經鼻應用SH2-Bβ抗體可抑制高脂飲食小鼠體質量增長
金韻,李新鳴,王效杰,臧晉,齊金萍,郭佳美
(沈陽醫學院1.解剖學教研室;2.微生物學教研室;3.2012級臨床醫學系,沈陽110034)
經鼻應用SH2-Bβ抗體可抑制高脂飲食小鼠體質量增長
金韻1,李新鳴2,王效杰1,臧晉1,齊金萍1,郭佳美3
(沈陽醫學院1.解剖學教研室;2.微生物學教研室;3.2012級臨床醫學系,沈陽110034)
目的探討經鼻應用SH2-Bβ抗體對高脂飲食小鼠體質量的影響。方法采用8周C57BL/6小鼠隨機分為正常組、肥胖組和SH2-Bβ抗體(1∶50)干預組,每組12只。測量每日攝食量及每周體質量變化,應用Western blot檢測SH2-Bβ在各組小鼠下丘腦和肝的表達,HE染色觀察肝的組織學改變。免疫組織化學染色檢測腎SH2-Bβ表達,并觀察其病理改變。結果肥胖組小鼠攝食量和體質量明顯高于SH2-Bβ抗體干預組和正常組(P<0.05)。Western blot檢測結果顯示,肥胖組小鼠SH2-Bβ蛋白在下丘腦表達下調,明顯低于SH2-Bβ抗體干預組和正常組(P<0.05);而在肝組織表達上調,明顯高于SH2-Bβ抗體干預組和正常組(P<0.05)。肝組織HE染色結果顯示,SH2-Bβ抗體干預組與肥胖組相比,肝細胞索排列整齊,肝細胞脂肪變性減輕,脂滴變小,炎性細胞減少。腎組織免疫組織化學染色結果顯示,肥胖組小鼠SH2-Bβ蛋白在腎組織表達上調,明顯高于SH2-Bβ抗體干預組和正常組(P<0.05)。炎性細胞浸潤明顯。結論經鼻應用SH2-Bβ抗體可使高脂飲食小鼠下丘腦內SH2-Bβ表達上調,降低攝食量而降低體質量;肝腎組織SH2-Bβ表達下調,使肝腎脂肪沉積減輕,進而減輕脂肪沉積所致的組織炎癥或損傷。這表明SH2-Bβ經中樞通過調節進食量,在周圍組織通過改變脂肪沉積量而對體質量進行調節。
SH2-Bβ;肥胖;體質量;小鼠;鼻腔給藥
近年肥胖患者數量迅速增長。SH2-Bβ是一種細胞內連接蛋白,在下丘腦內是重要的能量代謝和糖代謝的正向調節者[1]。腦內SH2Bβ基因敲除小鼠會導致嚴重的瘦素抵抗、胰島素抵抗、肥胖、2型糖尿病[2,3]。我們新近的研究發現高脂飲食可使小鼠下丘腦內SH2-Bβ表達下調進而導致肥胖[4]。尋找到能調節下丘腦內SH2-Bβ的藥物將成為肥胖治療的新靶點。
本研究通過鼻腔給予SH2-Bβ抗體,觀察小鼠下丘腦SH2-Bβ蛋白表達變化及其對攝食量和體質量的影響,并觀察體質量變化前后肝、腎SH2-Bβ蛋白表達變化,進一步了解SH2-Bβ蛋白在器官脂肪沉積中的作用。
1 材料與方法
1.1 材料和儀器
