DNA-PK基因沉默對卵巢癌細胞細胞凋亡的影響
趙 靜,陳 珂
DNA-PK基因沉默對卵巢癌細胞細胞凋亡的影響
趙 靜,陳 珂*
(南京明基醫院南京醫科大學附屬醫院婦產科,江蘇南京210000)
目的 探討DNA-PK基因對沉默卵巢癌細胞凋亡的影響及機制。方法 DNA-PK shRNA以脂質體介導轉染卵巢癌細胞株Skov3,24h后接受2Gy X射線照射,繼續培養48h。收集細胞,標記AnnexinⅤ/PI,流式細胞術分析細胞凋亡情況,同時,RT-PCR檢測p53和p21mRNA的表達量。結果 2Gy輻射引起2.40%±1.53%Skov3細胞凋亡,高于對照組(t=2.618,P=0.026),而轉染DNA-PK shRNA的Skov3細胞經2Gy照射,凋亡細胞為18.74%±4.15%,明顯高于單純2Gy照射組(t=9.052,P<0.001)及DNA-PK shRNA轉染組(2.77%±1.73%)(t=8.869,P<0.001)。RT-PCR檢測結果顯示,2Gy照射組,Skov3細胞p53mRNA表達量高于對照組,而DNA-PK shRNA轉染的Skov3細胞接受2Gy照射,其p53mRNA表達量降低,接近正常水平,單獨轉染DNA-PK shRNA組p53mRNA表達量無明顯變化。轉染DNA-PK shRNA的Skov3細胞經2Gy照射,其p21mRNA表達量低于2Gy照射組接近正常組水平,單獨轉染DNA-PK shRNA組Skov3細胞p21mRNA表達水平與正常對照組相當。結論DNA-PK shRNA可增加輻射后巢癌細胞株的細胞凋亡,其可能機制是通過下調p53和p21mRNA表達,而啟動細胞凋亡。
DNA-PK;干擾RNA;卵巢癌細胞Skov3;輻射損傷;DNA損傷
(Chin J Lab Diagn,2014:18:0200)
卵巢癌是婦科中致死率高、發病率高的惡性腫瘤,對放化療不敏感。這也是臨床腫瘤治療實踐及目前研究的熱點問題。
細胞受到射線照射后會產生DNA損傷,損傷的DNA激活損傷應答激酶ATM、ATR,進而調控細胞周期檢查,阻滯細胞周期進程,修復損傷的DNA。DNA-PK是DNA損傷識別與修復的關鍵酶,決定受損細胞的轉歸[1]。可見DNA損傷修復應答是細胞放化療抵抗的重要原因。因此本文擬以輻射作為損傷手段,制備細胞DNA損傷模型,干擾DNA損傷識別、修復關鍵酶DNA-PK,試圖探討DNA-PK對放射損傷的Skov3細胞凋亡的影響及機制,為臨床卵巢癌臨床治療的新方法新藥物開發提供新靶點。
1 材料和方法:
1.1 主要試劑和儀器
DMEM、LipofectimineTM2000,Invitrogen公司;小牛血清,Hyclone公司;胰酶、DEPC,Sigma公司;AnnexinⅤ/PI,碧云天生物技術研究所;TRIzaol,Invitrogen公司;Sensiscript RT Kit,QIAGEN公司;DNA marker DL2000,寶生物工程(大連)有限公司;引物由生工生物工程(上海)股份有限公司合成;卵巢癌細胞株Skov3,本室保存;DNA-PK shRNA,本室構建。FACScan流式細胞儀,BD公司;飛利浦深部X射線治療機,Philips;PCR儀,ABI;凝膠成像系統,Bio Rad。
1.2 方法
1.2.1 細胞培養及shRNA轉染 Skov3細胞常規培養,DMEM,加10%小牛血清、青霉素100U/ml、鏈霉素100μg/ml。37℃、5%CO2培養箱培養。DNA-PK shRNA以脂質體LipofectimineTM2000介導轉染Skov3細胞株,質粒與脂質體的比例為2 μg∶5μl。具體操作按說明書進行。
1.2.2 照射 收集細胞,計數,接種6孔培養板,每孔接種3×105個細胞,第二天采用深部X射線治療機進行照射,電壓200kV,電流10mA,濾板0.5 mm Cu和1.0mm Al。球靶距50cm,劑量率0.287Gy/min。
1.2.3 流式細胞術分析DNA-PK shRNA對Skov3細胞2Gy照射誘導凋亡的影響
DNA-PK shRNA載體轉染細胞后24h,照射2Gy,繼續培養48h,分別收集各組細胞,標記AnnexinⅤ/PI,流式細胞術分析其凋亡。
1.2.4 RT-PCR檢測DNA-PK shRNA轉染細胞p53和p21表達量的變化
收集細胞,應用TRIzol提取RNA,按Sensiscript RT Kit試劑盒說明進行RT-PCR。1.2%瓊脂糖凝膠電泳鑒定PCR產物,凝膠成像系統觀察并拍照。
1.2.5 統計學分析 應用SPSS14進行統計分析。
2 結果
2.1 DNA-PK shRNA對2Gy輻射誘導Skov3細胞凋亡的影響
2Gy輻射引起2.40%±1.53%Skov3細胞凋亡,高于對照組(t=2.618,P=0.026),而轉染DNA-PK shRNA的Skov3細胞經2Gy照射,凋亡細胞為18.74%±4.15%,明顯高于單純2Gy照射組(t=9.052,P<0.001)及DNA-PK shRNA轉染組(2.77%±1.73%)(t=8.869,P<0.001)(表1)。
