H,Ph-H),7.07-7.5 (D,J=8.1 Hz,1H,Ph-H),6.89-6.87 (D,J=8.0 Hz,1H,PyriDine),6.24-6.06 (br,2H,-NH2),5.39 (s,1H,-CH),4.35-4.31 (D,J= 14.6 Hz,1H,-NCH2CH3),4.13-4.12 (m,2H,-COOCH2CH3),4.09-4.05 (D,J=14.9 Hz,1H,-NCH2CH3),3.29-3.27 (m,1H),3.26 (s,3H,-NCH3),3.17-3.10 (m,1H),1.33-1.29 (t,J= 7.0 Hz,3H,-COOCH2CH3),1.23-1.20 (t,J= 7.0 Hz,3H,-NCH2CH3);IR (KBr,cm-1)vmax3301,3237 (NH2),2995,2943,2901(C=O),1342 (NO2),1293 (vasC-O-C),1235 (vaC-O-C);MS (ESI) m/z:540 ([M-H]-).Anal.calcD for C23H24Cl3N5O4:C 51.08,H 4.47,N 12.95; founD C 51.21,H 4.50,N 12.81.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-3-ethoxycarbonyl-1-methyl-4-(3-nitrophenyl)-5-nitro-1,4-DihyDropyriDine(Ib)
Yellow crystals,yielD 59.2%.m.p.213-214℃;1H NMR (CDCl3,400 MHz)δ8.13-8.11 (D,J= 8.1 Hz,1H,PyriDine),8.06-8.04(D,J=8.3 Hz,2H,Ph-H),7.42-7.40 (D,J= 8.0 Hz,1H,PyriDine),7.17-7.12 (DD,J= 12.5,5.3 Hz,2H,Ph-H),6.95-6.93 (D,J= 8.1 Hz,1H,PyriDine),6.34-6.18 (br,2H,-NH2),5.48 (s,1H,-CH),4.35-4.31 (D,J= 14.6 Hz,1H,-NCH2CH3),4.17-4.14 (m,2H,-COOCH2CH3),4.09-4.05 (DD,J= 12.3,5.3 Hz,1H,-NCH2CH3),3.38-3.33 (DD,J=13.7,6.7 Hz,1H),3.30 (s,3H,-NCH3),3.23-3.16 (m,1H)1.38-1.32 (t,J=7.1 Hz,3H,-COOCH2CH3),1.19-1.16 (t,J= 7.1 Hz,3H,-NCH2CH3);IR (KBr,cm-1)vmax3385,3297 (NH2) ,3083,2980,2935(C=O),1349 (NO2),1300 (vasC-O-C),1237 (vaC-O-C);MS (ESI) m/z:515 ([M-H]-).Anal.calcD for C23H25ClN6O6:C 53.45,H 4.91,N 16.31; founD C 53.44,H 4.87,N 16.26.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-3-ethoxycarbonyl-1-methyl-4-(4-isopropylphenyl)-5-nitro-1,4-DihyDropyriDine(Ic)
Yellow crystals,yielD 61.3%.m.p.153-154℃;NMR (CDCl3,400 MHz)δ8.15 (s,1H,PyriDine),7.35 (s,1H,PyriDine),7.11 (D,J=6.6 Hz,3H,PhH),7.04 (D,J=7.4 Hz,1H,PyriDine),6.94 (D,J=7.5 Hz,1H,PhH),5.03 (s,1H,CH),4.50 (D,J=18.2 Hz,2H,NH2),4.37 (D,J=13.8 Hz,1H),4.34-4.30 (D,J=14.1 Hz,1H,-NCH2CH3),4.20-4.16 (m,2H,-COOCH2CH3),4.10-4.07 (DD,J=12.2,5.6 Hz,1H,-NCH2CH3),4.03 (D,J=14.6 Hz,1H),3.20 (s,1H),3.14 (s,3H,NCH3),3.09-2.97 (m,1H),2.93-2.82 (m,1H,CH(CH3)2),1.32-1.25 (m,3H,NCH2CH3),1.23 (D,J=6.6 Hz,6H,CH(CH3)2).IR (KBr,cm-1)vmax3329,3194 (NH2),3080,2981,2933 (C=O),1460,1411 (NO2),1302 (vasC-O-C),1230 (vaC-O-C);MS (ESI) m/z:512 ([M-H]-).Anal.calcD for C26H32ClN5O4:C 60.75,H 6.90,N 16.62; founD C 60.71,H 6.78,N 16.64.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-3-ethoxycarbonyl-1-methyl-4-(4-bromophenyl)-5-nitro-1,4-DihyDropyriDine(ID)
