Pu在Gd2Zr2O7基質中的模擬固化: (Gd1–xCex)2Zr2O7+x的熱物理性能研究
夏祥來 李林艷 郭 放 蘇 偉 劉 艷陳曉謀 潘社奇
(1遼寧大學化學院, 沈陽 110036; 2清華大學核能與新能源技術研究院, 北京 100084;3中國工程物理研究院, 四川 綿陽 621900)
Pu在Gd2Zr2O7基質中的模擬固化: (Gd1–xCex)2Zr2O7+x的熱物理性能研究
夏祥來1,2李林艷2,*郭 放1,*蘇 偉3劉 艷3陳曉謀3潘社奇3
(1遼寧大學化學院, 沈陽 110036;2清華大學核能與新能源技術研究院, 北京 100084;3中國工程物理研究院, 四川 綿陽 621900)
采用高溫固相反應, 以NaF作助熔劑, 在1000 °C的溫度下合成了錒系元素Pu的模擬固化體(Gd1–xCex)2Zr2O7+x(0 ≤ x ≤ 0.7). 研究了模擬固化體的物相、熱膨脹系數(TEC)、熱導率(TC)隨溫度及組成的變化規律. 粉末X射線衍射(XRD)測試結果表明: Gd2Zr2O7基質本身呈弱有序燒綠石結構, 而用Ce4+取代Gd3+的模擬固化體都呈缺陷螢石結構. (Gd1–xCex)2Zr2O7+x的Ce(3d) X射線光電子能譜(XPS)有六個峰, 結合能分別位于 881.7, 888.1, 897.8, 900.4, 907.1, 916.1 eV處, 與CeO2的XPS圖譜非常相似, 說明Ce為四價. 隨著溫度的升高, 所有樣品的熱膨脹系數總體上呈增大趨勢. 在室溫至750 °C附近, 大部分樣品的熱導率隨溫度的升高而降低, 之后熱導率又呈小幅上升. 在相同溫度下, 固化體(Gd1–xCex)2Zr2O7+x(0 ≤ x ≤ 0.7)的熱膨脹系數及熱導率隨組成變化呈相同趨勢: 在0 ≤ x ≤ 0.1范圍內隨x的增大而增大, 隨后在x = 0.1–0.7時逐漸減小.
核廢料固化; 燒綠石結構; 螢石結構; 熱膨脹系數; 熱導率
1 Introduction
With the increasing severe energy crisis and environment pollution, many countries were forced to develop nuclear power due to its higher energy density and less waste emission than traditional fossil fuel. However, the spent nuclear fuel (SNF) contains fission products, corrosion products, process contaminants, fuel components, and transmutation products.1These wastes would be a severe threat to ecological environment because they contain high radioactive and long-lived actinides, such as238Pu,239Pu,237Np,243Am and so on.2Therefore, safe and effective disposal of high level waste (HLW) is crucial to the public acceptance and sustainable development of nuclear energy. Substantial amounts of HLW will require to be immobilized in an inert matrix for geological disposal. Many different types of glass and ceramic materials have been investigated for the immobilization of HLW,3such as borosilicate glasses,4phosphate glasses,5aluminosilicate glasses,6tianate or zirconate pyrochlore,7–13phosphate ceramics,14,15etc.
However, the glass solidified form has a tendency to get devitrified in the presence of water and steam at elevated pressure and temperature in geological repositories, which results in water soluble salt and increases the leachability of radioactive elements.16Studies about heavy-ion irradiation on the Gd2(ZrxTi1–x)2O7system demonstrated that amorphization decreases systematically with increasing Zr content. For the end member Gd2Zr2O7, no amorphization occurred at extremely high ion fluences, which indicated that Gd2Zr2O7is a very stable matrix and suitable for HLW immobilization.8–10
However, Gd2Zr2O7is difficult to be synthesized. For example, fluorite Gd2(ZrxTi1–x)2O7was prepared by a sol-gel route: the Gd-Ti-Zr-O gels were calcined at 700 °C for 1 h, then the calcine was wet ball-milled, pressed into pellets, and sintered at 1600 °C for 50 h in air.11Pyrochlore Gd2Zr2?xCexO7was prepared by grinding, pelletizing, and calcining the stoichiometric oxide mixture repeatedly at 1200 °C for 36 h, 1300 °C for 36 h, and 1400 °C for 48 h, respectively.17In addition, high pressure, high temperature, and microwave sintering were also used to synthesize Gd2Zr2O7.18,19Tetravalent cerium is very similar to tetravalent plutonium in many aspects, such as oxidation state, ionic radius, hydrolysis, and redox behavior. As the host of actinides, the thermal expansion coefficient should not be very high but the thermal conductivity should be high enough.20
This work used Ce as an analogue of Pu to simulate its immobilization in Gd2Zr2O7.21–23(Gd1–xCex)2Zr2O7+x(0 ≤ x ≤ 0.7) were synthesized at relatively low temperature compared with traditional high-temperature solid-state reaction,24and the thermophysical properties of these simulated solidified forms were also studied.