SH2-Bβ羊多克隆IgG抗體(Santa Cruz,sc-10827),ECL發光試劑盒(美國Santa Cruz Biotechnology公司);辣根過氧化物酶標記羊抗小鼠IgGHRP,β-actin小鼠單克隆IgG抗體,免疫組化SABC試劑盒,多聚甲醛,DAB顯色劑(Diaminobenzidine),OCT包埋劑,RIPA試劑及硝酸纖維素膜(中國武漢博士德生物公司)。其余為國產分析純。電子天平(BS110S),德國Startorius公司。內切式電動組織勻漿器,瑞士Kinematica公司。紫外分光光度計(DU70型),美國Beckman公司。電泳轉印儀,美國Bio-Rad公司。Metamoph圖像分析系統,北京中科恒業創新科技有限公司。
1.2 實驗動物及飼料配制
選用健康、雌性、出生8周的C57BL/6小鼠,體質量21 g左右。普通飼料:玉米粉30%,豆餅21.0%,麩皮10.0%,次粉28.0%,魚粉9.0%,酵母粉1.0%,魚肝油1%(g/100 g)等。高脂飼料:豬油12.0%,奶粉8.0%,雞蛋10.0%,花生5.0%,麻油3.0%,以普通飼料補足100 g混成含12%豬油的高脂飼料,食用前2 d配好,低溫保存。
1.3 動物分組
將C57BL/6小鼠適應性喂養1周后,按體質量隨機分為3組:(1)對照組(normal control group,NC)12只;(2)肥胖組(obese group,OB)12只;(3)SH2-B β抗體干預組(anti-SH2-Bβ intervene group,anti-SH2-Bβ)12只。對照組喂普通飼料,肥胖組和抗體干預組喂高脂飼料。SH2-Bβ抗體干預組每天下午經鼻腔滴入SH2-Bβ抗體1∶50稀釋液(PBS)25 μL,每天1次,其余2組鼻腔滴入等量IgG抗體。自由飲水,共飼養4周。
1.4 攝食量和體質量測定
每天測量攝食量,每1周稱體質量1次,以體質量超過對照組體質量2 g作為肥胖標準。
1.5 Western blot分析SH2-Bβ表達
實驗4周結束后,各組取6只小鼠用1%戊巴比妥鈉腹腔麻醉(40 mg·kg-1),取下丘腦和肝,-70℃儲存。用RIPA試劑抽提可溶性蛋白,制備SDS-聚丙烯酰胺凝膠(0.12),樣品加熱煮沸(100℃)5 min變性后每孔加50 μg蛋白樣品,電泳后濕轉至硝酸纖維素膜上。50 g·L-1脫脂奶粉TTBS緩沖液(TBS緩沖液500 mL+TWEEN-20 0.5 mL)室溫振蕩封閉3 h后洗膜,一抗SH2-Bβ(1∶300)或β-actin(1∶300)室溫孵育2 h,TTBS漂洗膜,辣根過氧化物酶標記羊抗小鼠IgG-HRP(1∶5 000)室溫孵育1 h,洗膜后加入ECL反應1 min,暗室曝光顯影后沖洗膠片。凝膠成像分析系統上攝像分析,計算出相對光密度值。
1.6 免疫組織化學反應(SABC法)檢測SH2-Bβ表達,HE染色觀察肝臟結構改變
