支氣管肺泡灌洗液4種新型腫瘤標志物檢測對周圍型肺癌的診斷價值
張實,張明周,吳學玲
·臨床研究·
支氣管肺泡灌洗液4種新型腫瘤標志物檢測對周圍型肺癌的診斷價值
張實,張明周,吳學玲
目的 探討支氣管肺泡灌洗液(BALF)和血清中4種新型腫瘤標志物熱休克蛋白90α(HSP90α)、谷胱甘肽S
轉移酶P1(GSTP1)、泛素特異性肽酶8(USP8)、幾丁質酶3樣蛋白1(CHI3L1)在周圍型肺癌診斷中的臨床價值。方法采用酶聯免疫吸附法檢測100例周圍型肺癌住院患者及50例肺部良性疾病患者BALF和血清中HSP90α、GSTP1、USP8、CHI3L1的濃度。分析4種腫瘤標志物含量與不同細胞類型、患者年齡、肺癌分期之間的相關性,以及對周圍型肺癌的診斷敏感性和特異性。結果 肺癌患者BALF中HSP90α平均濃度顯著高于肺部良性疾病顯著(P<0.05),診斷敏感性為77.0%,特異性為82.0%;肺癌患者血清中HSP90α平均濃度與肺部良性疾病患者比較差異無統計學意義(P>0.05)。肺癌患者BALF中CHI3L1平均濃度顯著高于肺部良性疾病顯著(P<0.05),診斷敏感性為72.0%,特異性為80.0%;肺癌患者血清中CHI3L1平均濃度與肺部良性疾病患者比較差異無統計學意義(P>0.05)。小結節肺癌(原發病灶≤1cm)患者BALF 中HSP90α及CHI3L1平均濃度與肺部良性疾病患者比較差異無統計學意義(P>0.05)。肺癌患者BALF及血清中USP8、GSTP1平均濃度與肺部良性疾病患者比較差異無統計學意義(P>0.05)。肺癌患者BALF及血清中4種腫瘤標志物濃度與年齡、肺癌分型(腺癌或鱗癌)、肺癌分期(Ⅰ-Ⅳ期)等因素無相關性;CHI3L1在肺癌患者BALF中的濃度與原發灶直徑有一定相關性(P<0.05),Pearson相關系數為0.203;其他3種肺癌標志物在BALF、血清中的濃度與腫瘤原發灶直徑無相關性。結論 檢測BALF中HSP90α及CHI3L1濃度對周圍型肺癌的診斷有一定價值,且優于血清腫瘤標志物。
支氣管肺泡灌洗液;腫瘤標記,生物學;肺腫瘤;診斷
肺癌是全球發病率最高的惡性腫瘤之一,死亡率在各類腫瘤中居首位,全球每年約有100萬人死于肺癌[1]。盡管現代診療水平不斷上升,但肺癌的長期生存時間與過去20年相比并無明顯改善[2-3]。提高肺癌生存期的關鍵手段是早期診斷[4],纖維支氣管鏡是目前診斷中央型肺癌的主要工具[5],氣管鏡獲取組織、支氣管肺泡灌洗液(bronchoalveolar lavage fluid,BALF)、纖刷物等可為腫瘤細胞學檢查提供標本[6]。然而,周圍型肺癌超出了氣管鏡視野,纖維支氣管鏡往往不能獲得組織標本[7],部分腫塊靠近肺門或者患者存在肺大泡、肺氣腫等客觀因素導致肺穿刺取活檢風險大,造成此類患者確診困難[8]。取肺癌患者的血液標本檢測腫瘤標志物也是一種積極有效的檢查方法[9],其中的一些指標如癌胚抗原(CEA)、細胞角蛋19片段CYFRA21-1、神經特異性烯醇化酶(NSE)等已作為臨床常用腫瘤標志物廣泛應用[10-11]。然而目前尚無一種敏感性、特異性均高的血清標志物[12-13]。
有研究表明,從腫瘤原發病變部位獲取標本檢測腫瘤標志物,其敏感性高于血清學檢測[14],如對肺癌組織分泌至肺泡中的物質進行檢測,其診斷敏感性高于血液標本,可對周圍型肺癌的診斷起輔助作用。本研究對肺癌患者BALF中4種新型腫瘤標志物熱休克蛋白90α(HSP90α)、谷胱甘肽S轉移酶P1(GSTP1)、泛素特異性肽酶8(USP8)、幾丁質酶3樣蛋白1(CHI3L1)進行篩查,以期發現比血液標本更為敏感的腫瘤標志物。