2 Experimental
Polycrystalline samples of (Gd1–xCex)2Zr2O7+x(0 ≤ x ≤ 0.7) were prepared by solid-state reaction using NaF as a flux. AR grade pow
ders of Gd(NO3)36H2O (99.9%), Zr(NO3)4(99.5%), Ce(NO3)36H2O (99.9%) in stoichiometric ratio and 5% (w, mass fraction) NaF (98.0%) were ground adequately. The mixtures were put in alumina crucibles and calcined in furnace (SX3-8-13, Tianjin City Central Experimental Electric Furnace Co., Ltd. China) at 1000 °C for 10 h in air.24The resulted calcines were washed by distilled water for 3–4 times to remove NaF, and dried at 150 °C for 3 h. The obtained powders were uniaxially cold pressed into pellets with 190 MPa and pressurelessly sintered at 1400 °C for 6 h. The bulk samples were cut and polished into cuboids or disks for the measurement of thermophysical properties.
Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku D/max-2000 diffractometer with graphite monochromatized Cu-Kαradiation on 40 kV, 100 mA at a scanning rate of 4 (°)min–1.
Thermal expansion coefficients (TECs) of the specimens (6 mm × 4 mm × 25 mm) were measured with a high-temperature dilatometer (Model NETSCH DIL 402EP, Germany). The data were recorded continuously at a scanning rate of 5 °Cmin–1in the range of ambient temperature to 1000 °C in N2atmosphere.
Thermal diffusivities (λ) and the specific heat capacities (Cp) of the specimens with 12.7 mm in diameter and 2 mm in thickness were measured with laser-flash method (Anter(TA)FL4010, USA) from ambient temperature to 1000 °C with every 250 °C as an interval. Densities (ρ) of the specimens were measured on a densimeter (QL-120C, MatsuHaku, Taiwan). Thermal conductivities (k) were calculated by Eq.(1) with λ, Cp, and ρ:

3 Results and discussion
3.1 Syntheses and phase identification
In our previous work, it proved that (Gd1–xCex)2Zr2O7+xcould be synthesized at relatively low temperature by using nitrate as active raw materials and NaF as a flux, compared with traditional high-temperature solid-state reaction.24As for many A2B2O7compounds, if the ratio of cation ionic radii lies in the range rA/rB= 1.46–1.78, they prefer pyrochlore structure withLa2Zr2O7as a typical example which crystallizes in Fd3m space group (S.G.) and a = 1.079 nm; for a lower radius ratio, the defect fluorite structure is stabilized, such as Yb2Zr2O7which crystallizes in Fm3m space group and a = 0.517 nm. In general, Gd2Zr2O7(rA/rB= 1.46) crystallizes in weakly ordered pyrochore structure (S.G.: Fd3m, a = 1.052 nm) which is closed related to fluorite structure (S.G.: Fm3m, a = 0.526 nm). The main lattice of the two structures produces a set of identical diffraction peaks (2θ): 29.62°, 34.38°, 49.34°, 58.67°, 61.48°. In pyrochore structure, the arrangements of cations and anion vacancies are ordered, which constitutes a super lattice featured in a set of weak diffraction peaks at 2θ ≈ 14.65° (111), 28.52° (311), 37.51° (331), 44.89° (511). The decrease of rA/rBcaused by other cation doping will result in the structure transformation from pyrochlore to fluorite.
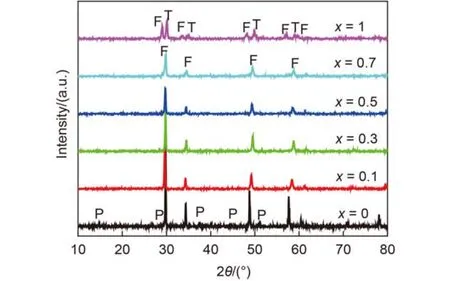
Fig.1 XRD patterns of (Gd1–xCex)2Zr2O7+x
XRD patterns of the as-synthesized (Gd1–xCex)2Zr2O7+xare shown in Fig.1. It can be seen that pure Gd2Zr2O7indeed exhibits weakly ordered pyrochore structure (JCPDS 79-1146). Partial Ce4+substitution for Gd3+leads to the structural transformation from pyrochlore to defect fluorite (JCPDS 80-0471) even if x is as low as 0.1. When x reaches 0.7, XRD peaks of the product widen obviously, indicating that high Ce4+substitution rate for Gd3+would lead to lattice distortion. When x = 1, that is, full substitution of Ce4+for Gd3+leads to the product into two phases, (Zr0.88Ce0.12)2O2(P42/nmc, JCPDS 82-1389) and (Ce0.75Zr0.25)2O2(Fm3m, JCPDS 28-0271).
The XPS Ce(3d) spectrum of the (Gd1–xCex)2Zr2O7+x(x = 0.5) sample is shown in Fig.2. It can be seen that there are six peaks at 881.7, 888.1, 897.8, 900.4, 907.1, 916.1 eV respectively, which is almost identical to those of CeO2.25Among them, the three peaks of 881.7, 888.1, 897.8 eV arise from the terminal state of 3d94f1, 3d94f2, and 3d94f0. The corresponding spin-orbit splitting peaks occur at 900.4, 907.1, and 916.1 eV. The XPS Ce(3d) spectrum reveals that Ce specimen in (Gd1–xCex)2Zr2O7+xshould be in tetravalent.
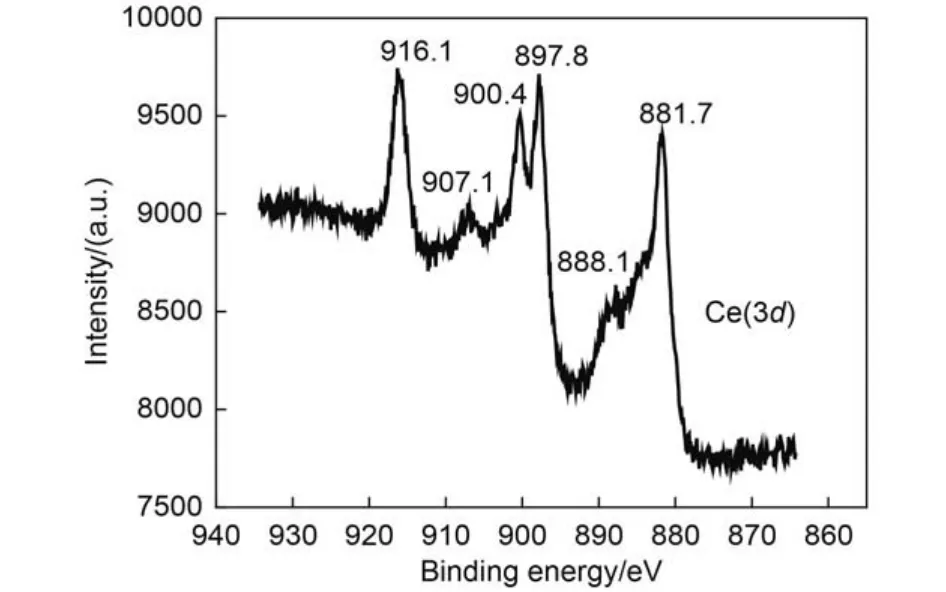
Fig.2 XPS Ce(3d) spectrum of the (Gd1–xCex)2Zr2O7+x(x = 0.5) splitting peaks occur at 900.4, 907.1 and 916.1eV.
The coordination numbers of Gd3+and Zr4+in Gd2Zr2O7lattice were 8 and 6, respectively. The relevant ionic radii for 8-fold and 6-fold coordinations are: Gd3+0.105 and 0.094, Ce4+0.097 and 0.087, Zr4+0.084 and 0.072 nm, respectively. As a result, partial Ce4+substitution for Gd3+with smaller ion radius leads to the decrease of rA/rB, which in turn causes the structural transformation from pyrochlore (Gd2Zr2O7) to defect fluorite ((Gd1–xCex)2Zr2O7+x(0.1 ≤ x ≤ 0.7)).
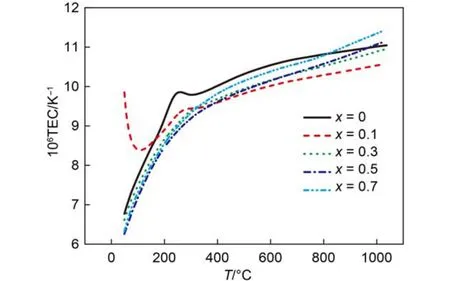
Fig.3 Temperature dependence of the thermal expansion coefficient of (Gd1–xCex)2Zr2O7+x
3.2 Thermal expansion
The dependence of linear TECs of (Gd1–xCex)2Zr2O7+x(0 ≤x ≤ 0.7) on temperature is shown in Fig.3. It can be seen that the TECs of the samples with any composition increase with the increasing temperature in general trend, which is attributed to the increasing atomic distance with the increase of temperature. The TECs of the Ce-doping sample are all lower than that of pure Gd2Zr2O7in the range of 200–750 °C. When the temperature is higher than 750 °C, the TECs of the samples with high Ce-doping rate (x = 0.5, 0.7) are slightly higher than those of pure Gd2Zr2O7. Composition dependence of the thermal expansion coefficient of (Gd1–xCex)2Zr2O7+xat the same temperature, such as 1000 °C is presented in Fig.4. It can be seen that the TECs of (Gd1–xCex)2Zr2O7+xdecrease from x = 0 to 0.1, then increase constantly from x = 0.1 to 0.7. The influences of Ce4+substitution for Gd3+on Gd2Zr2O7lattice mainly reflect in two aspects: ① In order to compensate the excess positive charges brought about by Ce4+substitution for Gd3+, the amount of oxygen vacancies in Gd2Zr2O7lattice decreases, which in turn increases the amount of Zr―O bonds. ② On the other hand, partial Ce4+may migrate to Zr4+nearby, which in turn weakensZr―O bonds. The former effect would decrease the TECs of (Gd1–xCex)2Zr2O7+x, whereas the latter effect on TECs is opposite because normal Zr―O bond is short and strong, which is negatively correlated with the TECs. Experimental results on the TECs of (Gd1–xCex)2Zr2O7+xshow that the former effect plays a dominant role at low temperature and low Ce4+substitution rate and vice versa.
3.3 Thermal conductivities
The measured specific heat capacities, thermal diffusivities at different temperatures of (Gd1–xCex)2Zr2O7+xsample at ambient temperature are shown in Fig.5 and Fig.6. The bulk densities of (Gd1–xCex)2Zr2O7+xsamples are 5.79, 5.89, 5.88, 5.90, 5.76gcm–1for x = 0, 0.1, 0.3, 0.5, 0.7 samples. According to Eq.(1), the calculated thermal conductivities of (Gd1–xCex)2Zr2O7+xat different temperatures are plotted in Fig.7. It can be seen from Fig.7 that the TCs of most (Gd1–xCex)2Zr2O7+xsamples decrease with the increasing temperature up to 750 °C nearby, followed by a slight increase from 750 to 1000 °C. According to the thermal conduction theory, heat conduction in crystals is mainly carried out by means of phonons, and the lattice thermal conductivity is proportional to the mean free path of phonon. With temperature increasing, lattice vibrations become more intense, which would shorten the mean free path of phonon, and TC decreases as a result. However, with temperature further going up, the mean free path of phonon could not be shortened infinitely, and the contribution of photon conduction to heat conduction increases with temperature increasing and cannot be neglected, which results in a slight increase of TC.
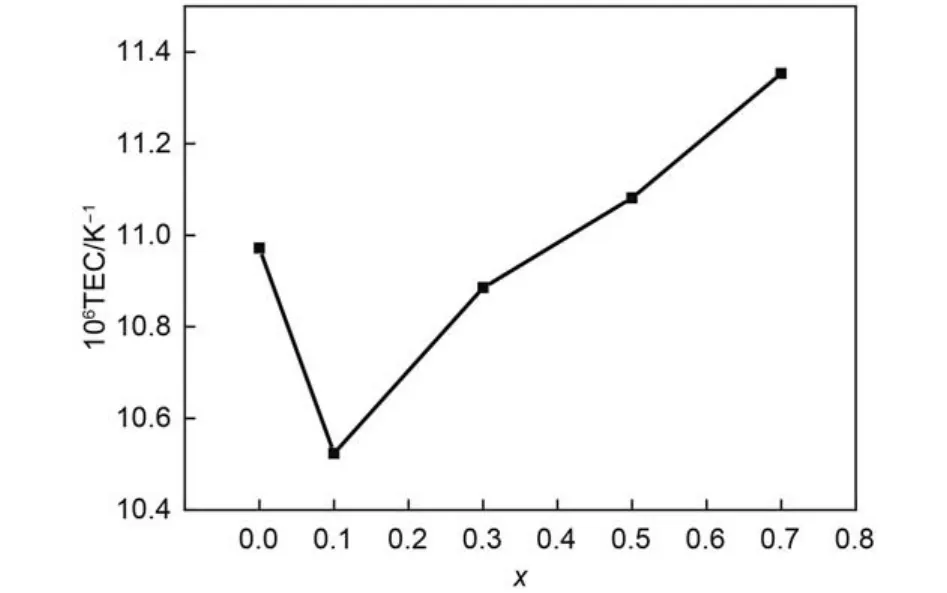
Fig.4 Composition dependence of the thermal expansion coefficient of (Gd1–xCex)2Zr2O7+xat 1000 °C
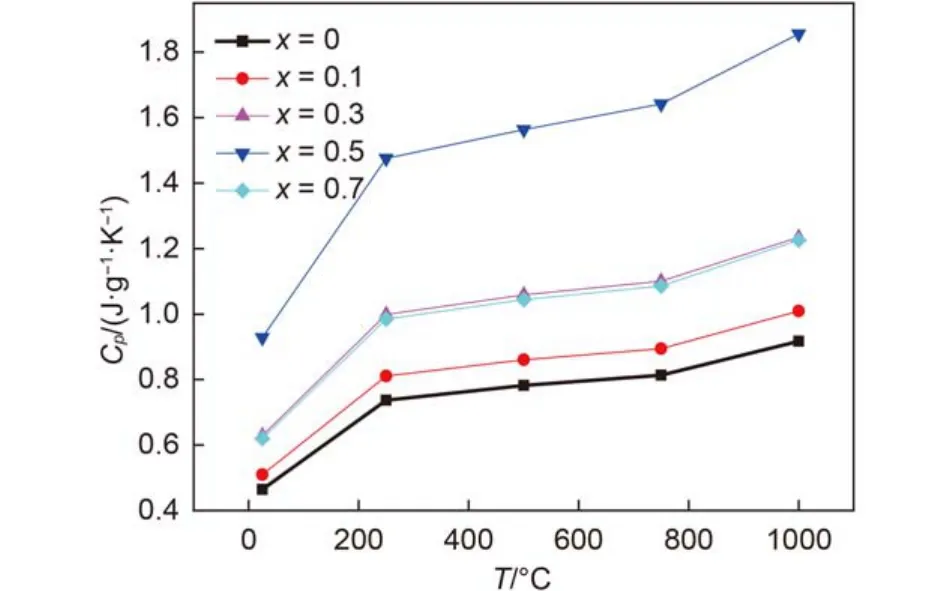
Fig.5 Temperature dependence of the specific heat capacity of (Gd1–xCex)2Zr2O7+x
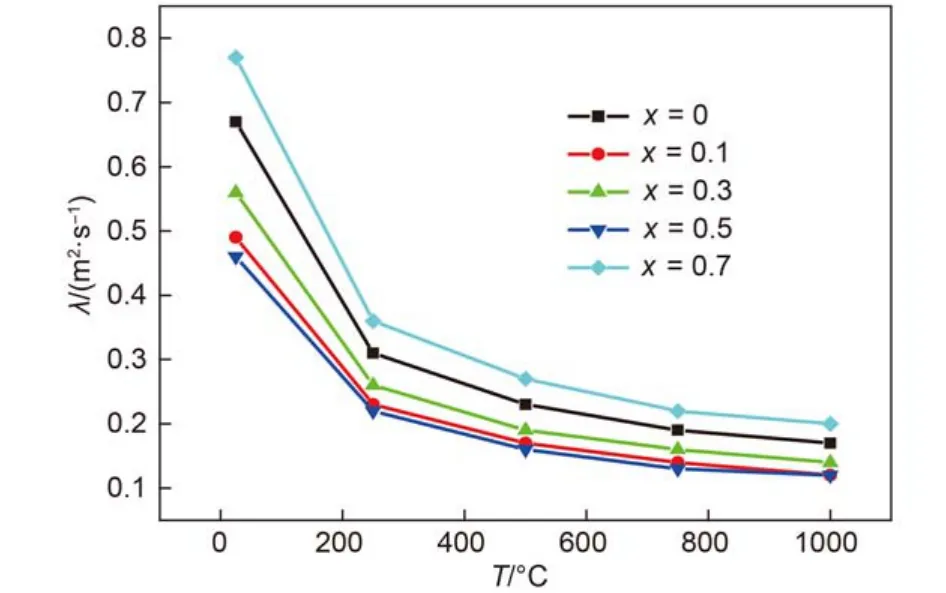
Fig.6 Temperature dependence of the thermal diffusivities of (Gd1–xCex)2Zr2O7+x
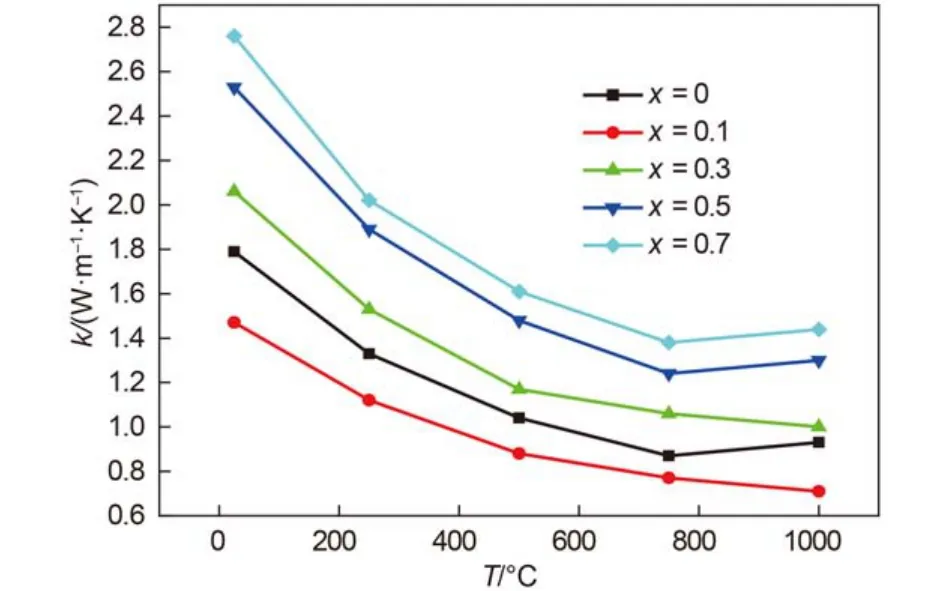
Fig.7 Temperature dependence of the thermal conductivities of (Gd1–xCex)2Zr2O7+x
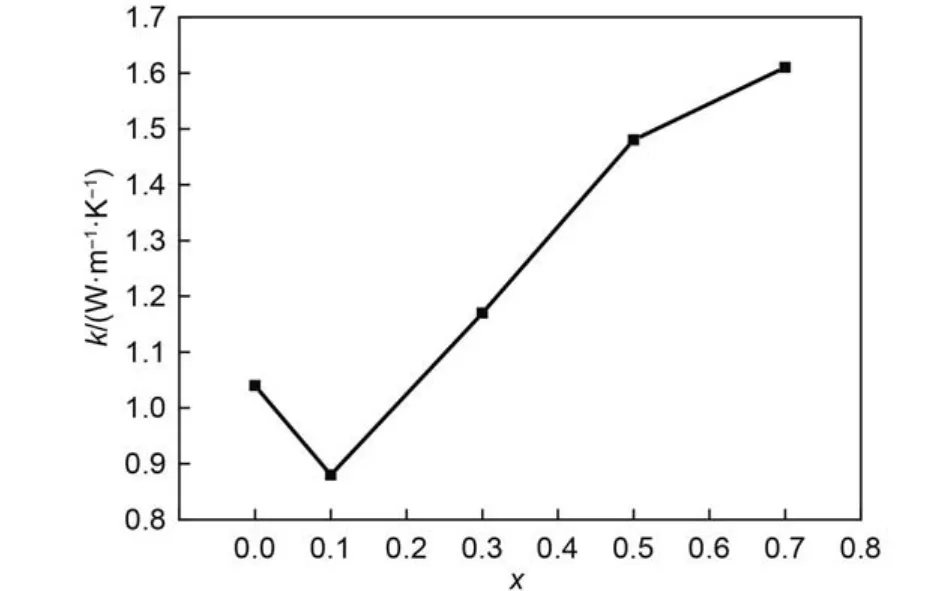
Fig.8 Composition dependence of the thermal conductivities of (Gd1–xCex)2Zr2O7+xat 500 °C
At the same temperature, the variation trend TCs of (Gd1–xCex)2Zr2O7+xwith their composition, taking 500 °C as an example, is given in Fig.8. It can be found that the TCs of (Gd1–xCex)2Zr2O7+xalso decrease from x = 0 to 0.1, then increase constantly from x = 0.1 to 0.7. In real crystal structure, scattering of phonons occurs when they interact with lattice defects, such as vacancies, dislocations, grain boundaries, substitutionby other atoms, and so on.26Substation of Ce4+with higher charge and smaller ion radius for Gd3+will produce replacement defectand reduce the amount of oxygen vacancies simultaneously in Gd2Zr2O7lattice. The replacement defectwould enhance the scattering of phonons, which in turn decreases TC, and the influence of the decrease of oxygen vacancies on TC is opposite. It could be concluded from Fig.8 that the replacement defectdominates the TC variation when Ce-doping amount is less than 10%, then the influence of the decrease of oxygen vacancies on TC gets more and more obviously.
4 Conclusions
(1) Polycrystalline samples of (Gd1–xCex)2Zr2O7+xwere prepared by solid-state reaction using NaF as a flux at 1000 °C for 10 h. XRD results show that pure Gd2Zr2O7exhibits weakly ordered pyrochore structure. Ce4+substitution for Gd3+leads to the structural transformation from pyrochlore to defect fluorite even if x is as low as 0.1. When x reaches 0.7, XRD peaks of the products widen obviously.
(2) The TECs of (Gd1–xCex)2Zr2O7+x(0 ≤ x ≤ 0.7) with any composition increase with the temperature increasing in general trend. The TECs of the Ce-doping samples are all lower than that of pure Gd2Zr2O7in the range of 200–750 °C. When the temperature is higher than 750 °C, the TECs of the samples with high Ce-doping rate (x = 0.5, 0.7) are higher than that of pure Gd2Zr2O7. At the same temperature, the TECs of (Gd1–xCex)2Zr2O7+xdecrease from x = 0 to 0.1, then increase constantly from x = 0.1 to 0.7.
(3) The TCs of most (Gd1–xCex)2Zr2O7+xsamples decrease with the increasing temperature up to 750 °C nearby, followed by a slight increase from 750 to 1000 °C. At the same temperature, the TCs of (Gd1–xCex)2Zr2O7+xalso decrease from x = 0 to 0.1, then increase constantly from x = 0.1 to 0.7.
(1)Stefanovsky, S. V.; Yudintsev, S. V.; Livshits, T. S. IOP Conf. Series: Mater. Sci. Eng. 2010, 9, 012001-1. doi: 10.1088/1757-899X/9/1/012001
(2)Yudintsev, S. V.; Stefanovsky, S. V.; Ewing, R. C. Actinide Host Phases as Radioactive Waste Forms. In Structural Chemistry of Inorganic Actinide Compounds; Krivovichev, S. V., Burns, P. C., Tananaev, I. G. Eds.; Elsevier: Amsterdam, 2007; pp 457–490.
(3)Donald, I. W.; Metcalfe, B. L.; Taylor, R. N. J. J. Mater. Sci. 1997, 32, 5851. doi: 10.1023/A:1018646507438
(4)Lutze, W.; Ewing, R. C. Radioactive Waste Forms for the Future; Lutze, W., Ewing, R. C. Eds.; Elsevier: Amsterdam, 1988; pp 1–159.
(5)Kumar, B.; Lin, S. J. Am. Ceram. Soc. 1991, 74, 226. doi: 10.1111/jace.1991.74.issue-1
(6)Stefanovsky, S. V.; Ivanov, I. A.; Gulin, A. N. Scientific Basis for Nuclear Waste Management XVIII; Murakami, T., Ewing, R. C. Eds.; Materials Research Society: Pittsburgh, PA, 1995; pp 101–106.
(7)Sickafus, K. E.; Minervini, L.; Grimes, R. W.; Valdez, J. A.; Ishimaru, M.; Li, F.; McClellan, K. J.; Hartmann, T. Science 2000, 289, 748. doi: 10.1126/science.289.5480.748
(8)Weber, W. J.; Ewing, R. C. Science 2000, 289, 2051. doi: 10.1126/science.289.5487.2051
(9)Lu, X. R.; Dong, F. Q.; Hu, S.; Wang, X. L.; Wu, Y. L. Acta Phys. Sin. 2012, 61, 152401-1. [盧喜瑞, 董發勤, 胡 淞, 王曉麗, 吳彥霖. 物理學報, 2012, 61, 152401-1.]
本研究中2例患者分別在使用唑來膦酸72、60 h后出現雙眼急性葡萄膜炎,在第二次使用唑來膦酸前,先給予地塞米松及潑尼松龍滴眼液共預處理6 d,患者再次啟動唑來膦酸治療后,未出現任何眼部癥狀[5]。PATEL等[22]的回顧性調查中,有3例患者在初次發病18個月后在未給予任何預處理的情況下再次給予唑來膦酸靜脈輸注,未出現急性葡萄膜炎的癥狀或體征。這些研究提示急性葡萄膜炎不應成為再次使用唑來膦酸的臨床禁忌,但應做好臨床監測和預防工作。
(10)Zhang, F. X.; Wang, J. W.; Lian, J.; Lang, M. K.; Becker, U.; Ewing, R. C. Phys. Rev. Lett. 2008, 100, 045503-1. doi: 10.1103/PhysRevLett.100.045503
(11)Mandal, B. P.; Pandey, M.; Tyagi, A. K. J. Nucl. Mater. 2010, 406, 238. doi: 10.1016/j.jnucmat.2010.08.042
(12)Kutty, K. V. G.; Asuvathraman, R.; Madhavan, R. R.; Jena, H. J. Phys. Chem. Solids 2005, 66, 596. doi: 10.1016/j.jpcs.2004.06.066
(13)Weber, W. J.; Wald, J. W.; Matzke, H. J. Nucl. Mater. 1986, 138, 196. doi: 10.1016/0022-3115(86)90006-1
(14)Kerdaniel, E. D. F.; Clavier, N.; Dacheux, N.; Terra, O.; Podor, R. J. Nucl. Mater. 2007, 362, 451. doi: 10.1016/j.jnucmat. 2007.01.132
(15)Metcalfe, B. L.; Donald, I. W.; Fong, S. K.; Gerrard, L. A.; Strachan, D. M.; Scheele, R. D. J. Nucl. Mater. 2009, 385, 485. doi: 10.1016/j.jnucmat.2008.12.035
(16)Ringwood, A. E.; Kesson, S. E.; Ware, N. G.; Hibberson, W.; Major, A. Nature 1979, 278, 219. doi: 10.1038/278219a0
(17)Patwe, S. J.; Ambekar, B. R.; Tyagi, A. K. J. Alloy. Compd. 2005, 389, 243. doi: 10.1016/j.jallcom.2004.06.094
(19)Lu, X. R.; Ding, Y.; Dan, H.; Yuan, S. B.; Mao, X. L.; Fan, L.; Wu, Y. L. Ceram. Int. 2014, 40, 13191. doi: 10.1016/j.ceramint.2014.05.024
(20)Mandal, B. P.; Garg, N.; Sharma, S. M.; Tyagi, A. K. J. Nucl. Mater. 2009, 392, 95. doi: 10.1016/j.jnucmat.2009.03.050
(21)Reid, D. P.; Stennettn, M. C.; Hyatt, N. C. J. Solid State Chem. 2012, 191, 2. doi: 10.1016/j.jssc.2011.12.039
(22)Dickson, C. L.; Glasser, F. P. Cem. Concr. Res. 2000, 30, 1619. doi: 10.1016/S0008-8846(00)00362-8
(23)Lu, X. R.; Fan, L.; Shu, X. Y.; Su, S. J.; Ding, Y.; Yi, F. C. Ceram. Int. 2015, 41, 6344. doi: 10.1016/j.ceramint.2015.01.068
(24)Zhao, P. Z.; Li, L.Y.; Xu, S. M.; Zhang, Q. Acta Phys. -Chim. Sin. 2013, 29, 1168. [趙培柱, 李林艷, 徐盛明, 張 覃. 物理化學學報, 2013, 29, 1168.] doi: 10.3866/PKU.WHXB201304013
(25)Zhao, L. Z. Acta Phys. Sin. 1989, 38, 987. [趙良仲. 物理學報, 989, 38, 987.]
(26)Zhang, H. S.; Chen, X. G.; Li, G.; Wang, X. L.; Dang, X. D. J. Eur. Ceram. Soc. 2012, 32, 3693. doi: 10.1016/j.jeurceramsoc. 2012.06.003
Simulation of the Immobilization of Pu in the Gd2Zr2O7Matrix by Investigating the Thermophysical Properties of (Gd1?xCex)2Zr2O7+x
XIA Xiang-Lai1,2LI Lin-Yan2,*GUO Fang1,*SU Wei3LIU Yan3CHEN Xiao-Mou3PAN She-Qi3
(1College of Chemistry, Liaoning University, Shenyang 110036, P. R. China;2Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, P. R. China;3China Academy of Engineering Physics, Mianyang 621900, Sichuan Province, P. R. China)
Polycrystalline samples of (Gd1?xCex)2Zr2O7+x(0 ≤ x ≤ 0.7) were synthesized by solid-state reaction using NaF as a flux at 1000 °C to simulate Pu-immobilization in the Gd2Zr2O7matrix. Phase transformation and variation of the thermal expansion coefficients (TECs) and thermal conductivities (TCs) of the samples with temperature and composition were investigated. Powder X-ray diffraction (XRD) patterns show that pure Gd2Zr2O7has a weakly ordered pyrochlore structure, whereas Ce-containing samples (i.e., the Pu-simulated solidified samples) exhibit a defect fluorite structure even if x is as low as 0.1. When x reaches 0.7, the XRD peaks of these samples widen. In the Ce 3d X-ray photoelectron spectrum (XPS) of (Gd1?xCex)2Zr2O7+xthere are six peaks located at binding energies of 881.7, 888.1, 897.8, 900.4, 907.1, and 916.1 eV, which are almost the same as the peaks of CeO2. The Ce 3d XPSreveals that the Ce species in (Gd1?xCex)2Zr2O7+xare tetravalent. The TECs of (Gd1?xCex)2Zr2O7+x(0 ≤ x ≤0.7) generally increase with increasing temperature. At the same temperature, the TECs and TCs exhibit the same variation trend with the composition of the simulated solidified forms: they decrease from x = 0 to 0.1 and then linearly increase from x = 0.1 to 0.7.
Immobilization of nuclear waste; Pyrochlore structure; Defect fluorite structure; Thermal expansion coefficient; Thermal conductivity
O642
10.3866/PKU.WHXB201507203
Received: March 26, 2015; Revised: July 16, 2015; Published on Web: July 20, 2015.
*Corresponding authors. LI Lin-Yan, Email: lilinyan@tsinghua.edu.cn; Tel: +86-10-89796097. GUO Fang, Email: fguo@lnu.edu.cn.
The project was supported by NSAF (11176014) and National Natural Science Foundation of China (21471088).
國家自然科學基金委員會和中國工程物理研究院聯合基金(11176014)和國家自然科學基金(21471088)資助項目
? Editorial office of Acta Physico-Chimica Sinica