實驗4周結束后,各組取6只小鼠用1%戊巴比妥鈉腹腔麻醉(40 mg·kg-1),用0.1 mol·L-1PBS配制的含有40 g·L-1多聚甲醛灌流液灌流固定后取下腎和肝,后固定24 h后移入0.87 mol·L-1的蔗糖緩沖液中至標本沉降。OCT包埋,恒冷箱連續切片(厚14 μm),冷風干燥后腎組織切片行免疫組織化學染色(SABC法)。一抗SH2-Bβ工作濃度為1∶150,DAB顯色。經SH2-Bβ免疫組織化學染色的切片,每塊組織隨機取3~4張切片,且各組所選部位相同,每張切片平均選取4個視野,使用Metamoph圖像分析系統,進行光密度測定。測量值與相應陰性對照切片測量值相減后的平均值為各區域最終平均光密度值(mean optical density,MOD)。肝組織切片行HE染色。
1.7 統計學處理
2 結果
2.1 各組小鼠平均每日攝食量比較
肥胖組小鼠平均每日攝食量(高脂飼料)(7.12± 0.79)g,明顯高于正常組(普通飼料)(5.87±0.35)g和SH2-Bβ抗體干預組(3.96±0.82)g差異有統計學意義(P<0.05)。
2.2 各組小鼠體質量比較
實驗4周后肥胖組小鼠體質量明顯高于正常組和SH2-Bβ抗體干預組(P<0.05),差異有統計學意義(表1)。
2.3 Western blot檢測下丘腦和肝臟SH2-Bβ的表達
在下丘腦,肥胖組SH2-Bβ表達量(SH2-Bβ/β-actin:0.102)低于對照組(SH2-Bβ/β-actin:0.379)(P<0.05)和抗體干預組(SH2-Bβ/β-actin:0.324)(P<0.05);而在肝臟,肥胖組SH2-Bβ表達量(SH2-Bβ/ β-actin:0.456)高于對照組(SH2-Bβ/β-actin:0.204)和抗體干預組(SH2-Bβ/β-actin:0.094)(P<0.05)(圖1)。
表1 各組小鼠體質量比較Tab.1 Comparison of body weight of mice in each group
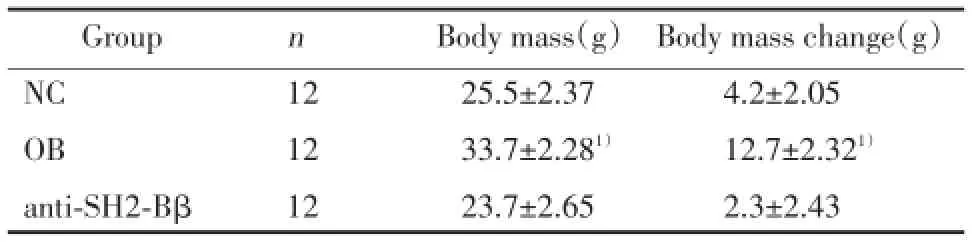
表1 各組小鼠體質量比較Tab.1 Comparison of body weight of mice in each group
1)P<0.05,vs groups NC and anti-SH2-Bβ.
?

圖1 Western blot檢測的各組下丘腦和肝臟SH2-BβFig.1 SH2-Bβexpression in hypothalamus and liver as determined by Western blot
2.4 HE染色觀察肝組織結構改變
正常組肝組織(2A)肝細胞索整齊,肝細胞內無空泡;肥胖組肝組織(圖2B)肝細胞固縮,大量脂肪空泡,空泡較大,肝細胞索斷裂,炎性細胞較多;抗體干預組肝組織(圖2C)有較少量小脂滴,肝細胞索較完整。