1 資料與方法
1.1 研究對象 150例患者均為第三軍醫大學新橋醫院2013年7月-2014年5月呼吸內科住院患者。肺癌組100例,均為周圍型肺癌,無法進行氣管鏡鉗取活檢,均經肺穿刺活檢與手術病理切片檢查確診為肺癌(鱗癌與腺癌)。對照組為同期住院的肺部良性疾病(包括肺炎、肺結核、塵肺、支氣管擴張、慢性嗜酸粒細胞肺炎)患者,共50例。兩組患者一般情況見表1。
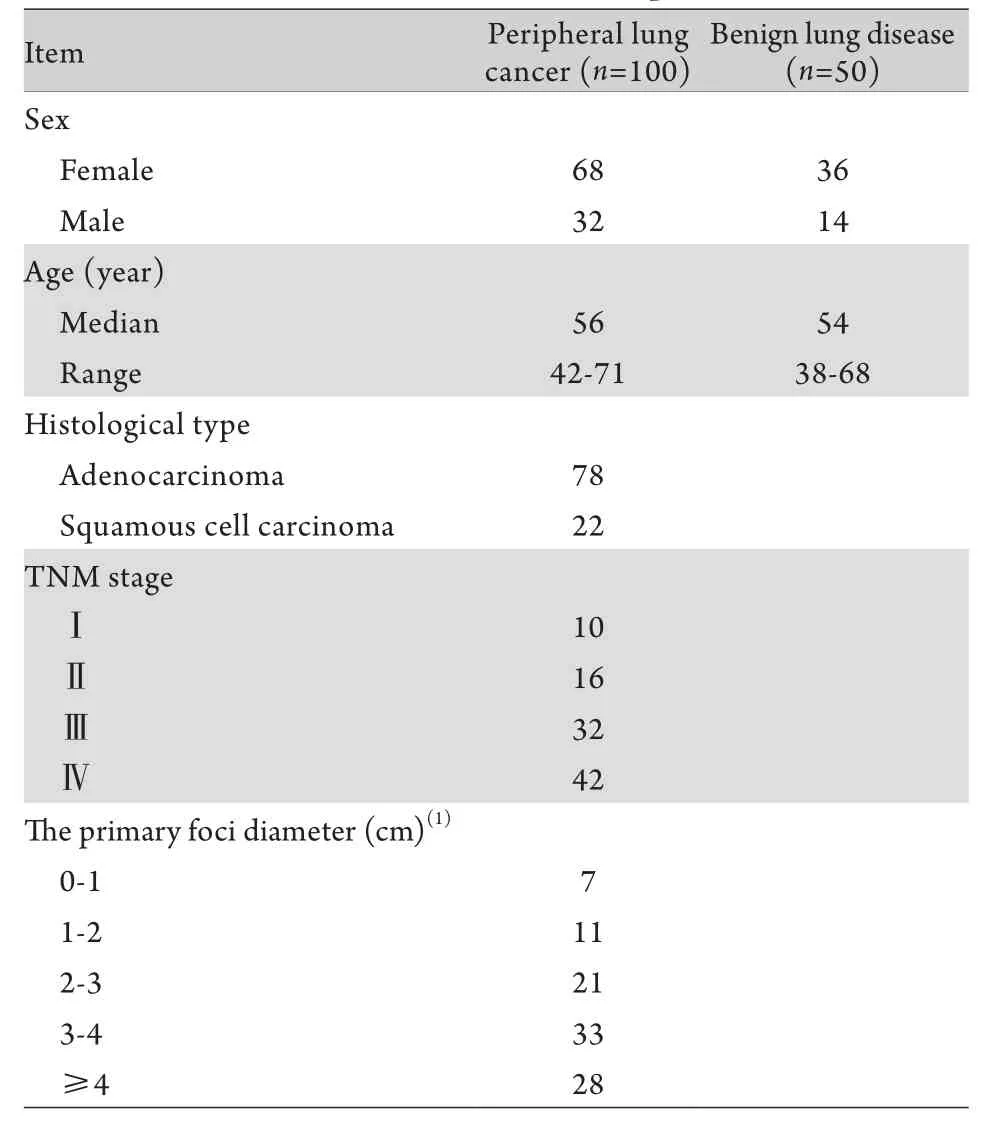
表1 入組患者基本信息Tab. 1 Clinical data of the patients
1.2 標本收集 血清:清晨取患者空腹靜脈血4ml,2000r/min離心20min,上清–80℃保存。BALF:兩組患者均按纖維支氣管鏡常規操作技術,將纖維支氣管鏡遠端楔于腫瘤或其他病變所在的段支氣管開口處,彌漫病變則沖洗右中葉或左舌葉。注入室溫生理鹽水,50ml/次,灌洗2次,以50~100mmHg負壓吸引,回收40%~60%的BALF,經雙層無菌紗布過濾后2000r/min離心10min,上清–80℃保存。
1.3 檢測方法 4種腫瘤標志物USP8、HSP90α、CHI3L1和GSTP1均采用酶聯免疫分析法,檢測試劑盒由上海滬尚生物科技有限公司提供,按照試劑盒說明書步驟進行操作。
1.4 統計學處理 采用SPSS 13.0軟件進行統計分析。計量資料以±s表示,經方差齊性檢驗,4種腫瘤標志物在兩組患者BALF及血清中的濃度均服從正態分布(P>0.05)。兩組比較采用兩獨立樣本t檢驗,多組間比較采用單因素方差分析;腫瘤標志物含量與年齡、原發灶直徑的關系采用Pearson相關分析,與肺癌TNM分期的關系用Spearman相關分析。用受試者工作特征曲線(ROC曲線)來定義診斷界值,計算曲線下面積并描述其診斷敏感性及特異性。P<0.05為差異有統計學意義。
2 結 果
2.1 兩組患者BALF與血清中4種腫瘤標志物濃度比較 肺癌患者BALF中HSP90α及CHI3L1平均濃度顯著高于肺部良性疾病患者(P<0.05),而血清中HSP90α和CHI3L1的平均濃度與肺部良性疾病患者比較差異無統計學意義(P>0.05);肺癌患者BALF及血清中USP8和GSTP1的平均濃度與肺部良性疾病患者比較差異均無統計學意義(P>0.05,表2)。

表2 4種腫瘤標志物在肺癌組與肺部良性疾病組的比較Tab. 2 Comparison of the levels of 4 tumor markers between peripheral lung cancer (PLC) and benign lung disease (BLD) patients
肺癌患者BALF中HSP90α及CHI3L1平均濃度顯著高于肺部良性疾病患者。為進一步明確HSP90α和CHI3L1能否為周圍型肺癌的早期診斷提供幫助,進一步比較小結節肺癌(原發病灶直徑≤1cm)與肺部良性病變之間的差異性。HSP90α 及CHI3L1在小結節肺癌組的濃度服從正態分布(P>0.05),統計學分析顯示,小結節肺癌組BALF中HSP90α及CHI3L1濃度(分別為150.05±54.10pg/ml 和18.67±6.20ng/ml)雖然稍高于肺部良性疾病組(分別為141.63±49.40pg/ml和15.85±11.32ng/ml),但兩組間差異無統計學意義(P>0.05)。