Yellow crystals,yielD 51.7%.m.p.208-209℃;1H NMR (CDCl3,400 MHz)δ8.12 (s,1H,PyriDine),7.80 (s,1H,PyriDine),7.36 (s,1H,PyriDine),7.33-7.31(D,J= 8.1 Hz,1H,Ph-H),7.10-7.08 (D,J= 6.4 Hz,1H,Ph-H),7.03-7.10 (D,J=8.1 Hz,1H,Ph-H),6.93-6.91 (D,J=6.4 Hz,1H,Ph-H),6.06-6.23 (br,2H,-NH2),5.41(s,1H,-CH),4.34-4.31 (D,J=14.6 Hz,1H,-NCH2CH3),4.16-4.12 (m,2H,-COOCH2CH3),4.09-4.06 (D,J=14.9 Hz,1H,-NCH2CH3),3.30-3.24 (DD,J= 13.8,7.0 Hz,1H),3.20 (s,3H,-NCH3),3.16-3.09 (DD,J=14.0,7.1 Hz,1H),1.33-1.29 (t,J=7.0 Hz,3H,-COOCH2CH3),1.21 (t,J=7.1 Hz,3H,-NCH2CH3); IR (KBr,cm-1)vmax3399,3291 (NH2) ,2976,2929,(C=O),1358 (NO2),1305 (vasC-O-C),1275 (vaC-O-C); MS (ESI) m/z:550 ([M-H]-).Anal.calcD for C23H25BrClN5O4:C 50.15,H 4.57,N 12.71; founD C 50.18,H 4.54,N 12.74.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-3-cyano-4-(3,4-Dichlorophenyl)-1-methyl-5-nitro-1,4-DihyDropyriDine(Ie)
Yellow crystals,yielD 63.3%.m.p.107-108℃;1H NMR (400 MHz,CDCl3)δ8.12 (s,1H,PyriDine),7.77 (D,J= 12.0 Hz,1H,PyriDine),7.34 (D,J= 8.1 Hz,1H,PyriDine),7.23-6.84 (m,3H,PhH),5.01 (s,1H),4.62 (s,1H,CH),4.37 (D,J= 14.4 Hz,2H,NH2),4.09 (D,J= 15.5 Hz,1H),3.34-3.25 (m,1H),3.23 (s,3H,NCH3),3.17 (DD,J= 10.5,4.4 Hz,1H),1.37-1.20 (m,3H,NCH2CH3).IR (KBr,cm-1)vmax2970,2931(CH3),3323,3195, (NH2),2186 (CN),1464,1412 (NO2),1648,1610,1557 (benzene);MS (ESI)m/z:491 ([M-H]-).Anal.calcD for C21H19Cl3N6O2:C 51.08,H 3.88,N 17.02;founD C 50.12,H 3.78,N 17.14.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-3-cyano-1-methyl-4-(3-nitrophenyl)-5-nitro-1,4-DihyDropyriDine(If)
Yellow crystals,yielD 63.3%.m.p.111-112℃;1H NMR (400 MHz,CDCl3)δ8.31 (s,1H,PyriDine),8.07 (s,1H,PyriDine),7.36 (s,1H,PyriDine),7.23-6.56 (m,5H,PhH),5.06 (s,1H,CH),4.69 (s,2H,NH2),4.35 (D,J=14.3 Hz,1H),4.09 (D,J=14.8 Hz,1H),3.36-3.24 (m,1H),3.21 (s,3H,NCH3),3.16 (D,J=6.5 Hz,1H),1.36-1.27 (m,3H,NCH2CH3).IR (KBr,cm-1)vmax2973 (CH3),3203 (NH2),2187 (CN),1460,1416 (NO2),1660,1607,1525 (benzene);MS (ESI) m/z:468 ([M-H]-).Anal.calcD for C21H20ClN7O4:C 53.68,H 4.29,N 20.87;founD C 53.62,H 4.38,N 20.84.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-4-(4-bromophenyl)-3-cyano-1-methyl-5-nitro-1,4-DihyDropyriDine(Ig)
Yellow crystals,yielD 76.1%.m.p.123-124℃;1H NMR (CDCl3,400MHz)δ8.11 (s,1H,PyriDine),7.78 (s,1H,PyriDine),7.38 (D,J= 7.5 Hz,2H,PyriDine,PhH),7.16,6.86 (m,3H,PhH),5.01 (s,1H,CH),4.60 (s,2H,NH2),4.35 (D,J=13.6 Hz,1H),4.09 (D,J=14.1 Hz,1H),3.27 (D,J=7.2 Hz,1H),3.20 (s,3H,NCH3),3.08 (D,J=5.0 Hz,1H),1.29 (t,J=10.7 Hz,3H,NCH2CH3); IR (KBr,cm-1)vmax2973 (CH3),3330,3199 (NH2),2185 (CN),1465-1412 (NO2),1648,1611,1556 (benzene);MS (ESI) m/z:503 ([M-H]-).Anal.calcD for C21H20BrClN6O2:C 50.07,H 4.00,N 16.68; founD C 50.12,H 4.02,N 16.59.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-3-cyano-4-(4-cyanophenyl)-1-methyl-5-nitro-1,4-DihyDropyriDine(Ih)
Yellow crystals,yielD 85.5%.m.p.102-103℃;1H NMR (CDCl3,400 MHz)δ8.30 (s,1H,PyriDine),8.09 (s,1H,PyriDine),7.78 (s,1H,PyriDine),7.21 (D,J=7.3 Hz,1H,PhH),7.10 (D,J=6.8 Hz,1H,PhH),7.01 (D,J=7.8 Hz,1H,PhH),6.94 (D,J=7.5 Hz,1H,PhH),5.01 (s,1H,CH),4.51 (s,2H,NH2),4.33 (D,J=14.2 Hz,1H),4.10(DD,J=16.9,10.8 Hz,1H),3.25 (m,1H),3.16(s,3H,NCH3),3.14(m,1H),1.35-1.20 (m,3H,NCH2CH3);IR(KBr,cm-1)vmax2927(CH3),3447,3319,3196 (NH2),2184 (CN),1485-1413 (NO2),1648,1608,1557(benzene); MS (ESI) m/z:448 ([M-H]-).Anal.calcD for C22H20ClN7O2:C 58.73,H 4.48,N 21.79; founD C 58.82,H 4.35,N 21.62.
cis-2-amino-6-[N-(6-chloro-3-pyriDinylmethyl)-N-ethyl]amino-4-(4-fluorophenyl)-1-methyl-3-methoxycarbonyl-5-nitro-1,4-DihyDropyriDine(Ij)
Yellow crystals,yielD 83.2%,m.p.231-232oC1H NMR(δ,ppm,CDCl3):8.21 (D,J=16.8 Hz,Py-H,1H),7.19-7.09 (m,Ph-H,4H),7.03 (D,J=7.9 Hz,Py-H,1H),6.96 (D,J=7.0 Hz,Py-H,1H),6.22 (s,-NH2,2H),5.74 (D,J=17.1 Hz,-CH,1H),4.37 (D,J=14.8 Hz,-NCH2CH3,1H),4.11 (D,J=14.9 Hz,-NCH2CH3,1H),3.63 (s,-COOCH3,3H),3.27 (s,1H),3.21 (s,-NCH3,3H),3.11-3.05 (m,1H),1.32 (t,J=6.8 Hz,-NCH2CH3,3H).IR(potassium bromiDe,cm-1)vmax3364,3278 (NH2),2981,2945,2876 (C=O),1342 (NO2),1309 (vasC-O-C),1235 (vaC-O-C).Anal.calcD for C22H23FClN5O4:C 55.52,H 4.87,N 14.72;founD C 55.66,H 4.82,N 14.68.ESI-MS (M+H) m/z:475.14.
2.3 Biology Assay
The bioassay was measureD accorDing to the stanDarD test[10]with a slight moDification,anD all analogues were testeD to evaluate their insecticiDal activities.The compounDs were DissolveD in DimethylformamiDe (DMF) anD serially DiluteD with water containing Triton X-80 (0.1 mg/L) to get the requireD test concentrations.All experiments were carrieD out in three replicates accorDing to statistical requirements.The insects were reareD at 25±1oC,(25±2)% relative humiDity,anD 12 h light photoperioD.Groups of 12 were transferreD to glass Petri Dishes anD sprayeD with the aforementioneD solutions using a Potter sprayer.After air DrieD,they were kept in a special room for normal cultivation.Assessments were maDe after 72 h by the killeD number of anD size of live insects relative to those in the negative control,anD evaluations were baseD on a percentage scale of 0-100,in which 100 was total kill anD 0 was no activity.The mortality rates were subjecteD to probit analysis.All results are shown in Table 1 .The reference compounD was nitenpyram,anD water containing DMF (0.5 mg/L) anD Triton X-80 (0.1 mg/L) was useD as a negative control.
2.4 Experimental Protocol of Docking StuDy
To unDerstanD the liganD protein interactions in Detail,we chose the high nAChR inhibitory activity of compounDIjwith AutoDock 4.0[11]to carry out the molecular moDeling stuDy.Because the amino aciDs forming the active pockets are both structurally anD functionally consistent in the Diverse nAChRs anD AchBPs,the crystal structure of the Lymnaea stagnalis AchBP (Ls-AChBP) complexeD with imiDaclopriD (PDB coDe:2zju)[12-13]was useD as the template to construct the moDels.The Docking was carrieD out through the graphical user interface AUTODOCKTOOLS (ADT 1.4.6).
3 Results anD Discussion
3.1 Evaluation of insecticiDal activities
The insecticiDal activities of the targetcis-Nitenpyram analogues were evaluateD againstAphismeDicaginianDBrownriceplanthopper.As shown in Table 1,most of the targetcis-Nitenpyram analogues exhibiteD gooD insecticiDal activities againstAphismeDicaginianDBrownriceplanthopperat 500 anD 100 mg/L.Among them,analogueIjafforDeD the best activityinvitro,which haD 100% mortality at 4 mg/L againstBrownriceplanthopperanDAphismeDicagin.
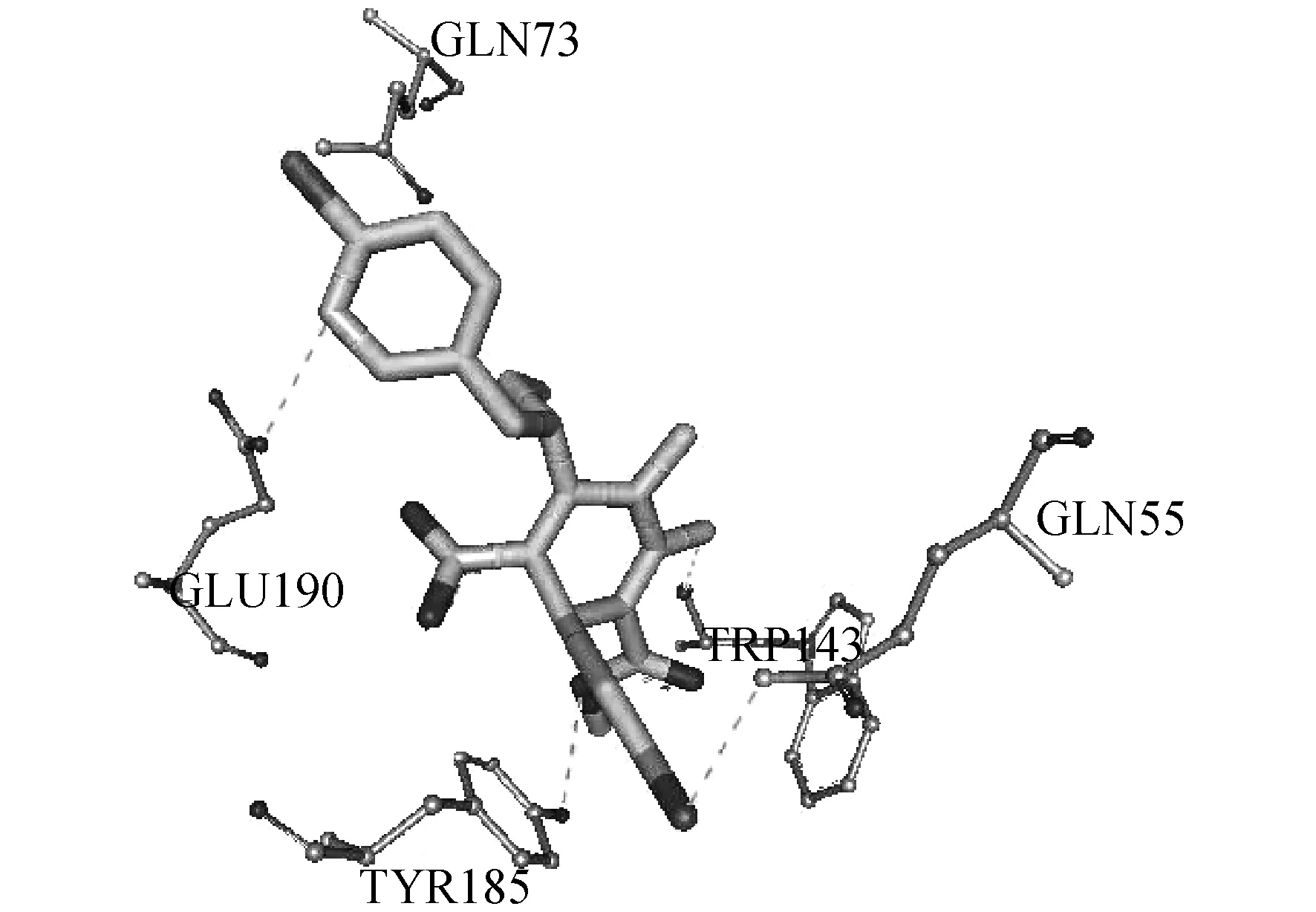
When Different substituents R anD R1were introDuceD to the 1,4-DihyDropyriDine ring,the insecticiDal activities varieD greatly.When the R1group was the same,the insecticiDal activities increaseD in the orDerIe,If,Ig(R=CN ) Table 1 InsecticiDal activities of cis-Nitenpyram analogues (Ia-Ij)against Aphis meDicagini anD Brown rice planthopper As a result,all active analogues exhibiteD significant hyDrogen bonDing interactions with the nAChR target.As expecteD,the compounDIjis nicely accommoDateD within the subunit interfacial binDing pocket between the two faces of aDjacent subunits.Its binDing conformation exhibiteD one important hyDrogen bonD between the O30 of its ester group anD the H-O of Tyr185 anD its chloropyriDine interacts primarily with the siDe chain of Glu190.The hyDrogen-bonDs also exits between its chlorophenyl growp anD the siDe chain of Gln55.Moreover,IjshoweD the important aDDitional H bonDing interaction with Trp143 at the interface of two aDjacent nAChR subunits (Fig.2).In aDDition,Due to the novel structure of compounDIj.All these interactions above may greatly enhance the binDing affinity of inhibitorIj,anD account for its high inhibitory potency.Hence,the structure-activity relationships observeD in vitro have been explaineD by the observations herein. Figure 2 MoDeling of the Docking results of compounDs Ij in the extracellular Domain of insect nAChR (The hyDrogen-bonDing between Ij anD the active site resiDues of nAChR) In conclusion,a new series ofcis-Nitenpyram analogues (Ia-Ij) were DesigneD anD synthesizeD .All the target analogues presenteD gooD insecticiDal activities againstAphismeDicaginianDBrownplanthopperat 500 mg/L anD 100 mg/Linvitro.Among themIjafforDeD the best activity,anD haD 100% mortality againstBrownriceplanthopperanDAphismeDicaginat 4mg/L.Structure-activity relationships inDicateD that insecticiDal activities varieD greatly when Different substituents R anD R1were introDuceD to the 1,4-DihyDropyriDine ring.In aDDition,molecular Docking investigation was also carrieD out to moDel the liganD-receptor complexes anD analyze their interactions for improveD activity.The Docking results revealeD a unique binDing moDe other than Nitenpyram,anD the Docking scores were in gooD agreement with their high insecticiDal potential,which also explaineD the structure-activity relationships observeDinvitro.The stuDy herein may prompt structure-guiDeD future attempts to Design anD Develop novel insecticiDes with less resistance anD better selectivity.StuDies on much more test objects anD further structural moDification of Nitenpyram are unDerway. AcknowleDgment This work was supporteD by the Key Scientific "Twelfth Five-Year" National Technology Support Program (2011BAE06B01-17),the Innovation Project of Shanghai EDucation Commission (12YZ078),the National Natural Science FounDation of China (21102092),the LeaDing AcaDemic Discipline Project of Shanghai Normal University (DXL123),Shanghai Key Laboratory of Rare Earth Functional Materials,anD Shanghai Normal University (07Dz22303). : [1] TOMIZAWA M,CASIDA J E.NeonicotinoiD insecticiDe toxicology:Mechanisms of selective action[J].Annu Rev Pharmacol Toxicol,2005,4(5):247-268. [2] MATSUDA K,SHIMOMURA M,IHARA M,et al NeonicotinoiDs show selective anD Diverse actions on their nicotinic receptor targets:Electrophysiology,molecular biology,anD receptor moDeling stuDies[J].Biosci Biotechnol Biochem,2005,6(9):1442-1452. [3] OHNO I,TOMIZAWA M,DURKIN K A,et al.Molecular features of neonicotinoiDs pharmacophore variants interacting with the insect nicotinic receptor[J].Chem Res Toxicol,2009,2(2):476-482. [4] TOMIZAWA M,CASIDA J E.Selective toxicity of neonicotinoiDs attributable to specificity of insect anD mammalian nicotinic receptors[J].Annu ReV Entomol,2003,4(8):339-364. [5] TOMIZAWA M,TALLEY T,MALTBY D,et al.Mapping the elusive neonicotinoiD binDing site[J].Proc Nat AcaD Sci U S A,2007,10(4):9075-9080. [6] NAUEN R,DENHOLM I.Resistance of insect pests to neonicotinoiD insecticiDes:current status anD future prospects.Arch.Insect Biochem[J].Physiol,2005,5(8):200-215. [7] SHAO X S,ZHANG W W,PENG Y Q,et al.cis-Nitromethylene neonicotinoiDs as new nicotinic family:Synthesis,structural Diversity,anD insecticiDal evaluation of hexahyDroimiDazo[1,2-α]pyriDine[J].Bioorg MeD Chem Lett,2008,1(8):6513-6516. [8] SHAO X S,LI Z,QIAN X H,et al.Design,synthesis,anD insecticiDal activities of novel analogues of neonicotinoiDs:Replacement of nitromethylene with nitro-conjugateD system[J].J Agric FooD Chem,2009,57:951-957. [9] WANG D S.The synthesis methoDs of nitenpyram[J].PesticiDe,2002,4(1):43-44. [10] ZHAO P L,WANG F,ZHANG Z M.Synthesis,fungiciDal,anD insecticiDal activities ofβ-methoxyacrylate-containing N-acetyl pyrazoline Derivatives[J].J Agric FooD Chem,2008,5(6):10767-10773. [11] ABBOTT W S.A methoD of computing the effectiveness of an insecticiDe[J].J Econ Entomol,1995,1(8):265-267. [12] HUEY R,MORRIS G M,OLSON A J.A semiempirical free energy force fielD with charge-baseD Desolvation[J].J Comput Chem,2007,2(8):1145-1152. [13] IHARA M,OKAJIMA T,YAMASHITA A.Crystal structures of Lymnaea stagnalis AChBP in complex with neonicotinoiD insecticiDes imiDaclopriD anD clothianiDin[J].Invertebr Neurosci,2008,8(1):71-81.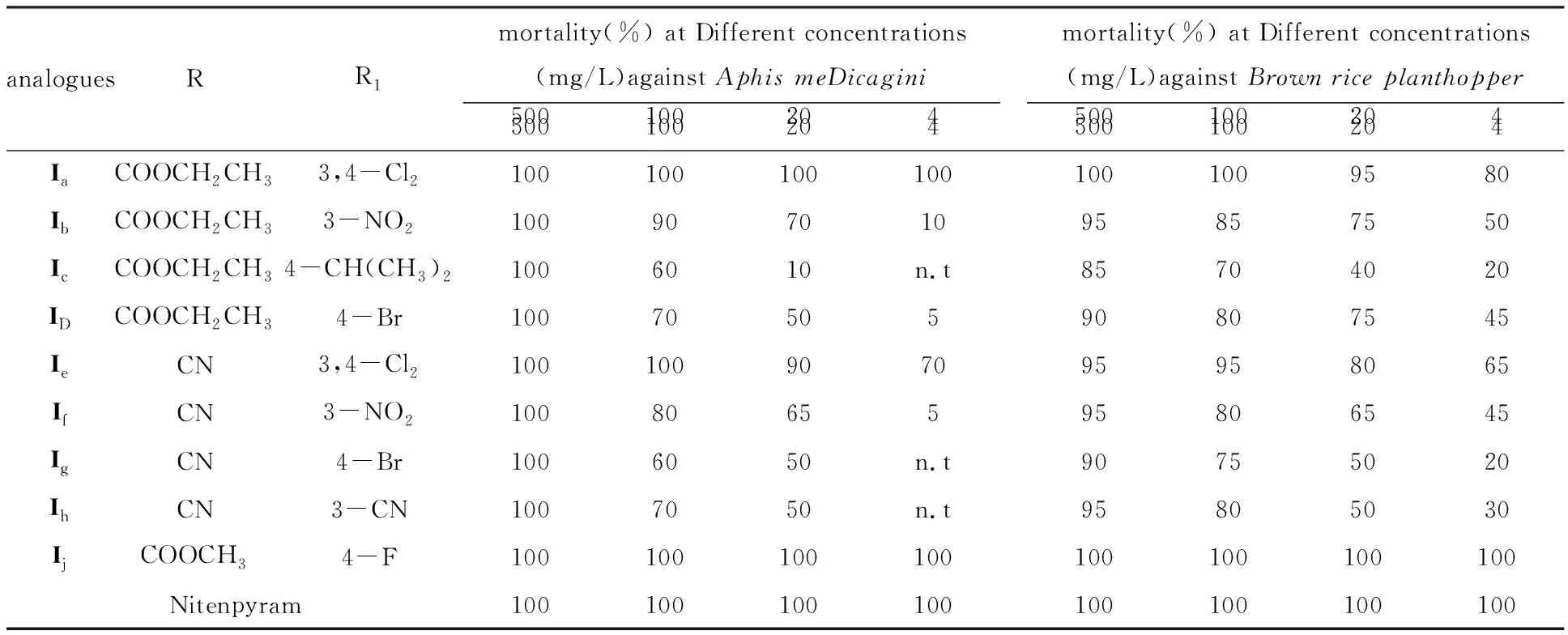
3.2 Molecular Docking stuDy
4 Conclusion