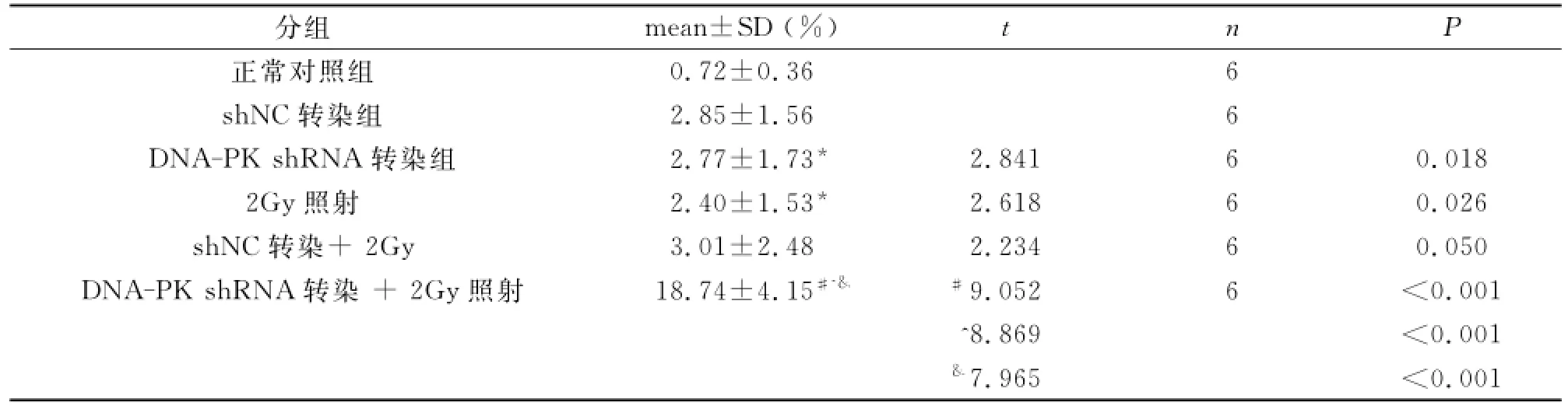
表1 DNA-PK shRNA對2Gy輻射誘導Skov3細胞凋亡的影響
2.2 RT-PCR檢測DNA-PK shRNA轉染細胞p53和p21表達量的變化
RT-PCR檢測結果顯示,2Gy照射組,Skov3細胞p53mRNA表達量高于對照組,而DNA-PK shRNA轉染的Skov3細胞接受2Gy照射,其p53 mRNA表達量降低,接近正常水平,單獨轉染DNA-PK shRNA組p53mRNA表達量無明顯變化,接近正常組水平(圖1a)。轉染DNA-PK shRNA的Skov3細胞經2Gy照射,其p21mRNA表達量低于2Gy照射組接近正常組水平,單獨轉染DNA-PK shRNA組Skov3細胞p21mRNA表達水平與正常對照組相當(圖1b)。
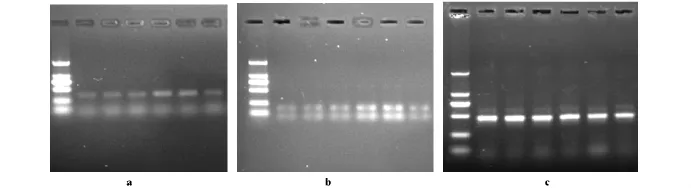
圖1 RT-PCR檢測DNA-PK shRNA轉染細胞p53和p21表達量的變化
3 討論
卵巢癌臨床治療的難題是其對放化療具有抵抗性,而影響疾病的轉歸。放化療對腫瘤細胞殺傷效應主要是造成腫瘤細胞的DNA損傷,而細胞具有DNA損傷修復能力[2-4]。當損傷發生時,細胞周期檢查點被激活[5],指導周期特異性修復機制(HR和NHEJ等)[6]。因此,如果能有效的干擾細胞周期檢查點和修復系統,則可以誘使大量的腫瘤細胞進入凋亡程序。
在DNA損傷修復中DNA-PK是關鍵酶。為此,本文應用放射方法,體外建立細胞DNA損傷模型,干擾DNA-PK沉默其表達,觀察其對卵巢癌細胞Skov3凋亡的影響。流式細胞術分析顯示,轉染DNA-PK shRNA的Skov3細胞經2Gy照射,凋亡細胞為18.74%±4.15%,明顯高于單純2Gy照射組(2.40%±1.53%)(t=9.052,P<0.001)及DNA-PK shRNA轉染組(2.77%±1.73%)(t=8.869,P<0.001)。表明DNA-PK shRNA可提高輻射誘導的Skov3細胞凋亡。
如果修復失敗,檢查點通過啟動p53-依賴或p53-非依賴途徑使細胞發生凋亡。在本研究中DNA-PK shRNA轉染的Skov3細胞接受2Gy照射,與單純2Gy照射組對比其p53mRNA表達量下降,減少其下游p21轉錄,使其mRNA表達量下降,致使Cdk2-cyclinE復合物活性增加,解除細胞周期阻滯,增加細胞凋亡。提示干擾DNA-PK,通過下調p53和p21mRNA表達量,啟動細胞凋亡,從而增加放射誘導的細胞凋亡。本研究結果提示在臨床卵巢癌治療時短期大劑量照射,降低腫瘤細胞修復后的增殖機會,可提高治療效果。
[1]Chapman JR,Taylor MR,Boulton SJ.Playing the end game:DNA double-strand break repair pathway choice[J].Mol Cell,2012,47(4):497.
[2]Kinsella TJ.Understanding DNA damage response and DNA repair pathways:applications to more targeted cancer therapeutics[J].Semin Oncol,2009,36(2Suppl 1):S42.
[3]Smith J,Tho LM,Xu N,et al.The ATM-Chk2and ATR-Chk1 pathways in DNA damage signaling and cancer[J].Adv Cancer Res,2010,108:73.
[4]Deckbar D,Jeggo PA,L?brich M.Understanding the limitations of radiation-induced cell cycle checkpoints[J].Crit Rev Biochem Mol Biol,2011,46(4):271.
[5]Lossaint G,Besnard E,Fisher D,et al.Chk1is dispensable for G2 arrest in response to sustained DNA damage when the ATM/p53/p21pathway is functional[J].Oncogene,2011,30(41):4261.
[6]Landsverk KS,Patzke S,Rein ID,et al.Three independent mechanisms for arrest in G2after ionizing radiation[J].Cell Cycle,2011,10(5):819.
The effects of DNA-PK gene silencing to cell apoptosis on ovarian cancer
ZHANG Jing,CHEN Ke.
(Affiliated Hos-pital of Nanjing Medical University,Nanjing Mingji Hospital,Obstetrics and Gynecology,Nanjing210000,China)
Objective To explore the effects and mechanism that DNA-PK gene silencing to cell apoptosis on ovarian cancer cells.Methods Constructed DNA-PK shRNA and applied liposome-mediated transfection to ovarian cancer cells Skov3,after 20h,giving 2Gy X-ray irradiation and continued to train until 48h.Then the cells were collected,and AnnexinⅤ/PI was labeled,flow cytometry was applied to analyze the apoptosis of cells,while RT-PCR was used to detecte of p53and p21mRNA expression.Results After 2Gy radiation,2.40%±1.53%Skov3turn out cells apoptosis,higher than the control group,the differences were significant(t=2.618,P=0.026),and the Skov3cells were transfected DNA-PK shRNA that after 2Gy irradiation,apoptotic cells was 18.74%±4.15%,significantly higher than only 2Gy irradiation group(t=9.052,P<0.001)and single DNA-PK shRNA transfection group(2.77%± 1.73%),the differences were significant(t=8.869,P<0.001).The results of RT-PCR showed that the mRNA expression of Skov3cells p53in 2Gy irradiation group was higher than the normal group,while the mRNA expression of p53in the DNA-PK shRNA transfected Skov3cells and received 2Gy irradiation group decreased to near normal levels,the mRNA expression of p53in single transfection DNA-PK shRNA group did not change.DNA-PK shRNA transfected Skov3cells were given 2Gy irradiation,the mRNA expression of p21levels was lower than the 2Gy irradiation group,and it’s close to the level of the normal group,the mRNA expression of p21in only DNA-PK shRNA transfected Skov3cells group is near the control group.Conclusion DNA-PK shRNA can increase ovarian cancer cell line apoptosis after radiation,and its possible mechanism is that the mRNA expression of p53and p21decreased and then initiate apoptosis.
DNA-PK;Interfering RNA;Ovarian cancer Skov3;Radiosensitization;DNA damage
R737.31
A
2013-01-25)
1007-4287(2014)02-0200-03
*通訊作者