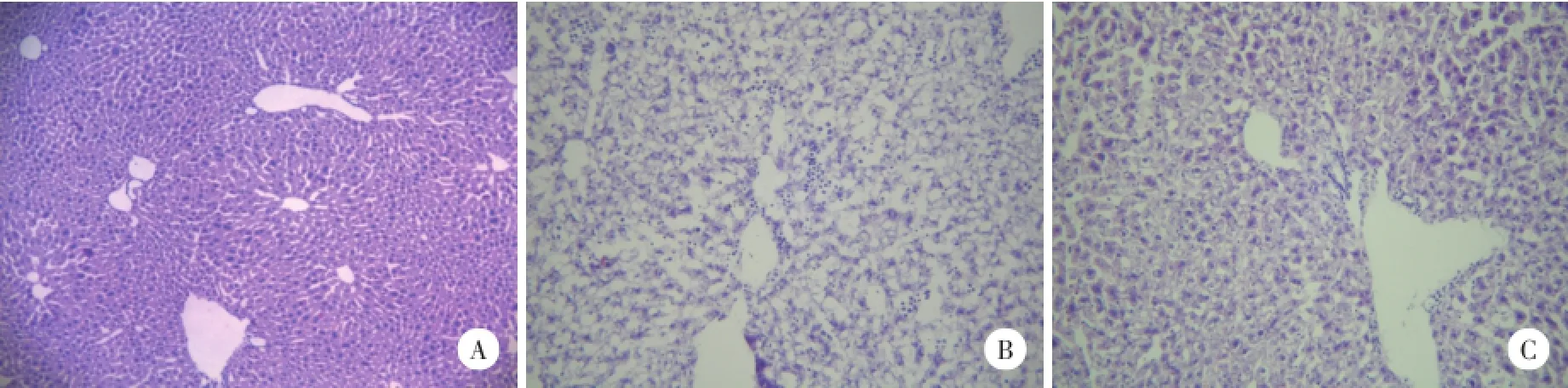
圖2 高脂飲食對肝組織結構的影響及SH2-Bβ抗體的干預作用HE×200Fig.2 Influence of high fat diet to the structures of the liver of and the effect of SH2-Bβantibody intervene HE×200
2.5 免疫組織化學染色檢測SH2-Bβ在腎組織表達
正常組腎組織(圖3A)腎小管上皮SH2-Bβ少量表達;肥胖組腎組織(圖3B)出現SH2-Bβ高表達,腎小管上皮SH2-Bβ表達上調,炎性細胞浸潤。抗體干預組腎組織(圖3C),腎小管上皮SH2-Bβ表達明顯下調,炎性細胞減少。
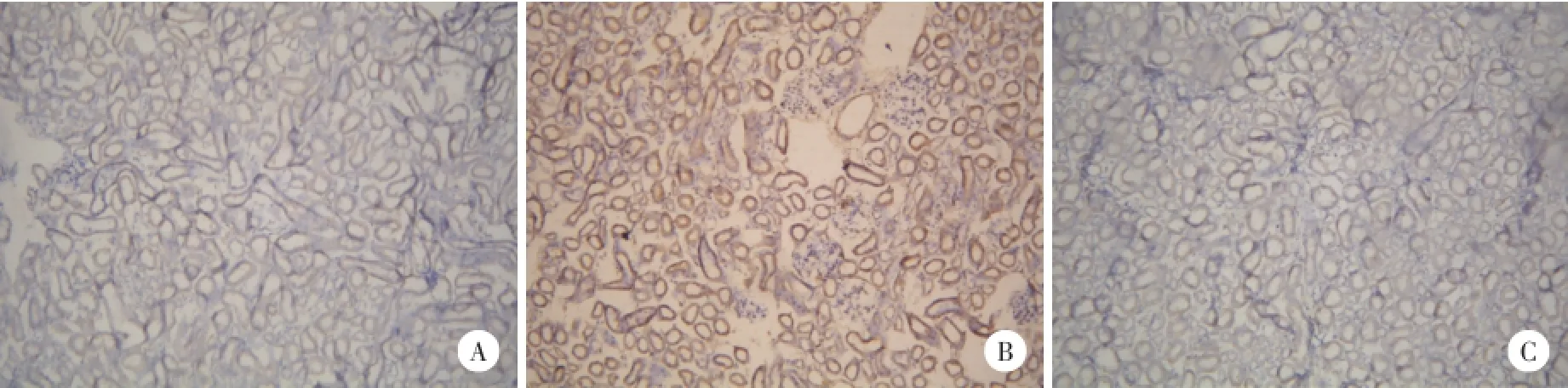
圖3 SH2-Bβ在腎組織表達免疫組化SABC法×200Fig.3 Expression of SH2-Bβin kidney Immunocytochemistry SABC method×200
3 討論
SH2-Bβ是細胞內一種連接蛋白,在多種組織表達,從昆蟲到人類的研究表明它是非常重要的代謝調節者[1,2,5]。在人類SH2-Bβ功能受損與肥胖和2型糖尿病密切相關[6]。那么,SH2-Bβ在肥胖者體內的表達變化以及其變化與體質量改變之間的關系值得關注。C57BL/6J小鼠被認為非常適合用于高脂飲食誘導營養性肥胖小鼠模型制備[7]。C57BL/6J小鼠經高脂飼料誘導4周后可形成良好肥胖模型[8]。本研究采用C57BL/6小鼠,經鼻應用SH2-Bβ抗體,觀察了SH2-Bβ蛋白在小鼠下丘腦、肝和腎的表達變化及其對攝食量及體質量的影響。
SH2-Bβ直接連接并激活JAK2,在細胞受到生長激素、瘦素、紅細胞生成素和催乳素作用時,加強JAK2信號[9,10]。SH2-Bβ也連接IRS1和IRS2,在瘦素和胰島素作用下,加強IRS蛋白介導的PI 3激酶通路激活[11]。SH2-Bβ也介導成纖維細胞生長因子(fibroblast growth factor,FGF)、神經生長因子(nerve growth factor,NGF)和血小板源性生長因子(plateletderived growth factor,PDGF)的信號轉導[12]。從SH2-Bβ參與的傳導通路可見,它可能與肥胖以及由肥胖引發的慢性炎癥密切相關,值得關注。本研究經鼻腔應用SH2-Bβ抗體,引起了下丘腦內SH2-Bβ表達上調,降低了攝食量,并降低體質量,這是首次被發現的非常重要的結果。這說明SH2-Bβ抗體可能經嗅細胞直接吸收入腦或經其他途徑到腦內發揮作用。鼻腔給藥的應用被認為存在巨大的潛在的應用價值,它使得通過口服或靜脈給藥不能通過血腦屏障的藥物得以進入腦內發揮作用[13,14]。