2.2 4種腫瘤標志物的含量與不同細胞類型、患者年齡、肺癌分期之間相關性的分析 肺腺癌患者與肺鱗癌患者BALF、血清中4種腫瘤標志物平均濃度無明顯差別(P>0.05),表明4種腫瘤標志物的濃度與肺癌分型無明顯相關性(鱗癌與腺癌)。對肺癌患者BALF及血清中4種腫瘤標志物濃度與患者年齡、原發灶直徑、肺癌分期(Ⅰ-Ⅳ期)的關系進行分析,結果顯示僅BALF中CHI3L1的濃度與原發灶直徑有一定相關性,Pearson相關系數為0.203(P<0.05),關聯性較低(表3)。
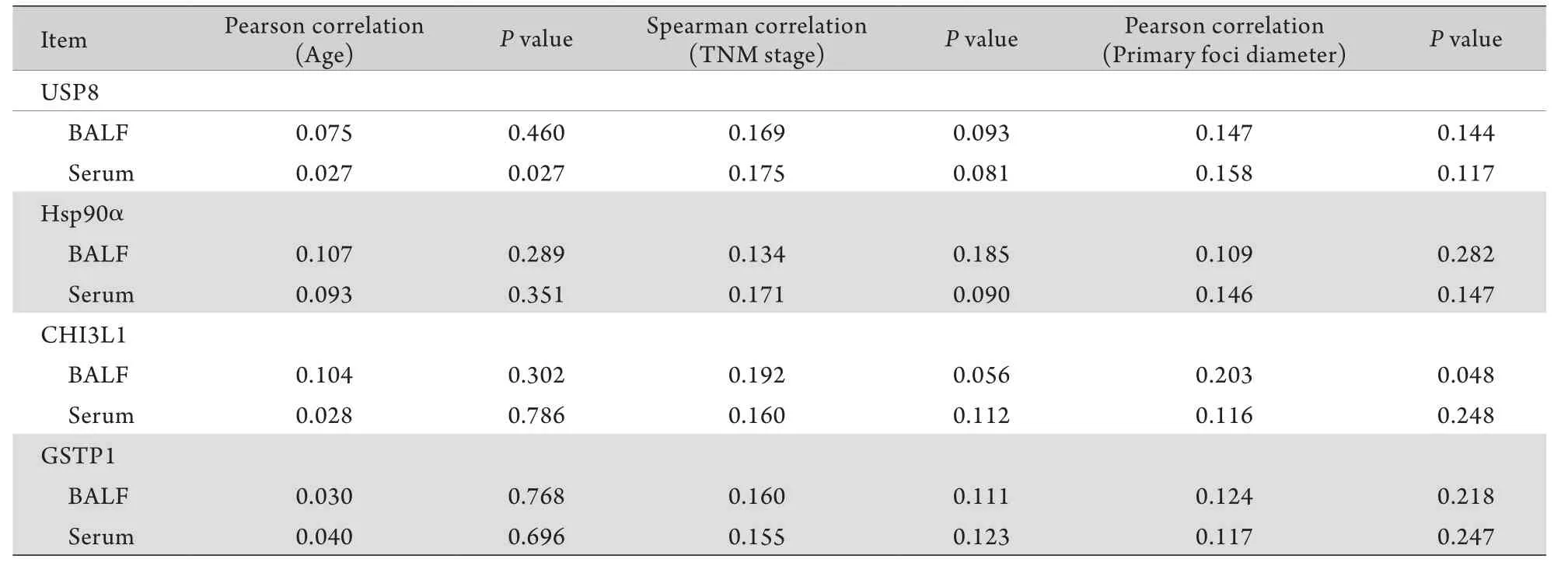
表3 4種腫瘤標志物濃度與肺癌患者年齡、腫瘤分期、原發灶直徑的相關性分析Tab.3 Correlation analysis of tumor marker concentrations to patients' age, TNM stage and primary foci diameters
2.3 CHI3L1、HSP90α對周圍型肺癌的診斷敏感性及特異性 BALF和血清中CHI3L1及HSP90α的ROC曲線如圖1所示,計算曲線下面積,并通過曲線拐點即敏感性+特異性最大值處確定診斷二者對周圍型肺癌的診斷界值。通過診斷界值,確定兩種標志物的診斷敏感性及特異性(表4)。
3 討 論
腫瘤標志物是細胞在癌變的發生、發展、浸潤及轉移過程中所分泌、產生的一些活性物質,它們存在于癌組織和宿主體液中,其實質是由腫瘤組織產生的反映腫瘤自身存在的化學物質[15]。腫瘤標志物通過腫瘤細胞分泌或腫瘤細胞凋亡后釋放到體液(血液、痰等)中,因此在體液標本中檢測腫瘤標志物可以為肺癌的診斷提供幫助。一些經典的標志物如CEA、NSE、CYFRA21-1等已經廣泛應用于臨床[16-17]。同時,一些有潛力的新型肺癌標志物如USP8等引發了很多學者的興趣,并取得了很多進展。
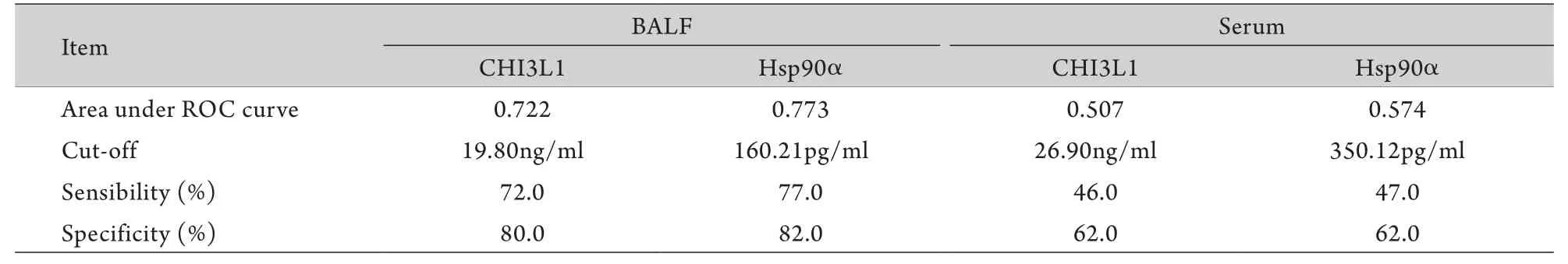
表4 BALF及血清CHI3L1和HSP90α診斷周圍型肺癌的相關參數Tab. 4 Corresponding parameters of CHI3L1 and HSP90α in BALF and serum in diagnosis of peripheral lung cancer
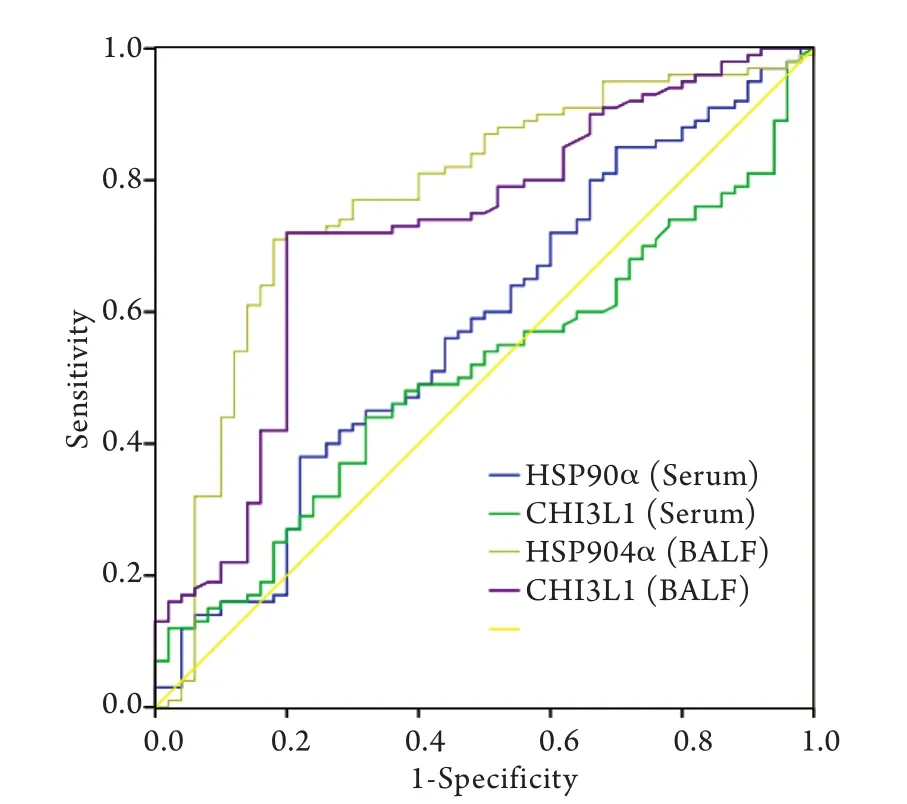
圖1 BALF及血清標本中CHI3L1、HSP90α的ROC曲線Fig. 1 The ROC curve of CHI3L1 and HSP90α in BALF and serum
泛素-蛋白酶體通路是人體內降解蛋白質的主要途徑之一,該過程可以被泛素特異性肽酶(USP)介導的去泛素化抑制[18]。腫瘤細胞內的蛋白質也可以經過泛素-蛋白酶體通路降解為氨基酸。然而,腫瘤細胞中富含很多泛素特異性肽酶如USP8,這些USP可以抑制泛素-蛋白酶體通路降解腫瘤細胞中的蛋白質,延長腫瘤細胞壽命[19]。Baykara等[20]的研究表明,肺癌患者血清中的USP8水平顯著高于健康志愿者(P<0.01),可作為肺癌標志物。Byun等[21]的研究表明,USP8抑制劑可有效殺死小鼠腫瘤細胞,且對正常細胞損害不大,提示USP8可作為肺癌靶向治療的目標。
