大口黑鱸GHRH基因啟動子區域序列分析及其活性檢測
馬冬梅,韓林強,白俊杰
(1.中國水產科學研究院珠江水產研究所,農業部熱帶亞熱帶魚類選育與養殖重點開放實驗室,廣州 510380;2.淡水水產健康養殖湖北省協同創新中心,武漢 430070)
大口黑鱸GHRH基因啟動子區域序列分析及其活性檢測
馬冬梅,韓林強,白俊杰
(1.中國水產科學研究院珠江水產研究所,農業部熱帶亞熱帶魚類選育與養殖重點開放實驗室,廣州 510380;2.淡水水產健康養殖湖北省協同創新中心,武漢 430070)
生長激素釋放激素(growth hormone releasing hormone,GHRH)是下丘腦弓狀核合成和分泌的小分子多肽,其主要功能是調節垂體細胞合成和釋放生長激素。為研究大口黑鱸(Micropterus salmoides)GHRH基因5’側翼啟動子區域的活性和該區域中潛在的轉錄因子對GHRH基因表達的調控作用,對該基因5’端啟動子區域約1400 bp長度的片段進行序列分析,預測順式作用元件,獲得了Oct-1、SP1、NF-1、C/EBPalp和C/EBP等多個潛在的調控GHRH基因表達的調節因子結合位點序列。在包括外顯子1和內含子1的GHRH基因5’側翼區兩側加入兩個限制性酶切位點Xho I和Bam H I,對其進行改造,并將該片段插入紅色熒光蛋白報告基因載體pDsRed2-1,構建了重組表達質粒pGHRH1-RFP。同時,用不含有外顯子1和內含子1的GHRH基因5’側翼區構建重組表達質粒pGHRH-RFP。將質粒pGHRH1-RFP和pGHRH-RFP轉染鯉(Cyprinus carpio)上皮細胞(epithelioma papillosum cyprinid,EPC)。經過48 h的培養,在pGHRH1-RFP轉染的部分細胞中檢測到紅色熒光蛋白表達。又將pGHRH1-RFP或pGHRH-RFP質粒注射到斑馬魚(Danio rerio)一細胞或二細胞期的胚胎中,注射了pGHRH1-RFP的胚胎在受精后48 h約有22.5%能檢測到有紅色熒光蛋白表達,受精后72 h約有29%的仔魚檢測到紅色熒光蛋白表達。實驗結果表明,目前分離到的GHRH基因5’側翼序列具有啟動基因表達的活性,且該基因的內含子1和外顯子1是啟動子的活性所必需的。另外,pGHRH1-RFP質粒注射的斑馬魚胚胎只能在胚胎和仔魚的脊椎和肌肉中檢測到RFP的表達,而在腦中沒有檢測到表達。推測擴增到的大口黑鱸GHRH啟動子序列1407 bp(-1043 bp~362 bp)只是起到了驅動RFP脊椎和骨骼肌表達的作用,而不包括驅動在腦組織中特異性表達的啟動子,本研究為GHRH基因功能的深入分析奠定了基礎。
生長激素釋放激素;啟動子活性;大口黑鱸
生長激素釋放激素(growth hormone releasing hormone,GHRH)是一種小分子多肽,為PACAP/胰高血糖素(pituitary adenylate cyclase-activating polypeptide,PACAP/glucagon)超家族成員,主要由丘腦下部的弓狀核合成和分泌[1-2],其主要的功能是通過與GHRH受體結合刺激垂體合成和分泌生長激素(growth hormone,GH)[3]。GHRH在胚胎期對調節生長激素細胞增殖分化、腦垂體形成等有重要作用[4];外源適度增加動物體內GHRH的含量能夠加快動物的生長[5];人類GHRH受體基因的突變或缺失會導致侏儒癥、巨人癥等疾病[6],但人類GHRH基因本身的突變或缺失引起的疾病還未見報道[7]。GHRH的精確表達以及對GH時空表達的準確調節,對于動物器官的形成和發育來說是必不可少的,該基因啟動子區域順式調控元件的突變極可能會影響到啟動子的活性,從而影響到基因的正確表達,因此分析GHRH基因啟動子的序列和活性是十分有意義的一項工作,可為分析啟動子序列突變對其活性的影響奠定基礎。
在魚類啟動子的研究中,管家基因β-actin的啟動子研究得最為深入[8-9],并在轉基因魚研究中得到了較為廣泛應用[10-11]。而熱激蛋白70(HSP 70)[12]、肌肉生長抑制素(myostatin)[13]、促甲狀腺激素(TSHβ)[14]、胰島素樣生長因子-II(IGF-II)[15]等多種基因的啟動子也得到了研究,為深入研究基因功能、基因表達調控和基因之間相互作用奠定了基礎。目前,GHRH基因啟動子在人(Homo sapiens)、小鼠(Musmusculus)和大鼠(Rattus norregicus)中都有研究[16-19],且Gsh-1基因對大鼠GHRH表達的調控功能得到了深入的分析[19]。但魚類GHRH啟動子研究較少,對大口黑鱸(Micropterus salmoides)GHRH基因的研究發現,其5’側翼序列中存在一個66 bp的插入/缺失位點(c.-923_-858del),該插入/缺失位點為隱性致死位點[20]。
為進一步探討大口黑鱸GHRH基因啟動子區域突變對其功能的影響,本研究分析了大口黑鱸GHRH基因5’側翼約1400 bp序列潛在的順式作用調控元件,并構建了重組紅色熒光蛋白質粒,在鯉(Cyprinus carpio)上皮細胞(EPC)和斑馬魚(Danio rerio)體內初步分析了該片段的啟動子活性,以為深入分析GHRH基因功能奠定基礎。
1 材料和方法
1.1 實驗魚和細胞系
用于實驗的AB品系斑馬魚由本實驗室繁育、飼養。選擇6月齡斑馬魚成魚雌魚5 ind、雄魚5 ind,平均體質量1.2 g,平均全長4.5 cm,雌雄分別飼養,繁殖斑馬魚時一雌一雄配對,收集受精卵用于顯微注射。實驗用鯉上皮細胞系(EPC)由中國水產科學研究院珠江水產研究所實驗室保存。
1.2 啟動子區域的轉錄元件分析
用Transcription Element Search System軟件(http://www.cbil.upenn.edu/cgi-bin/tess/tess)預測大口黑鱸GHRH基因5’側翼區[20]1451 bp片段上順式作用元件,所有參數設置均使用默認值,核心序列矩陣相似度與序列矩陣相似度比值均大于0.8。
1.3 表達質粒的構建
根據大口黑鱸GHRH基因的5’側翼序列[20]設計特異引物,轉錄啟始位點定義為 +1,GHRH1-pF:5’-CCGCTCGAGCGGGCTGGTCTGTT AAATACAAGGT-3’和GHRH1-pR:5’-CGGGATC CAGCTAGTCGTGGAGAAGAATGGACAG-3’(-1043 bp~362 bp,擴增產物包括內含子1和外顯子1);GHRH-pR:5’-CGGGATCCTTCACTCTCAT CTCTCATCCTC-3’(-1043 bp~50 bp,擴增產物不包括完整的內含子1和外顯子1),在擴增引物兩端引入XhoI和BamH I酶切位點,以大口黑鱸基因組為模板進行PCR擴增。擴增產物經限制性內切酶Xho I和Bam H I(Fermentas公司)酶切純化后,插入紅色熒光蛋白報告基因載體pDsRed2-1(Clontech公司),轉化大腸桿菌E.coliDH5α,測序驗證重組質粒。得到的重組質粒命名為pGHRH1-RFP和pGHRH-RFP。
1.4 細胞培養和轉染
用質粒DNA提取試劑盒(北京天根公司)提取并純化質粒pGHRH1-RFP和pGHRH-RFP,溶解于H2O中至終濃度為50μg·mL-1。將凍存的鯉上皮瘤細胞(epithelioma papulosum cyprini,EPC)復蘇后,在CO2培養箱中用含有10%小牛血清(Gibco公司)的M199培養基37℃培養至單層,然后將EPC細胞接種到24孔板內,每孔約1 ×105cell細胞,37℃培養22~24 h后用于轉染。當24孔板的細胞密度達到90%時進行轉染,分別將800 ng的pGHRH1-RFP和pGHRH-RFP質粒與2μL脂質體2000轉染試劑(Invitrogen公司)混合,加入Opti-MEM培養基至100μL用于每一孔的轉染,同時以800 ng不含GHRH基因啟動子的pDsRed2-1質粒作為對照。轉染方法參照轉染試劑說明書。培養48 h,在Nikon熒光顯微鏡下觀察紅色熒光蛋白在EPC細胞中的表達。
1.5 顯微注射
將重組質粒注射到斑馬魚受精卵內瞬時表達進行啟動子活性分析。在斑馬魚受精卵一細胞或二細胞時分別注射約2 nL pGHRH1-RFP或pGHRH-RFP質粒DNA溶液至細胞質,同時以注射空載體pDsRed2-1為對照。胚胎在曝氣水中培養,用MS222麻醉后,在Zeiss熒光顯微鏡下觀察紅色熒光蛋白在斑馬魚體內的表達。
2 結果與分析
2.1 大口黑鱸GHRH基因5’側翼區域轉錄因子作用位點預測
用Transcription Element Search System軟件預測分析了GHRH基因從-1803 bp到362 bp(以轉錄起始位點記為+1)潛在啟動子區域上的順式作用元件,預測結果見圖1。在GHRH基因5’側翼及內含子1和外顯子1區域內存在啟動子轉錄元件TATA框和GATA框各1個,八聚體轉錄因子1(Oct-1)結合位點6個,核轉錄因子SP1結合位點4個,肝細胞核因子HNF-1結合位點2個,核轉錄因子NF-1結合位點4個,Homeobox結合位點3個,脂肪形成轉錄因子C/EBPalp結合位點13個,增強子結合蛋白C/EBP結合位點8個,垂體特異性轉錄因子Pit-1a和Pit-1結合位點3個,同源轉錄因子Gsh-1、上游激活因子USF、激活蛋白AP-1轉錄因子、生物鐘基因REV-ErbA、環磷腺苷效應元件結合蛋白CREB、小眼畸形相關轉錄因子MITF、抑制糖皮質激素受體GR、原癌基因c-Jun、轉錄因子PU.1和轉錄因子Oct-2.1結合位點各1個。
2.2 pGHRH1-RFP質粒在真核細胞中的表達
利用脂質體2000轉染試劑將重組質粒pGHRH1-RFP、pGHRH-RFP和pDsRed2-1(陰性對照)轉染EPC細胞。轉染24 h后,熒光顯微鏡下鏡檢觀察,可以看到經pGHRH1-RFP轉染的細胞孔中,部分細胞發出明顯的紅色熒光,而質粒pGHRH-RFP轉染的細胞和陰性對照組沒有表達紅色熒光的細胞,結果見圖2。
2.3 pGHRH1-RFP質粒在斑馬魚體內的表達
將pGHRH1-RFP質粒注射到斑馬魚的受精卵中,48 h后在熒光顯微鏡下觀察,約200 cell胚胎中有45個表達紅色熒光;72h斑馬魚胚胎出膜后在熒光顯微鏡下觀察,56 ind活仔魚中有16 ind表達紅色熒光,約占存活個體的29%。紅色熒光蛋白主要表達在斑馬魚的肌肉和脊椎骨中(圖3),而腦中的表達不顯明。用pGHRH-RFP和pDsRed2-1質粒注射的斑馬魚沒有觀察到有紅色熒光蛋白的表達。
3 討論
本研究經軟件預測大口黑鱸GHRH基因5’側翼約1400 bp區域序列中存在多個順式調控元件,這些順式調控元件中包括多個Oct-1、SP1、C/EBPalp和C/EBP結合位點,推測GHRH受到多種因子的精密調控,Oct-1、SP1、C/EBPalp和C/EBP可能在大口黑鱸GHRH基因的時空表達活性調節中起到重要作用。另外,順式作用元件中還包括調節因子CREB與Gsh-1的結合位點各1個,小鼠的GHRH啟動子的研究表明,CREB與Gsh-1的共表達對于GHRH啟動子的活性具有重要的調節作用[19]。
完整的內含子1和外顯子1序列是GHRH啟動子活性所必需的,用不完整的內含子1和外顯子1序列構建的重組質粒,經細胞轉染和顯微注射兩種方法,都檢測不到RFP的表達,說明內含子1和外顯子1區域中含有GHRH啟動子活性所必須的重要調節因子結合位點,具體的調節機制還有待進一步研究。人類的金屬蛋白酶1基因組織抑制劑基因(TIMP-1)的內含子1和外顯子1序列對于該基因在成纖維細胞中的轉錄活性也是必須的[21]。
利用紅色熒光蛋白報告載體pDsRed2-1,以包括外顯子1和內含子1在內的1407 bp的GHRH基因5’側翼區序列為啟動子序列,構建GHRH啟動子紅色熒光蛋白表達質粒,注射斑馬魚受精卵后,檢測到紅色熒光蛋白主要在其胚胎中部分脊椎骨和骨骼肌有表達,而沒有檢測到在腦組織中的明顯表達。迄今為止,尚未見魚類GHRH早期基因表達的研究報道,可見GHRH早期基因表達的復雜性,而本研究為魚類GHRH早期基因表達的研究提供了一定的數據與參考。目前,有研究者用RT-PCT的方法,分別在斑點叉尾鮰(Ictalurus punctatus)的骨骼肌中檢測到了GHRH-LP基因的表達[22]及在中國林蛙的脊椎中檢測到GHRH-LP明顯的表達[23]。而關于GHRH基因的進化研究認為,GHRH基因、GHRH-LP基因以及PHI-VIP基因是由一個共同的祖先基因進化而來的[24]。推測GHRH和GHRH-LP這一類同源基因在進化過程中仍保留了啟動子功能區域的相似性,時空表達上也具有相似的特征。

圖1 大口黑鱸GHRH基因5’側翼片段轉錄因子結合位點分析Fig.1 Analysis of transcription factor binding sites in 5’flanking region of GHRH gene from Micropterus salmoides
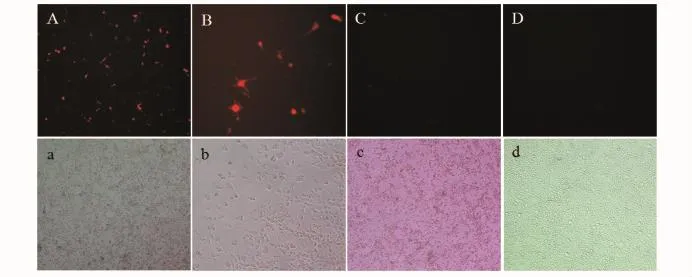
圖2 pGHRH1-RFP質粒在EPC細胞中的表達(24 h)Fig.2 Expression of pGHRH-RFP in the EPC cells(24 h)
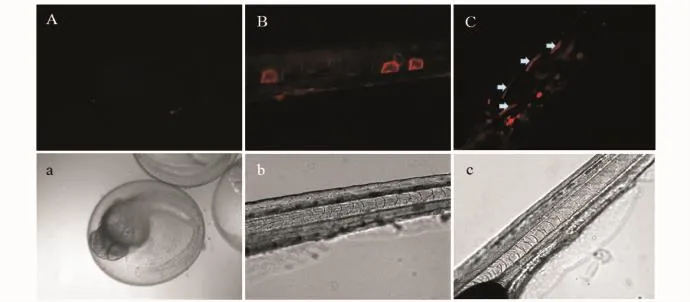
圖3 pGHRH1-RFP質粒在斑馬魚胚胎和仔魚中的表達Fig.3 Expression of pGHRH1-RFP in the embryos and larvae of zebrafish Danio rerio
對大口黑鱸GHRH基因mRNA的組織分布研究只在延腦和前腦組織檢測到了GHRH的表達,而在肌肉中未見表達[25];對金魚(Carassiusauratus)的研究中也只在大腦(brain)內檢測到了GHRH基因的表達[24]。本研究在斑馬魚的腦中沒有檢測到有規律的明顯的表達,推測本研究擴增到的大口黑鱸GHRH啟動子序列1407 bp(-1043 bp~362 bp)只是起到了驅動RFP脊椎和骨骼肌表達的作用,而不包括驅動在腦組織中特異性表達的啟動子。對大鼠GHRH啟動子的研究表明,GHRH mRNA在精巢、胎盤和下丘腦中具有不同的剪切方式,精巢和下丘腦的轉錄起始位點相距約10.7 kb,說明該基因不同組織特異調控區域分布于GHRH基因5’側翼序列很大范圍內的不同位置[18]。
本研究沒有采用雙熒光素酶方法檢測啟動子活性的方法,而是采用了構建紅色熒光蛋白報告重組質粒細胞轉染和顯微注射斑馬魚受精卵的方法,在轉染EPC細胞體外直接觀察的檢測方法更為簡便、直接,而顯微注射斑馬魚胚胎的體內檢測方法則可以為研究基因的表達和功能提供更為詳盡的依據。
[1] NAM B H,MOON JY,KIM Y O,etal.Molecular and functional analyses of growth hormone-releasing hormone(GHRH)from olive flounder(Paralichthysolivaceus)[J].Comparative Biochemistry and Physiology Part B Biochemistry and Molecular Biology,2011,159(2):84-91.
[2] MAYO K E,MILLER T,DEALMEIDA V,et al.Regulation of the pituitary somatotroph cell by GHRH and its receptor[J].Recent Progress in Hormone Research,2000(55):237-266.
[3] MAYO K E,CERELLIG M,LEBO R V,et al.Gene encoding human growth hormone-releasing factor precursor:structure,sequence,and chromosomal assignment[J].Proceedings of the National Academy of Sciences USA,1985,82(1):63-67.
[4] BILLESTRUP N,SWANSON LW,VALEW.Growth hormone releasing factor stimulates proliferation of somatostrophsin vitro[J].Proceedings of the National Academy of Sciences USA,1986,83(18):6854-6857.
[5] BAUMANN G.Mutations in the growth hormone releasing hormone receptor:a new form of dwarfism in humans[J].Growth Hormone&IGF Research,1999,9(SUPPL.B):24-30.
[6] DESAIM P,UPADHYE P S,KAMIJO T,et al.Growth hormone releasing hormone receptor(GHRH-r)gene mutation in Indian children with familial isolated growth hormone deficiency:a study from western India[J].Journal of Pediatric Endocrinology&Metabolism,2005,18(10):955-973.
[7] ZHANG Y,ZHU Y J,LIZ,etal.Injection of porcine growth hormone releasing hormone gene plasmid in skeletalmuscle increase piglets’growth and whole body protein turnover[J].Livestock Science,2008,115(2-3):279-286.
[8] FREDERICKSON R M,MICHEAU M R,IWAMOTO A,etal.5'flanking and first intron sequences of the human beta-actin gene required for efficient promoter activity[J].Nucleic Acids Research,1989,17(1):253-270.
[9] LIU Z J,MOAV B,FARASA J,et al.Functional analysis of elements affecting expression of the betaactin gene of carp[J].Molecular and Cellular Biology,1990,10(7):3432-3440.
[10] FENG J,LIG,LIU X,et al.Functional analysis of the promoter region of amphioxusβ-actin gene:a useful tool for driving gene expressionin vivo[J].Molecular Biology Reports,2014,41(10):6817-6826.
[11] JIAN Q,CHEN M,BAI J J,et al.Generation and characterization of a stable red fluorescent transgenic Tanichthys albonubes line[J].African Journal of Biotechnology,2012,11(30):7756-7765.
[12] QI J,LIU X D,LIU J X,et al.Molecular characterization of heat shock protein 70(HSP 70)promoter in Japanese flounder(Paralichthys olivaceus),and the association of Pohsp70 SNPs with heat-resistant trait[J].Fish&Shellfish Immunology,2014,39(2):503-511.
[13] 孫科軍,劉希良,王開卓,等.斑鱖myostatin基因及其啟動子的克隆與序列[J].基因組學與應用生物學,2012,31(2):133-140.
SUN K J,LIU X L,WANG K Z,etal.Cloning and sequence analysis of the myostatin gene and its promoter in golden mandarin fish(Siniperca scherzeri)[J].Genomics and Applied Biology,2012,31(2):133-140.
[14] WANG Y,SUN ZH,ZHOU L,et al.Grouper tshβ promoter-driven transgenic zebrafish marks proximal kidney tubule development[J].Plos One,2014,9(6):1-10.
[15] TSE M C,CHAN K M,CHENG C H.Cloning,characterization and promoter analysis of the common carpIGF-IIgene[J].Gene,2008,412(1-2):26-38.
[16] SOLLOSO A,BARREIRO L,SEOANE R,et al.GHRH proliferative action on somatotrophs is celltype specific and dependent on Pit-1/GHF-1 expression[J].Journal Cell Physiol,2008,215(1):140-50.
[17] NOGUéSN,DELRíO JA,PéREZ-RIBA M,etal.Placenta-specific expression of the rat growth hormone-releasing hormone gene promoter in transgenic mice[J].Endocrinology,1997,138(8):3222-3227.
[18] SRIVASTAVA CH,MONTSB S,ROTHROCK JK,et al.Presence of a spermatogenic-specific promoter in the rat growth hormone-releasing hormone gene[J].Endocrinology,1995,136(4):1502-1508.
[19] MUTSUGA N,IWASAKIY,MORISHITA M,etal.Homeobox protein Gsh-1-dependent regulation of the rat GHRH gene promoter[J].Molecular Endocrinology,2001(12):2149-2156.
[20] MA D M,HAN L Q,BAI J J,et al.A 66-bp deletion in growth hormone releasing hormone gene 5’-flanking region with largemouth bass recessive embryonic lethal[J].Animal Genetics,2014,45(3):421-426.
[21] CLARK IM,ROWAN A D,EDWARDSDR,etal.Transcriptional activity of the human tissue inhibitor ofmetalloproteinases1(TIMP-1)gene in fibroblasts involves elements in the promoter,exon 1 and intron 1[J].Biochemical Journal,1997,324(Pt2):611-617.
[22] SMALL B C,NONNEMAN D.Sequence and expression of a cDNA encoding both pituitary adenylate cyclase activating polypeptide and growth hormone-releasing hormone-like peptide in channel catfish(Ictalurus punctatus)[J].General and Comparative Endocrinology,2001,122(3):354-363.
[23] ALEXANDRE D,VAUDRY H,JéGOU S,et al.Structure and distribution of the mRNAs encoding pituitary adenylate cyclase-activating polypeptide and growth hormone-releasing hormone-like peptide in the frog,Rana ridibunda[J].Journal of Comparative Neurology,2000,421(2):234-246.
[24] LEE L T O,SIU F K Y,TAM J K V,et al.Discovery of growth hormone releasing hormones and receptors in nonmammalian vertebrates[J].Proceedings of the National Academy of Sciences USA,2007,104(7):2133-2138.
[25] 韓林強,白俊杰,李勝杰.大口黑鱸GHRH-LP和GHRH基因序列同源性、基因結構和時序表達研究[J].水生生物學報,2011,35(3):473-481.
HAN L Q,BAI J J,LI S J,Comparison of gene structure,sequence homology and expression pattern of largemouth bass GHRH-LP and GHRH[J].Acta Hydrobiologica Sinica,2011,35(3):473-481.
Sequence and activity analysis of GHRH promoter region from M icropterus salmoides
MA Dong-mei,HAN Lin-qian,BAIJun-jie
(1.Key Laboratory of Tropical&Subtropical Fishery Resource Application&Cultivation,Ministry of Agriculture,Pearl River Fisheries Research Institute,Chinese Academy of Fishery Sciences,Guangzhou510380,China;2.Freshwater Aquaculture Collaborative Innovation Center of Hubei Province,Wuhan430070,China)
Growth hormone releasing hormone(GHRH)is a smallmolecular weight peptide released from the arcuate nucleus of hypothalamus.It mainly plays the role to regulate the synthesis and release of growth hormone(GH)from the anterior pituitary somatotrophs.Non-pituitary GHRH has awide spectrum of activity,includingmodulating cell proliferation,especially in malignant tissues,regulating differentiation of some cell types,and promoting healing of skin wounds.In order to analyze the activity of GHRH 5’flanking region and themechanism of the potential transcription factors regulating GHRH gene expression in largemouth bass(Micropterus salmoides),the cis-acting elementswere predicted by the transcription element search system for the 1400 bp-length fragment in 5’flanking region.Many sites related to regulating the GHRH expression were identified,includingmultiple of Oct-1,SP1,NF-1,C/EBPalp and C/EBP binding sites,which indicated that these sites probably played the key roles to regulate the spatiotemporal expression of GHRH.The GHRH 5’flanking region fragmentwith integrate exon 1 and intron 1 wasmodified by adding to two restrict enzyme sites,XhoI andBamH I,and was inserted into the red fluorescent protein(RFP)reporter gene vector pDsRed2-1.Then the recombined plasmid pGHRH1-RFP was constructed.Meanwhile,the recombinant plasmid pGHRH-RFP was constructed using the fragment without exon 1 and intron 1.The plasmids pGHRH1-RFP and pGHRH-RFPwere transfected epithelioma papillosum cyprini(EPC)cells.After 48 h of culture,the expression of the RFP was detected in EPC cells with pGHRH1-RFP,but not in the cells as negative controls or the cells transfected by pGHRH-RFP.At the same time,the plasmid pGHRH1-RFP or pGHRH-RFPwas alsomicroinjected in zebrafish embryos at the one cell or two cell stages.Embryos without microinjection served as negative controls.A total of 22.5%(n=45/200)embryos injected with pGHRH1-RFP could be detected RFP 48hpf(hours post fertilization)and 29%(n=16/56)fries could be detected RFP 72hpf.And the RFP was not detected in the zebrafish embryos and larvae microinjected with plasmid pGHRH-RFP and negative controls.The results showed that the isolated GHRH 5’flanking region had the activity of starting GHRH gene expression.And there are indispensable cis-elements in exon 1 and intron 1 of GHRH gene for promoter activity.Additionally,it is interesting that the RFP is found in spine and muscle,not in brain.In rat,GHRH gene is spliced into alternative upstream promoters in brain,gonads and placenta.It suggests that the cis-acting elements responsible for GHRH spatially specific expression in brain of largemouth bass have not been discovered yet.The current study can lay foundations for analysis of the functions of GHRH gene.
growth hormone releasing hormone(GHRH);promoter activity;largemouth bass(Micropterus salmoides)
Q 954
A
1004-2490(2016)04-0383-08
2015-08-27
國家自然科學基金項目(31001107);948計劃重點項目(2011-G12);廣東省省級科技計劃項目(2015A020209035);國家科技支撐計劃(2012BAD26B03)
馬冬梅(1978-),女,遼寧撫順人,博士,主要研究方向為魚類遺傳育種。Tel:020-81616127,E-mail:madongmei2003@163.com
白俊杰,研究員。Tel:020-81616129,E-mail:jjbai@163.net