腦內SH2-Bβ基因敲除小鼠會導致嚴重的瘦素抵抗、胰島素抵抗,肥胖、2型糖尿病和脂肪肝[2,3],說明SH2-Bβ在中樞發揮著重要的體質量調節作用。中樞是體質量的調節者,而周圍組織是體質量調節的實現者。當腦內缺乏SH2-Bβ時,實際相當于停止了機體正常的反饋機制,高脂肥胖的信息不能被中樞感知,中樞便失去了對周圍組織的調節,包括降脂和儲脂,即能量的儲存和分解出現紊亂。而在整個外周組織敲除SH2-Bβ基因,則使高脂飲食誘導的胰島素抵抗加重[15]。對成年鼠而不是胚胎鼠進行特異性肝SH2-Bβ基因敲除,減弱了肝內甘油酰基轉移酶(DGAT2)表達,增加了甘油三酯脂酶(ATGL)表達,即減弱了肝內脂肪合成,增強了脂肪分解,導致小鼠肝內甘油三酯聚集量下降[16]。本研究經鼻腔應用SH2-Bβ抗體,引起了下丘腦內SH2-Bβ表達上調,而在肝腎組織表達下調,可以理解成SH2-Bβ抗體對中樞神經系統作用后的整體效應是攝食量降低,體質量減輕;而SH2-Bβ在肝腎組織表達下調,則增加了組織的脂肪分解,減弱了脂肪的合成。本實驗中具體表現為,抗體干預組肝組織SH2-Bβ表達下調,肝內脂質沉積為數量較少的小脂滴,肝細胞索較完整,而高脂組則出現SH2-Bβ高表達,肝細胞固縮,肝細胞索斷裂,脂滴大而多;對于腎,抗體干預組腎小管上皮SH2-Bβ表達明顯下調,而高脂組則出現SH2-Bβ高表達,炎性細胞浸潤。研究表明,高脂飼料長期大量食用造成肝腎損傷[17~20]。說明在周圍組織,脂質過氧化損傷等傷害性刺激可能會導致SH2-Bβ表達上調,進而導致更多的脂質沉積,進一步使細胞受損。所以對于周圍組織脂肪過多沉積所引起的疾病,如冠心病、脂肪肝等,可通過降低SH2-Bβ表達而得到治療。從本實驗可見,對于肥胖個體,減少攝食量可使肝腎組織SH2-Bβ表達下調,但不能排除經鼻腔應用SH2-Bβ抗體后,神經元功能發生變化而引起的神經內分泌調節,這方面還需進一步研究。
本研究表明高脂飲食經鼻腔應用SH2-Bβ抗體,可使高脂飲食小鼠下丘腦內SH2-Bβ表達上調,明顯降低了攝食量,降低體質量;同時使肝、腎內SH2-Bβ表達下調,降低了肝、腎組織的脂肪沉積,及其引發的炎性細胞浸潤。這表明SH2-Bβ在中樞通過調節進食量,在周圍組織通過改變脂肪沉積量而對體質量進行調節。
[1]Doche ME,Bochukova EG,Su HW,et al.Human SH2B1 mutations are associated with maladaptive behaviors and obesity[J].J Clin Invest,2012,122(12):4732-4736.