熱休克蛋白在機體受到應急等刺激時大量分泌。HSP90是熱休克蛋白中的一種,是細胞突變中的一個重要緩沖因子,可以糾正突變蛋白所發生的錯誤折疊[22]。HSP90與腫瘤關系密切,多項研究表明其在腫瘤細胞中高表達并可與P53等癌基因產物結合,使后者不受蛋白酶降解,半衰期延長,從而保護腫瘤細胞[23]。HSP90的抑制劑如替拉替尼等可作為腫瘤靶向治療藥物,目前正在被廣泛研究[24]。一些學者認為,HSP90α可以作為肺癌標志物用于臨床[25]。
谷胱甘肽S轉移酶(GST)是一種多功能代謝Ⅱ相酶,主要生理作用是促進體內有毒物質與谷胱甘肽結合,起到解毒作用[26]。很多致癌物質本身并沒有致癌毒性,在體內經代謝后與遺傳物質發生作用,才能起到致癌作用[27]。GST-P1為GST同工酶的一種,與腫瘤關系密切[28]。Eimoto等[29]采用免疫組化法證實了肺癌組織中GST-P1含量高于癌旁組織(P<0.05)。Hida等[30]的實驗證明NSCLC患者血清中GST-P1含量高于健康志愿者(P<0.05)。Howie等[31]的實驗也證實肺癌患者血漿中GST-P1含量高于健康志愿者(P<0.05)。
Chitinase-3-like蛋白1(CHI3L1)是一種分泌糖蛋白,由巨噬細胞、軟骨及細胞及某些腫瘤細胞分泌,具體生理作用尚不清楚,目前認為其參與炎癥及組織重塑過程[32]。Yan等[33]的實驗表明肺癌組小鼠BALF及血清中CHI3L1含量顯著高于正常組小鼠(P<0.01),可作為肺癌標志物。Th?m等[34]的研究表明血清中CHI3L1含量高的肺癌患者生存期短于CHI3L1含量低的肺癌患者,故可作為判斷肺癌預后的一項標志物。
周圍型肺癌系指發生于肺段以下支氣管直到細小支氣管的肺癌,臨床癥狀出現較晚,以腺癌、鱗癌多見[35]。應用纖維支氣管鏡通常無法取到病變部位的病理組織,但肺穿刺取病理組織風險高、術后并發癥較多。盡管支氣管鏡超聲和電磁導航支氣管鏡可以為孤立性肺結節的診斷提供幫助[36-38],但這些檢查在中國絕大多數醫院還沒有開展。尋找高診斷效能的檢測方法,可以為孤立性肺結節的診斷與鑒別診斷提供幫助,解決臨床實際問題。
在原發病灶獲取標本,其診斷效能必然高于循環標本(血液標本),這是由于從原發灶獲取的標本更能反映病變情況,且該標本沒有受到循環、代謝、溶血等因素的影響。該觀點在臨床已得到證實,如半乳甘露聚糖實驗(GM實驗)中BALF標本的敏感性遠遠高于血液標本,而抗酸染色時采用BALF標本可提高診斷效能。因此,在測定腫瘤標志物時BALF標本優于血液標本,可以為周圍型肺癌的臨床診斷提供幫助。例如,Deng等[39]和Li等[40]已經證實在BALF中檢測CEA、NSE、CYFRA21-1、鱗狀細胞癌抗原(SCC-Ag)對肺癌的診斷價值優于血清標本;Ghosh等[41]也證實了肺癌患者BALF中CA-125、CA-153、CA-199的平均濃度高于血清,在BALF中檢測CA-125、CA-153、CA-199對肺癌的診斷價值優于血清。然而,對于USP8等新型腫瘤標志物在BLAF中的檢測結果對周圍型肺癌的診斷價值目前尚不明確。本研究針對USP8等4種新型有潛力的腫瘤標志物進行BALF及血清濃度檢測,以探究其對周圍型肺癌的診斷價值。