冷誘導RNA結合蛋白參與小鼠H1N1甲型流感病毒感染的應答
聶培婷,湯 承,岳 華*
(1.西南民族大學生命科學與技術學院,成都 610041;2.新疆天康畜牧生物技術股份有限公司制藥事業部,烏魯木齊 830011)
?
冷誘導RNA結合蛋白參與小鼠H1N1甲型流感病毒感染的應答
聶培婷1,2,湯承1,岳華1*
(1.西南民族大學生命科學與技術學院,成都 610041;2.新疆天康畜牧生物技術股份有限公司制藥事業部,烏魯木齊 830011)
冷誘導RNA結合蛋白(CIRP)是哺乳動物間高度保守的多功能蛋白,但其在病毒感染中的作用尚不清楚,本研究旨在探討CIRP對甲型流感病毒感染的應答。用H1N1甲型流感病毒PR-8株滴鼻接種成年BALB/c小鼠,分別于感染后24、48、72、96、120 h隨機處死3只,用熒光定量RT-PCR和免疫組化法分別檢測小鼠心、肝、脾和肺中CIRP基因和蛋白質的表達水平。基因定量檢測結果顯示,感染小鼠各被檢器官中CirpmRNA的轉錄量顯著升高;免疫組化試驗結果顯示,CIRP在感染小鼠心肌細胞、肺泡上皮細胞、肺支氣管上皮細胞和脾淋巴細胞的細胞質中表達量均有不同程度的升高。綜上可見,CIRP參與機體對H1N1甲型流感病毒感染的應答,由于CIRP對炎性因子產生過程具有顯著的調節作用,其在流感病毒感染過程中的作用值得深入研究。
冷誘導RNA結合蛋白;甲型流感病毒;熒光定量RT-PCR;免疫組化
冷誘導RNA結合蛋白(cold induced RNA binding protein,CIRP)是RNA結合蛋白家族的異源核糖核蛋白(hnRNP)亞群成員[1],是哺乳動物中最早鑒定出的與冷應激相關的蛋白質[2],在多種應激狀態下發揮細胞保護作用[2-7]。最近的研究表明,在LPS刺激和敗血癥情況下,CIRP通過調節NF-κB信號通路影響多種炎性因子的產生[8],并通過調節TRL4促進炎癥發生[9],是一種新的促炎介質[10],在細菌感染中發揮重要作用。甲型流感病毒可引起人和多種動物嚴重的呼吸道感染[11],其引起的細胞因子風暴是引起重癥肺炎和致死的主要原因[12],肺上皮細胞和炎性浸潤在肺的病理性損傷中起重要作用[13]。CIRP與病毒感染的聯系尚不清楚,本研究旨在探討CIRP對甲型流感病毒感染的應答。
1 材料與方法
1.1材料
30只5周齡的雌性BALB/c小鼠,體重18 g±2 g,購自華西實驗動物中心;H1N1甲型流感病毒(PR/8)由四川省疾病預防控制中心惠贈,HA效價為28;RNA提取試劑盒購自美國Invitrogen公司;反轉錄試劑盒購自日本TaKaRa公司;兔抗人CIRP多克隆抗體購自美國Proteintech公司;生物素羊抗兔抗體、免疫組化ABC檢測試劑盒購自美國VECTOR公司;DAB顯色試劑購自武漢博士德公司。
1.2病毒感染模型的建立及樣本采集
將小鼠隨機等分為兩組,用干冰吸入麻醉后,感染組滴鼻接種PR/8,50 μL·只-1,對照組用等體積生理鹽水代替病毒液滴鼻,兩組小鼠隔離飼養。各組分別于感染后24、48、72、96、120 h隨機處死3只小鼠,采集心、肝、脾和肺,每個樣本分為兩份,一份用焦炭酸二乙酯溶液清洗后立即放入液氮中過夜,隨后轉入-80 ℃冰箱凍存,用于mRNA轉錄量的檢測,另一份用4%多聚甲醛固定,石蠟包埋切片。
1.3組織樣本總RNA的提取及cDNA合成
按照 RNA提取試劑盒說明書推薦程序提取各組織樣本的總RNA,用反轉錄試劑盒反轉錄合成cDNA。
1.4流感病毒感染的檢測
采用RT-PCR[14]檢測PR/8感染后48 h肺中H1N1甲型流感病毒M基因,以確定小鼠是否感染成功。
1.5小鼠組織器官中CirpmRNA的表達水平的測定
以“1.3”合成的組織cDNA為模板,熒光定量PCR[15]檢測CirpmRNA在感染小鼠組織器官中的時空表達水平,以在流感病毒感染過程中穩定表達的Ppia作為內參基因[16],用2-△△Ct法[17]進行相對定量,每個樣本重復測定3次,用SPSS軟件進行統計學分析。
1.6小鼠組織器官中CIRP的分布及表達水平測定
按照免疫組化試劑盒說明書對石蠟切片進行染色,用Nikon ECLIPSE 6200顯微數碼圖像采集系統采集免疫組化圖像信息,在每張切片隨機選取5個視野,用ImagePro-Plus6.0 (IPP6.0) 圖像分析軟件測定其陽性顆粒的累積光密度值(integrated optical density,IOD),用SPSS軟件對數據進行統計學處理。圖像分析判定標準:IOD≤100為陰性;100≤IOD≤2 000為弱陽性;2 000≤IOD≤5 000為陽性;IOD≥5 000為強陽性[18]。
2 結 果
2.1H1N1甲型流感病毒成功感染小鼠
RT-PCR從感染小鼠肺中擴增出一條約238 bp的條帶,大小與預期相符(圖1),說明PR-8感染了小鼠,成功建立了N1N1甲型流感病毒感染小鼠模型。
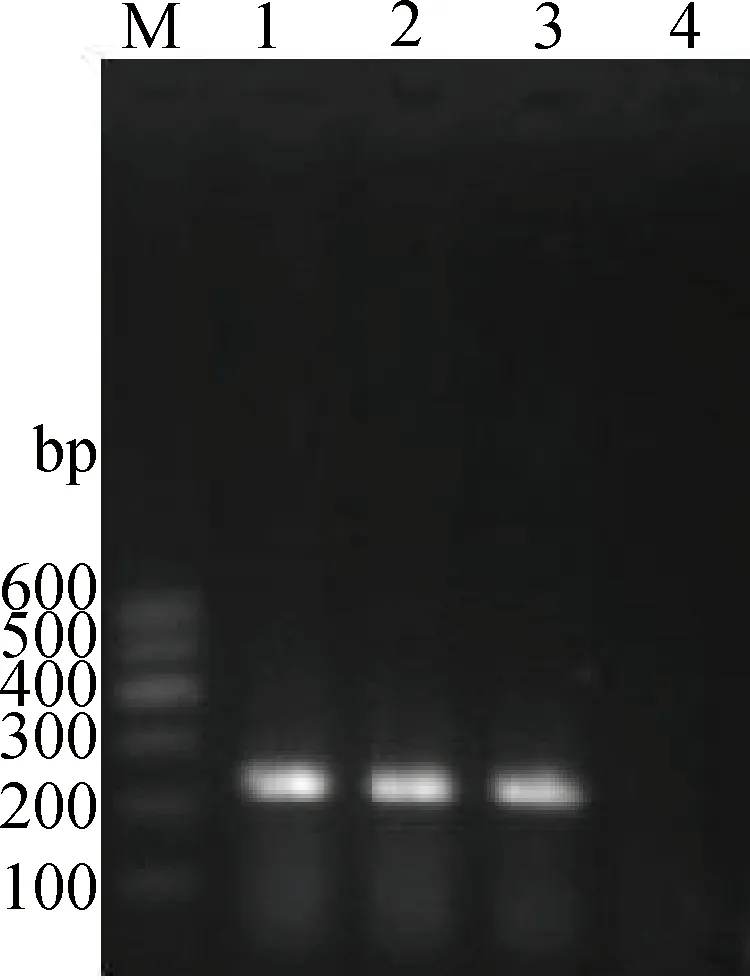
M.DNA相對分子質量標準Marker Ⅰ;1~3.感染小鼠肺組織樣本;4.陰性模板對照M.DNA marker Ⅰ;1-3.Lung samples of infected mice;4.Negative control圖1 RT-PCR檢測感染后48 h小鼠肺中PR-8 M基因的結果Fig.1 The result of PR-8 M gene in infected lungs 48 h PI detected by RT-PCR
2.2Cirp基因對H1N1甲型流感病毒感染的轉錄應答
熒光定量RT-PCR檢測結果顯示,CirpmRNA在健康小鼠的心、肝、脾和肺等器官中均有轉錄,在肝中轉錄量最低,其在心、脾和肺中的轉錄量分別為肝轉錄量的7.6、7.4和2.2倍(圖2)。CirpmRNA在感染H1N1甲型流感病毒后顯著升高,但在不同器官上升的時間點不同(圖3)。
2.3CIRP在小鼠組織器官中的定位及對H1N1甲型流感病毒感染的應答
免疫組化結果顯示,CIRP在健康小鼠和感染小鼠心肌細胞、肝細胞、肺泡上皮細胞、肺支氣管黏膜上皮細胞和脾淋巴細胞的細胞質中表達,甲型流感病毒感染未引起CIRP細胞定位的改變(圖4)。
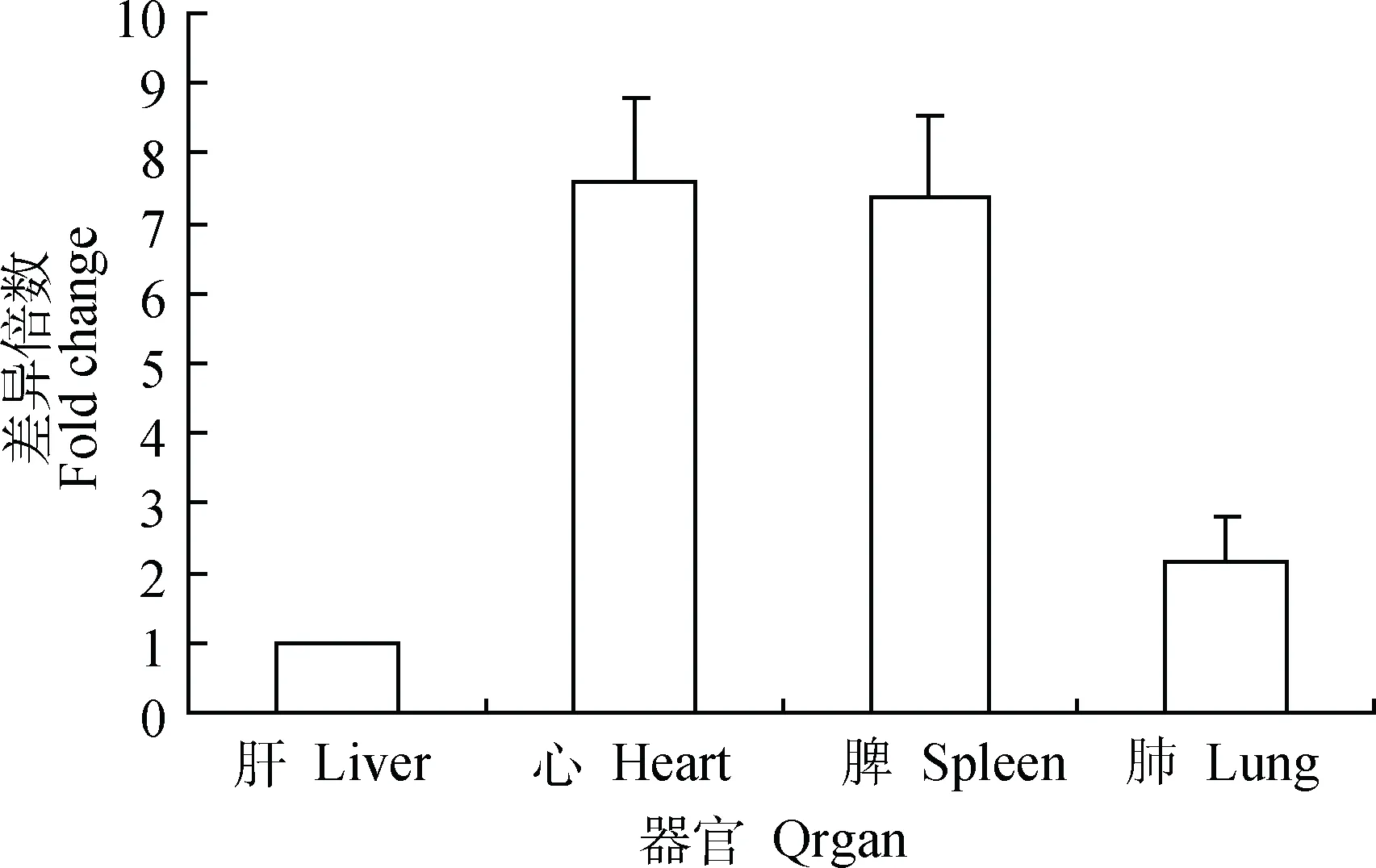
圖2 健康小鼠各組織器官中Cirp基因的相對轉錄水平Fig.2 The relative transcription level of Cirp gene in the organs of healthy mice

*.差異顯著(P<0.05)*.Significant difference (P<0.05)圖3 小鼠各臟器Cirp基因對H1N1甲型流感病毒感染的轉錄應答Fig.3 The transcription response of Cirp in mouse organs infected Influenza A Virus
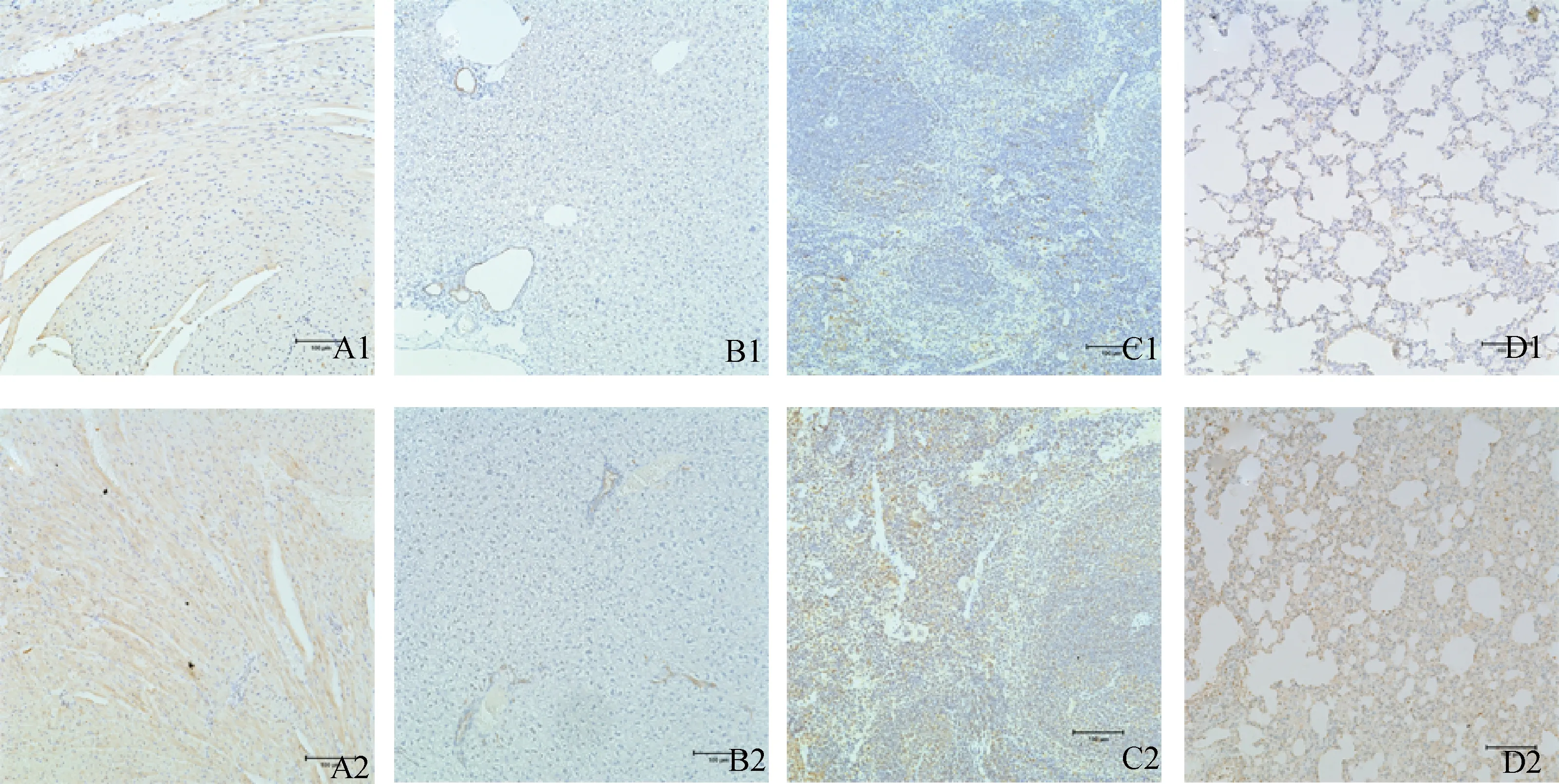
A1.對照組小鼠心;A2.感染后96 h小鼠心;B1.對照組小鼠肝;B2.感染后120 h小鼠肝;C1.對照組小鼠脾;C2.感染后120 h小鼠脾;D1.對照組小鼠肺;D2.感染后24 h小鼠肺A1.Control group of heart tissue;A2 Experimental mice of 96 h-PI heart tissue;B1.Control group of liver tissue;B2.Experimental mice of 120 h-PI liver tissue;C1.Control group of spleen tissue;C2.Experimental mice of 120 h-PI spleen tissue;D1.Control group of lung tissue;D2.Experimental mice of 24 h-PI lung tissue圖4 CIRP在健康及感染小鼠器官中的分布(免疫組化ABC法,20×)Fig.4 Distribution of CIRP in healthy and infected mice (ABC IHC staining,20×)

圖5 CIRP對H1N1甲型流感病毒感染的應答Fig.5 Response of CIRP to H1N1 influenza A virus infection
IPP6.0圖像定量分析結果顯示,CIRP在健康小鼠心和肺的表達呈強陽性,在脾中的表達呈陽性,在健康小鼠肝中的表達量為弱陽性;CIRP在感染小鼠心、肝、脾和肺中的表達量有升高趨勢,其中脾CIRP的表達量在感染后72 h顯著升高(圖5)。
3 討 論
已知CIRP存在于多種不同類型的細胞中,是哺乳動物種間高度保守的多功能蛋白,參與對溫度[2]、H2O2[3]、缺氧[4]、滲透壓[5]等多種應激的轉錄應答,多種應激因子誘導機體CIRP的表達量升高和/或發生核質遷移,發揮細胞保護作用[2-7,15]。此外還參與胚胎發育、神經調節[19]和生物鐘調節等生理過程,并參與機體對細菌的應答,但CIRP對病毒感染的應答未見報道。本研究發現CIRP在甲型流感病毒感染小鼠心、肝、脾、肺等器官中無論是基因還是蛋白質的水平均顯著升高,證明其具有參與機體對流感病毒感染應答的功能。研究證明機體在LPS刺激下CIRP表達量升高,通過激活NF-κB信號通路誘導產生炎性因子,流感病毒感染能引發機體的炎性損傷,H5N1高致病性禽流感病毒刺激肺上皮細胞和巨噬細胞釋放大量細胞因子,在細胞因子風暴中起重要作用[12]。在本研究中,CIRP在流感病毒感染小鼠體內表達量升高,極有可能通過調節炎性因子的產生,進而參與機體對流感病毒感染的應答過程。
RNA結合蛋白家族的某些成員在流感病毒感染中發揮著重要作用。S.D.Shapira等[20]發現一個RNA結合蛋白調控網絡,能通過影響IFN-β的合成來抵抗機體對流感病毒的免疫作用,而RNA結合蛋白3(RanBP3)可通過Ser58位點的磷酸化作用促進流感病毒vRNP的出核轉運[21]。ERK和NF-κB信號通路是流感病毒復制和機體起始免疫不可或缺的重要環節[22~25],因此推測作為這兩條信號通路的上游調控因子,CIRP在流感病毒感染過程中表達量升高也可能影響流感病毒的復制,其在流感病毒感染中的作用值得進一步研究。
[1]BURD C G,DREYFUSS G.Conserved structures and diversity of functions of RNA-binding proteins[J].Science,1994,265(5172):615-621.
[2]NISHIYAMA H,ITOH K,KANEKO Y,et al.A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth[J].JCellBiol,1997,137(4):899-908.
[3]XUE J H,NONOGUCHI K,FUKUMOTO M,et al.Effects of isehemia and H2O2:on the cold stress protein CIRP expression in rat neuronal cells[J].FreeRadicBiolMed,1999,27(11-12):1238-1244.
[4]WELLMANN S,BüHRER C,MODEREGGER E,et al.Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-l-independent mechanism[J].JCellSci,2004,117(Pt 9):1785-1794.
[5]PAN F,ZARATE J,CHOUDHURY A,et al.Osmotic stress of salmon stimulates upregulation of a cold inducible RNA binding protein (CIRP) similar to that of mammals and amphibians[J].Biochimie,2004,86(7):451-461.
[6]YANG C,CARRIER F.The UV-inducible RNA-binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress response[J].JBiolChem,2001,276(50):47277-47284.
[7]DE LEEUW F,ZHANG T,WAUQUIER C,et al.The cold-inducible RNA-binding protein migrates from the nucleus to cytoplasmic stress granules by a methylation-dependent mechanism and acts as a translational repressor[J].ExpCellRes,2007,313(20):4130-4144.
[8]BROCHU C,CABRITA M A,MELANSON B D,et al.NF-κb-dependent role for cold-inducible RNA binding protein in regulating interleukin 1β[J].PLoSOne,2013,8(2):e57426.
[9]QIANG X,YANG W L,WU R,et al.Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis[J].NatMed,2013,19(11):1489-1495.
[10]ZHOU M,YANG W L,JI Y,et al.Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia[J].BiochimBiophysActa,2014,1840(7):2253-2261.
[11]NEUMANN G,NODA T,KAWAOKA Y.Emergence and pandemic potential of swine-origin H1N1 influenza virus[J].Nature,2009,459(7249):931-939.
[12]TEIJARO J R,WALSH K B,RICE S,et al.Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection[J].ProcNatlAcadSciUSA,2014,111(10):3799-3804.
[13]LA GRUTA N L,KEDZIERSKA K,STAMBAS J,et al.A question of self-preservation:immunopathology in influenza virus infection[J].ImmunolCellBiol,2007,85(2):85-92.
[14]聶培婷,湯承,岳華.H1N1甲型流感病毒在BHK21中的增殖規律[J].西南民族大學學報·自然科學版,2012,38(5):764-769.
NIE P T,TANG C,YUE H.Study on the proliferation profile of influenza A virus in BHK21 cells[J].JournalofSouthwestUniversityforNationalities·NaturalScienceEdition,2012,38(5):764-769.(in Chinese)
[15]周鴻淼,湯承,岳華,等.冷誘導RNA結合蛋白參與小鼠對LPS的應答[J].畜牧獸醫學報,2014,45(8):1348-1354.
ZHOU H M,TANG C,YUE H,et al.Cold-inducible RNA binding protein involves in response to LPS in mice[J].ActaVeterinariaetZootechnicaSinica,2014,45(8):1348-1354.(in Chinese)
[16]吳巧,張斌,湯承,等.H5N1禽流感病毒感染小鼠后內參基因的篩選[J].中國畜牧獸醫,2013,40(9):55-60.
WU Q,ZHANG B,TANG C,et al.Selection of reference gene in mice infected with H5N1 avian influenza virus[J].ChinaAnimalHusbandry&VeterinaryMedicine,2013,40(9):55-60.(in Chinese)
[17]LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ctmethod[J].Methods,2001,25(4):402-408.
[18]蔡文琴,王伯沄.實用免疫細胞化學與核酸分子雜交技術[M].成都:四川科學技術出版社,1994.
CAI W Q,WANG B Y.Practical immunocytochemistry and nucleic acid hybridization techniques[M].Chengdu:Sichuan Science and Technology Press,1994.(in Chinese)
[19]SAITO K,FUKUDA N,MATSUMOTO T,et al.Moderate low temperature preserves the stemness of neural stem cells and suppresses apoptosis of the cells via activation of the cold-inducible RNA binding protein[J].BrainRes, 2010,1358:20-29.
[20]SHAPIRA S D,GAT-VIKS I,SHUM B O,et al.A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infections[J].Cell,2009,139(7):1255-1267.
[21]PREDICALA R,ZHOU Y.The role of Ran-binding protein 3 during influenza A virus replication[J].JGenVirol,2013,94(Pt 5):977-984.
[22]PLANZ O.Development of cellular signaling pathway inhibitors as new antivirals against influenza[J].AntiviralRes,2013,98(3):457-468.
[23]LUDWIG S,PLANZ O.Influenza viruses and the NF-κB signaling pathway—towards a novel concept of antiviral therapy[J].BiolChem,2008,389(10):1307-1312.
[24]CANNON G,CALLAHAN M A,GRONEMUS J Q,et al.Early activation of MAP kinases by influenza A virus X-31 in murine macrophage cell lines[J].PLoSOne,2014,9(8):e105385.
[25]NIMMERJAHN F,DUDZIAK D,DIRMEIER U,et al.Active NF- kappaB signalling is a prerequisite for influenza virus infection[J].JGenVirol,2004,85(Pt 8):2347-2356.
(編輯白永平)
Cold-inducible RNA Binding Protein of Mice Involved in Response to Influenza A Virus Infection in Mice
NIE Pei-ting1,2,TANG Cheng1,YUE Hua1*
(1.CollegeofLifeScienceandTechnology,SouthwestUniversityforNationalities,Chengdu610041,China;2.XinjiangTeconAnimalHusbandryBio-TechnologyCO.,LT,Urumqi830011,China)
Cold induced RNA binding protein (CIRP) is a highly conserved multifunctional protein among vertebrates,but its role in viral infection is unclear.The objective of this paper was to explore the response of CIRP to influenza virus infection.The BALB/c mice were intranasal inoculated with H1N1 influenza A virus.Three mice were randomly selected in each group and euthanized at 24,48,72,96,120 h post infection.The heart,liver,spleen and lung of each mouse were collected.Real-time RT-PCR and immunohistochemistry (IMH) were used to detect the transcription ofCirpmRNA and expression of CIRP in each organ.The results of Real-time RT-PCR exhibited that theCirpmRNA significantly increased in all detected organs.The results of IMH test showed that CIRP protein was highly expressed in cytoplasm of myocardial cells,alveolar epithelial cells,bronchial epithelium cells and spleen lymphocytes.In summary,CIRP is involved in the response to influenza A virus infection,because CIRP can significantly regulate the production of inflammatory factors,the role of CIRP in influenza virus infection should be further studied.
cold-inducible RNA binding protein;influenza A virus;real-time RT-PCR;immunohistochemistry
10.11843/j.issn.0366-6964.2016.08.016
2015-12-28
國家自然科學基金(31172307);四川省教育廳創新團隊(13TD0057)
聶培婷(1989-),女,新疆霍城人,主要從事感染與免疫學研究,E-mail:540016852@qq.com
岳華,教授,E-mail:yhua900@163.com
S852.23
A
0366-6964(2016)08-1652-06