[2]Duan C,Yang H,White MF,et al.Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance[J].Mol Cell Biol,2004,24(17):7435-7443.
[3]Ren D,Li M,Duan C,et al.Identification of SH2-B as a key regulator of leptin sensitivity,energy balance,and body weight in mice[J]. Cell Metabolism,2005,2(2):95-104.
[4]齊金萍,王效杰,金韻,等.SH2-B β在肥胖小鼠下丘腦和肺內表達及作用[J].中國公共衛生雜志,2012,28(10):1331-1333.
[5]Song W,Ren D,Li W,et al.SH2B regulation of growth,metabolism and longevity in both insects and mammals[J].Cell Metab,2010,11(5):427-437.
[6]Bachmann-Gagescu R,Mefford HC,Cowan C,et al.Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity[J].Genet Med,2010,12(10):641-647.
[7]Halade GV,Rahman MM,Fernandes G.Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57Bl/6J mice[J].J Nutr Biochem,2010,21(4):332-337.
[8]劉芳,高南南,楊潤梅,等.不同品系小鼠肥胖模型比較及C57BL/ 6J小鼠肥胖機制研究[J].中國藥理學通報,2013,29(3):360-365.
[9]Javadi M,Hofstatter E,Stickle N,et al.The SH2B1 adaptor protein associates with a proximal region of the erythropoietin receptor[J].J Biol Chem,2012,287(31):26223-26234.
[10]Rider L,Diakonova M.Adapter protein SH2B1beta binds filamin A to regulate prolactin-dependent cytoskeletal reorganization and cell motility[J].Mol Endocrinol,2011,25(7):1231-1243.
[11]Morris DL,Cho KW,Rui L.Critical role of the Src homology 2(SH2)domain of neuronal SH2B1 in the regulation of body weight and glucose homeostasis in mice[J].Endocrinology,2010,151(8):3643-3651.
[12]Kong M,Wang CS,Donoghue DJ.Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B.A role in STAT5 activation[J].J Biol Chem,2002,277(18):15962-15970.
[13]Derad I,Sayk F,Lehnert H,et al.Intranasal angiotensinⅡin humans reduces blood pressure when angiotensinⅡtype 1 receptors are blocked[J].Hypertension,2014,63(4):762-767.
[14]Peterson A,Bansal A,Hofman F,et al.A systematic review of inhaled intranasal therapy for central nervous system neoplasms:an emerging therapeutic option[J].J Neurooncol,2014,116(3):437-446.
[15]Morris DL,Cho KW,Zhou Y,et al.SH2B1 enhances insulin sensitivity by both stimulating the insulin receptor and inhibiting tyrosine dephosphorylation of insulin receptor substrate proteins[J]. Diabetes,2009,58(9):2039-2047.
[16]Liang S,Yan L,Lin J,et al.Hepatic SH2B1 and SH2B2 regulate liver lipid metabolism and VLDL secretion in mice[J].PLoS One,2013,8(12):e83269.