結果顯示,肺癌患者BALF中HSP90a及CHI3L1濃度顯著高于肺部良性病變患者(P<0.05);HSP90α、CHI3L1在兩組患者血清中差異無統計學意義,GSTP1、USP8在兩組患者BALF及血清中差異無統計學意義,考慮為樣本量不足或其診斷敏感性偏低所致。針對HSP90α及CHI3L1兩種標志物,通過ROC曲線獲得最佳界值,使每種標志物的診斷特異性高達80%以上,在這種特異性下的診斷敏感性是可靠的,其中HSP90α診斷敏感性為77.0%,特異性為82.0%,CHI3L1診斷敏感性為72.0%,特異性為80.0%,與CEA、NSE等經典標志物相比不相上下。通過ROC曲線計算出HSP90α 及CHI3L1兩種BALF標志物的曲線下面積分別為0.722與0.773,再次印證了兩種BALF標志物對周圍型肺癌的診斷效能比較可靠。同時,為進一步探究HSP90α及CHI3L1兩種BALF標志物對超早期肺癌的診斷價值,本研究將小結節肺癌組與肺部良性病變組進行比較,雖然統計結果未見顯著差異,但小結節肺癌組的平均濃度有高于肺部良性病變組的趨勢,可擴大樣本量進行進一步觀察。
本研究中,肺癌組BALF中HSP90α及CHI3L1濃度顯著高于肺部良性病變組,而兩組患者血清中兩種標志物濃度無明顯差異,再次印證了BALF腫瘤標志物標本檢測敏感性優于血清標本,與Li等[14]及Ghosh等[38]的結論一致。
總之,盡管本研究中腫瘤標志物的檢測方法并非診斷肺癌的金標準,但在BALF檢測腫瘤標記物可作為診斷周圍型肺癌的一種補充手段,為臨床醫生提供新的思路與方法。
[1] Thunnissen E, van der Oord K, den Bakker M. Prognostic and predictive biomarkers in lung cancer. A review[J]. Virchows Arch, 2014, 464(3): 347-358.
[2] Li WB, Gao DW, Lu WN, et al. Analysis of complications and outcomes after pulmonary resection in patients aged 80 years or over with non-small cell lung cancer[J]. Med J Chin PLA, 2014, 39(10): 823-825. [李文兵, 高德偉, 盧文寧, 等. 80歲以上老年非小細胞肺癌切除術后并發癥及預后分析[J]. 解放軍醫學雜志, 2014, 39(10): 823-825.]
[3] Zhang LL, Wang L, Chen Z, et al. Efficacy analysis of two drugs consisting platinum combined with first-line chemotherapeutics regimens on 117 elderly pateints with advanced non-small cell lung carcinoma[J]. Med J Chin PLA, 2013, 38(8): 644-648. [張黎黎, 王莉, 陳卓, 等. 一線含鉑兩藥化療方案治療117例老年晚期非小細胞肺癌的療效分析[J]. 解放軍醫學雜志, 2013, 38(8): 644-648.]
[4] Buettner R, Heydt C. Biomarker analysis from a pathologist's view. Founding the rationale for personalised treatment of lung cancer[J]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz, 2013, 56(11): 1502-1508.
[5] Gao L, Asmitanand T, Ren H, et al. Fiber-optic bronchoscope and detection of lung cancer: a five year study[J]. Neoplasma,2012, 59(2): 201-206.
[6] Li LD, Mo BW, Yu HN, et al. The Diagnostic Value of Sox2 mRNA Transcription Level in Bronchoscopy Biopsy Specimens in Lung Cancer[J]. Tianjin Med J, 2014, 42(4): 301-304. [李勞冬, 莫碧文, 于會娜, 等. 纖維支氣管鏡活檢組織Sox2基因對肺癌的診斷價值[J]. 天津醫藥, 2014, 42(4): 301-304.]
[7] Fukui T, Mitsudomi T. Small peripheral lung adenocarcinoma: clinicopathological features and surgical treatment[J]. Surg Today, 2010, 40(3): 191-198.
[8] Lal H, Neyaz Z, Nath A, et al. CT-guided percutaneous biopsy of intrathoracic lesions[J]. Korean J Radiol, 2012, 13(2): 210-226.
[9] Harmsma M, Schutte B, Ramaekers FC. Serum markers in small cell lung cancer: opportunities for improvement[J]. Biochim Biophys Acta, 2013, 1836(2): 255-272.
[10] Dragomir A, Moldoveanu E, Mihǎltan F. Update regarding the role of biomarkers in early diagnosis of non-small cell bronchopulmonary cancer[J]. Pneumologia, 2011, 60(1): 7, 9-13.
[11] Li YK, Wei S, Li XR, et al. Influence of preoperative peripheral blood CYFRA21-1 level on the prognosis of non-small-cell lung cancer patients[J]. Med J Chin PLA, 2014, 39(5): 406-410. [李旸凱, 魏晟, 李曉蓉, 等. 術前外周血CYFRA21-1水平對非小細胞肺癌手術患者預后的影響[J].解放軍醫學雜志, 2014, 39(5): 406-410.]
[12] Cagle PT, Allen TC, Olsen RJ. Lung cancer biomarkers: present status and future developments[J]. Arch Pathol Lab Med, 2013, 137(9): 1191-1198.
[13] Jiang BL, Sha H. Current Study and Progress of Serum Tumor Markers in Lung Cancer[J]. Tianjin Med J, 2014, 42(4): 393-395. [蔣貝蘭, 沙杭. 肺癌血清腫瘤標志物的研究現狀及進展[J]. 天津醫藥, 2014, 42(4): 393-395.]
[14] Cao C, Chen ZB, Sun SF, et al. Evaluation of VEGF-C and tumor markers in bronchoalveolar lavage fluid for lung cancer diagnosis[J]. Sci Rep, 2013, 3: 3473.
[15] Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer[J]. Lung Cancer, 2012, 76(2): 138-143.
[16] Cedres S, Nunez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC)[J]. Clin Lung Cancer, 2011, 12(3): 172-179.
[17] Li J, Liang XF, Zhai GL. Application of combined detection of serum levels of CA199, CA125 and CEA in diagnosis of pancreatic cancer[J]. J Jilin Univ (Med Ed) 2014, 40(6): 1252-1255. [李靜, 梁曉芳, 翟桂蘭. 血清CA199、CA125和CEA聯合檢測在胰腺癌診斷中的應用[J]. 吉林大學學報(醫學版), 2014, 40(6): 1252-1255.]
[18] Lill JR, Wertz IE. Toward understanding ubiquitin-modifying enzymes: from pharmacological targeting to proteomics[J]. Trends Pharmacol Sci, 2014, 35(4): 187-207.
[19] Burkhart RA, Peng Y, Norris ZA, et al. Mitoxantrone targets human ubiquitin-specific peptidase 11 (USP11) and is a potent inhibitor of pancreatic cancer cell survival[J]. Mol Cancer Res, 2013, 11(8): 901-911.
[20] Baykara M, Yaman M, Buyukberber S, et al. Clinical and prognostic importance of XIAP and USP8 in advanced stages of non-small cell lung cancer[J]. J Buon, 2013, 18(4): 921-927.
[21] Byun S, Lee S Y, Lee J, et al. USP8 is a novel target for overcoming gefitinib resistance in lung cancer[J]. Clin Cancer Res, 2013, 19(14): 3894-3904.
[22] Saibil H. Chaperone machines for protein folding, unfolding and disaggregation[J]. Nat Rev Mol Cell Biol, 2013, 14(10): 630-642.
[23] Chae YC, Angelin A, Lisanti S, et al. Landscape of the mitochondrial Hsp90 metabolome in tumours[J]. Nat Commun, 2013, 4: 2139.
[24] Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic[J]. Lancet Oncol, 2013, 14(9): e358-e369.
[25] Proia DA, Bates RC. Ganetespib and HSP90: translating preclinical hypotheses into clinical promise[J]. Cancer Res, 2014, 74(5): 1294-1300.
[26] Di Pietro G, Magno LA, Rios-Santos F. Glutathione S-transferases: an overview in cancer research[J]. Expert Opin Drug Metab Toxicol, 2010, 6(2): 153-170.
[27] Chang BX, Mao PY. The research progress and its relevance with tumor of glutathione S[J]. Med J Chin PLA, 2012, 37(8): 838-842. [常彬霞, 貌盼勇. 谷胱甘肽S轉移酶的研究進展及其與腫瘤的相關性[J]. 解放軍醫學雜志, 2012, 37(8): 838-842.]
[28] Lopez-Cima MF, Alvarez-Avellon SM, Pascual T, et al. Genetic polymorphisms in CYP1A1, GSTM1, GSTP1 and GSTT1 metabolic genes and risk of lung cancer in Asturias[J]. BMC Cancer, 2012, 12: 433.
[29] Eimoto H, Tsutsumi M, Nakajima A, et al. Expression of the glutathione S-transferase placental form in human lung carcinomas[J]. Carcinogenesis, 1988, 9(12): 2325-2327.
[30] Hida T, Kuwabara M, Ariyoshi Y, et al. Serum glutathione S-transferase-pi level as a tumor marker for non-small cell lung cancer. Potential predictive value in chemotherapeutic response[J]. Cancer, 1994, 73(5): 1377-1382.
[31] Howie AF. Measurement of glutathione S-transferase pi by radioimmunoassay: elevated plasma levels in lung cancer patients[J]. Br J Biomed Sci, 1993, 50(3): 187-199.
[32] Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V. CHI3L1 plays a role in cancer through enhanced production of proinflammatory/pro-tumorigenic and angiogenic factors[J]. Immunol Res, 2013, 57(1/3): 99-105.
[33] Yan C, Ding X, Wu L, et al. Stat3 downstream gene product chitinase 3-like 1 is a potential biomarker of inflammationinduced lung cancer in multiple mouse lung tumor models and humans[J]. PLoS One, 2013, 8(4): e61984.
[34] Th?m I, Andritzky B, Schuch G, et al. Elevated pretreatment serum concentration of YKL-40-An independent prognostic biomarker for poor survival in patients with metastatic nonsmall cell lung cancer[J]. Cancer, 2010, 116(17): 4114-4121.
[35] Kim HR, Kim TH, Chung JH, et al. The detection of peripheral lung cancer by MAGE A1-6 RT-nested PCR in bronchial washing specimens[J]. Lung Cancer, 2009, 65(2): 166-169.
[36] Port J, Harrison S. Electromagnetic navigational bronchoscopy[J]. Semin Intervent Radiol, 2013, 30(2): 128-132.
[37] Nabavizadeh N, Zhang J, Elliott DA, et al. Electromagnetic navigational bronchoscopy-guided fiducial markers for lung stereotactic body radiation therapy: analysis of safety, feasibility, and interfraction stability[J]. J Bronchology Interv Pulmonol, 2014, 21(2): 123-130.
[38] Vanderlaan PA, Wang HH, Majid A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUSTBNA): An overview and update for the cytopathologist[J]. Cancer Cytopathol, 2014, 122(8): 561-576.
[39] Deng LP, Dong W, Du YP. Combined determination of tumor markers in serum and bronchoalveolar lavage fluid for lung cancer diagnosis[J]. Acta Acad Med Mil Tert, 2008, 30(1): 78-80.
[40] Li J, Chen P, Mao CM, et al. Evaluation of diagnostic value of four tumor markers in bronchoalveolar lavage fluid of peripheral lung cancer[J]. Asia Pac J Clin Oncol, 2014, 10(2): 141-148.
[41] Ghosh I, Bhattacharjee D, Das AK, et al. Diagnostic Role of Tumour Markers CEA, CA15-3, CA19-9 and CA125 in Lung Cancer[J]. Indian J Clin Biochem, 2013, 28(1): 24-29.
Diagnostic value of 4 new tumor markers in bronchoalveolar lavage fluid in the diagnosis of peripheral lung cancer
ZHANG Shi, ZHANG Ming-zhou, WU Xue-ling*
Institute of Respiratory Disease, Xinqiao Hospital, Third Military Medical University, Chongqing 400037, China
*
, E-mail: wuxueling@aliyun.com
This work was supported by the National Natural Science Foundation of China (81270130)
ObjectiveTo investigate the clinical value of heat shock protein 90α (HSP90α), glutathione S-transferase P1 (GSTP1), ubiquitin specific peptidase 8 (USP8) and chitinase 3-like 1 (CHI3L1) in bronchoalveolar lavage fluid (BALF) and serum for diagnosis and evaluation of the extent of peripheral lung cancer.MethodsThe levels of HSP90α, GSTP1, USP8 and CHI3L1 of in BALF and serum were measured and compared among 100 patients with peripheral lung cancer (PLC) and 50 patients with benign lung diseases (BLD) by ELISA. The results were compared and analyzed.ResultsThe average content of HSP90α and CHI3L1 in BALF of PLC patients were higher than that in BLD patients (P<0.05). The diagnostic sensitivity of HSP90α and CHI3L1 in BLAF for the diagnosis of lung cancer were respectively 77.0% and 72.0%, and the specificity of them was respectively 82.0% and 80.0%. The levels of HSP90α and CHI3L1 in serum of PLC patients and BLD patients showed no significant difference (P>0.05). The levels of HSP90α and CHI3L1 in BALF of the patients with small nodular lung cancer group (primary focus diameter ≤1cm) and BLD patients showed no significant difference (P>0.05). The levels of GSTP1 and USP8 in BALF and serum of PLC patients and that of BLD patients showed no significant difference (P>0.05). The levels of HSP90α, GSTP1, USP8 and CHI3L1 in BALF and serum showed no significant correlation with other factors, such as the patient's age, pathological classification (adenocarcinoma or squamous carcinoma) and stage of lung cancer (phase Ⅰ to Ⅳ). The level of CHI3L1 in BALF was correlatedto the diameter of the primary foci (P<0.05), while the levels of other lung cancer markers in BALF and serum showed no significant correlation with diameter of primary focus.ConclusionDetection of tumor markers such as HSP90α and CHI3L1 from patients' BALF has a diagnostic value for PLC, and is superior to the examinations of patients' serum specimens. The measurement of HSP90α in BALF shows better clinical value, and it may contribute to the diagnosis of peripheral pulmonary carcinoma.
bronchoalveolar lavage fluid (BALF), tumor marker, peripheral lung cancer pulmonary carcinoma, diagnosis
R734.2
A
0577-7402(2015)03-0206-06
10.11855/j.issn.0577-7402.2015.03.07
2014-10-14;
2015-02-12)
(責任編輯:沈寧)
國家自然科學基金(81270130)
張實,醫學碩士。主要從事肺部疾病的臨床診斷及治療工作
400037 重慶 第三軍醫大學新橋醫院呼吸科(張實、張明周、吳學玲)
吳學玲,E-mail:wuxueling@aliyun.com