[17]Declèves AE,Zolkipli Z,Satriano J,et al.Regulation of lipid accumulation by AMK-activated kinase in high fat diet-induced kidney injury[J].Kidney Int,2014,85(3):611-623.
[18]Pinhal CS,Lopes A,Torres DB,et al.Time-course morphological and functional disorders of the kidney induced by long-term highfat diet intake in female rats[J].Nephrol Dial Transplant,2013,28(10):2464-2476.
[19]Liang Y,Huang B,Song E,et al.Constitutive activation of AMPK α 1 in vascular endothelium promotes high-fat diet-induced fatty liver injury:role of COX-2 induction[J].Br J Pharmacol,2014,171(2):498-508.
[20]Xia X,Ma Y,Xing X,et al.Antioxidant and hepatoprotective effect of different extracts of guizhencao(herba bidentis bipinnatae)against liver injury in hyperlipidemia rats[J].J Tradit Chin Med,2013,33(4):518-523.
(編輯 裘孝琦)
IntranasalAdministration ofSH2-BβAntibody Inhibitsthe Weight Gain in Mice with High FatDiet
JINYun1,LIXin-ming2,WANG Xiao-jie1,ZANGJin1,QIJin-ping1,GUO Jia-mei3
(1.Department of Anatomy,Shenyang Medical College,Shenyang 110034,China;2.Department of Microbiology,Shenyang Medical College,Shenyang 110034,China;3.2012 Clinic Medicine,Shenyang MedicalCollege,Shenyang 110034,China)
ObjectiveTo explore the effect of SH2-Bβ antibody intranasal administration on weight gain of mice with high fat diet.MethodsC57BL/6 mice(female)aged 8 weeks were randomized to normal control group(n=12),obese group(n=12)and anti-SH2-Bβ(1∶50)intervene group(n=12).The amount of the food intake everyday and the weight of mice were measured every week.SH2-Bβ expression in hypothalamus and liver was detected by Western blot,and the histology change of the liver was observed by HE staining.By means of immunohistochemistry stained,SH2-Bβ expression in the kidney and the pathological change were also recorded.ResultsThe food intake and weight of obese group were higher than those of normal control group and anti-SH2-Bβ intervene group(P<0.05).Western blot result showed SH2-Bβ expression in hypothalamus of obese group were decreased compared with the normal control group and anti-SH2-Bβ intervene group(P<0.05);but SH2-Bβ expression in liver of obese group were higher compared with the normal control group and anti-SH2-Bβ intervene group(P<0.05).Liver HE staining result showed that,the hepatic cord arranged in order,steatosis of the hepatic cells were decreased and lipid droplets became smaller,the inflammatory cells were decreased in anti-SH2-Bβ intervene group as compared with obese group.Immunohistochemistry result showed SH2-Bβ expression in kidney of obese group were reduced and the inflammatory of cells infiltrated obviously compared with the normal control group and anti-SH2-Bβ intervene group(P<0.05).ConclusionIntranasal administration of SH2-Bβ antibody upregulated SH2-Bβ expression in hypothalamus,decreased the amountofthe food intake and the weightofmice with high fatdiet;SH2-Bβexpression in liverand kidney was downregulated,fatdeposition was reduced in liver and kidney,and the inflammation and injury induced by fat deposition was also decreased.It is suggested that SH2-Bβ regulates the weightgain through the centraleffectto regulate food intake and through the peripheraleffectto change fatdeposition.
SH2-Bβ;obesity;weight;mice;intranasal administration
R392.5;R562.2
A
0258-4646(2014)09-0809-05
遼寧省教育廳高校科研計劃(20060890);沈陽醫學院優秀人才資助(20073022)
金韻(1963-),女,副教授,本科.
齊金萍,E-mail:qijinping2013@163.com
2014-05-09
網絡出版時間:

