蛇足石杉中的新內生真菌及其代謝產物的分離與鑒定
于飛雪, 楊銀河, 趙沛基, 陳 瑤
( 1. 昆明理工大學附屬醫院, 云南省第一人民醫院, 昆明 650500; 2. 中國科學院昆明植物研究所植物化學與西部植物資源持續利用國家重點實驗室, 昆明 650201 )
?
蛇足石杉中的新內生真菌及其代謝產物的分離與鑒定
于飛雪1,2, 楊銀河2, 趙沛基2, 陳瑤1*
( 1. 昆明理工大學附屬醫院, 云南省第一人民醫院, 昆明 650500; 2. 中國科學院昆明植物研究所植物化學與西部植物資源持續利用國家重點實驗室, 昆明 650201 )
從蛇足石杉內生菌的次級代謝產物中尋找活性成分,為進一步開發利用蛇足石杉藥用植物資源提供了新途徑,但至今其內生菌代謝產物的系統性研究較為少見。種類豐富的內生真菌普遍存在于各種植物中,但蕨類植物中內生真菌的研究較少。為了尋找蛇足石杉內生菌中的細胞毒活性成分,該研究從蛇足石杉根部分離得到一株球毛殼屬 (Chaetomiumsp.) 真菌M336,對其化學成分進行了研究。對蛇足石杉內生真菌M336采用PDA固體培養基擴大發酵,發酵物經提取及乙酸乙酯萃取后,通過正相硅膠柱色譜法、Sephadex LH-20凝膠柱色譜法、薄層制備、高效液相色譜等色譜手段對其發酵物中的化學成分進行分離純化,利用理化性質、質譜、核磁等波譜分析技術,并結合相關文獻數據鑒定化合物的結構。結果表明:從內生真菌M336發酵提取物的乙酸乙酯萃取部分分離并鑒定出8個化合物,分別為chaetoviridines F、chaetoviridines E、5′-epichaetoviridin A、5′-epichaetoviridin A、xanthoquinodins Al、xanthoquinodins A2、xanthoquinodins B1和毛殼菌素。從M336中分離得到8個化合物,化合物3有一定的抑菌作用,其余化合物有一定的細胞毒活性。該研究結果豐富了蛇足石杉內生真菌球毛殼屬中的天然細胞毒活性的化合物。
球毛殼屬, 內生真菌, 結構鑒定, 細胞毒活性
蛇足石杉(Huperziaserrata)又名千層塔、蛇足草、萬年杉等,屬蕨類石杉科 (Huperiaceae) 石杉屬 (Huperzia) 藥用植物 (余紅英等,2001)。民間用千層塔的全草治療癰癤腫毒、跌打損傷等 (程丹華和戴克敏,1992)。由于千層塔野生資源的匱乏及其良好的藥理活性,使得大多數研究學者的目光轉向了對其內生真菌的研究。植物的內生真菌是一個豐富多樣性的生物類群。在植物內生真菌中,有的內生真菌是對植物病原菌產生拮抗而保護宿主植物免受病原菌侵害的衛士,有的內生真菌對宿主體內某些活性成分的形成產生重要影響,也有能產生與宿主相同或相似的生理活性成分的內生真菌,還有一部分內生真菌與宿主只是共生或伴生關系而作用不明等 (谷蘇等,2001; Petrini,1991;Tan & Zhou,2001)。自然界中內生真菌普遍存在于各種植物中,其分布廣泛,且種類繁多。但蕨類中內生真菌研究較少見 (鄒文欣和譚仁祥,2001)。有報道表明蕨類植物內生菌具有多樣性及蕨類植物內生真菌產生的次級代謝產物具有豐富的生物學活性 (張君誠等,2010)。Phonkerd et al(2008)研究發現從球毛殼屬中分離得到的化合物chaetoviridines F、chaetoviridines E具有抗人表皮樣癌 (KB) ,人乳腺癌 (BC1) 和人的小細胞肺癌 (NCI-H187) 作用。Li et al (2013) 報道從羅布斯塔維氏菊的球毛殼屬內生真菌中分離得到的化合物chaetoviridines E和5′-epichaetoviridin A對人類癌癥細胞株HepG2具有高細胞毒性。從腐質霉屬中分離得到的化合物xanthoquinodins Al、xanthoquinodins A2、xanthoquinodins B1具有抗人髓細胞性白血病HL-60,肝細胞癌SMMC-7721,肺癌A-549,乳腺癌MCF-7和結腸癌SW480細胞系作用 (Tabata et al, 1993)。另外,有文獻報道毛殼菌素具有明顯的細胞毒活性(Kung et al, 2004; Staab et al, 2007; Cook et al, 2009)。蛇足石杉內生真菌次級代謝產物具有細胞毒活性的報道很少見,但以上報道表明,來源于蛇足石杉的球毛殼屬內生真菌的次級代謝產物可能具有一定的細胞毒性。因此,具有重要經濟價值的蛇足石杉內生真菌將成為篩選新的細胞毒活性物質的重要來源。
本研究從蛇足石杉根部分離并篩選得到1株具細胞毒活性的內生真菌Chaetomiumsp. M336。我們采用了固體發酵形式,對其次級代謝產物進行了研究,從中分離并鑒定了8個化合物,利用化合物各自的理化性質以及質譜、核磁等波譜分析技術,并結合相關文獻數據,結構鑒定為chaetoviridines F(1), chaetoviridines E (2),5′-epichaetoviridin A (3),5′-epichaetoviridin A (4),xanthoquinodins Al (5),xanthoquinodins A2 (6),xanthoquinodins B1(7),毛殼菌素 (8)。
1 材料與方法
1.1 儀器與試劑
由VG AutoSpec-3000,Finnigan LCQ-Advantage 型質譜儀測定質譜;通過Bruker AV-400,DRX-600,Avance III 600 核磁共振儀測得NMR譜,內標為TMS;半制備采用Waters996型-高效液相色譜儀完成;正相柱層析硅膠 (100~200目、200~300目、硅膠GF254) 以及GF254薄層硅膠板,均為青島海洋化工廠生產;Sephadex LH-20由瑞典Amersham Biosciences公司提供;顯色方法為待分離樣品在GF254薄層硅膠板上用適當展開劑展開后,將其置于熒光燈下波長254 nm和365 nm處觀察熒光, 并觀察碘蒸氣顯色以及5%硫酸乙醇溶液處理后加熱顯色。
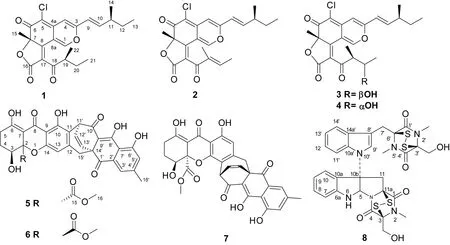
圖 1 化合物1-8的結構Fig. 1 Structures of compounds 1-8
Chaetomiumsp. M336來自蛇足石杉 (Huperziaserrata) 的根部,蛇足石杉為本課題組2013年采自云南省文山州西疇縣。
1.2 內生真菌的鑒定
Chaetomiumsp. M336的總DNA采用改良的CTAB法提取,使用通用引物ITS4和ITS5擴增ITS序列。
PCR擴增反應程序的熱循環參數:95 ℃ 15 min,15 min時加酶; 95 ℃ 40 s,55℃ 40 s,72 ℃ 90 s, 30個循環;72 ℃ 10 min。
PCR結束后,經膠回收獲得約700 bp的核酸片段,測序后通過NCBI與GenBank基因庫序列進行比對,確定菌株所屬類群。
1.3 培養基及培養條件
Chaetomiumsp. M336大批量發酵采用固體PDA培養基,固體PDA培養基配方:馬鈴薯20%,葡萄糖2%,瓊脂2%,蒸餾水1 000 mL,pH 自然。
培養條件:Chaetomiumsp. M336經PDA固體培養基發酵30 L,總共1 250個培養皿,25 ℃培養17 d。
1.4 發酵產物的分離純化
將劃分成小塊的固體培養基用乙酸乙酯∶甲醇∶冰醋酸 = (80∶15∶5,V/V/V) 混合有機溶劑浸泡提取4次,合并提取液經46 ℃減壓濃縮得到浸膏,將浸膏溶于蒸餾水后加入等體積的乙酸乙酯萃取至乙酸乙酯相顏色較淺,減壓濃縮得到提取物40.1 g。粗提物經正相柱層析 (200~300目),分別用石油醚∶乙酸乙酯 (100∶4→6∶4),氯仿∶甲醇 (100∶4→0∶100)進行梯度洗脫,得到11個組分Fr. 1 - Fr. 11。組分Fr. 3 (218 mg) 反復經GF254硅膠正相柱,分別以石油醚-丙酮 (200∶5→0∶100) ,氯仿-甲醇 (100∶0→0∶100) 梯度洗脫,再經Sephadex LH-20 (氯仿∶甲醇 = 1∶1) 和Sephadex LH-20 (甲醇) 凝膠柱得到化合物 1 (1.9 mg)。組分Fr. 4 (1.9 g) 反復經200 ~ 300目硅膠正相柱,以石油醚-乙酸乙酯 (100∶1→6∶4) 梯度洗脫得到5個亞組分,Fr. 4.2 (1.7 g) 經Sephadex LH-20 (氯仿∶甲醇 = 1∶1) 凝膠柱得到化合物 2 (15.0 mg)。組分Fr. 5 (1.8 g) 反復經正相柱、Sephadex LH-20 (氯仿∶甲醇 = 1∶1)及使用半制備柱:Waters 公司產品XterraTMRP-C187 μm 7.8×300 mm (檢測波長為210 nm) 經甲醇∶水 (75%∶25%) (水和甲醇均加0.3%的甲酸) 洗脫30 min,分離得到化合物 3 (6.0 mg)。組分Fr. 6 (2.5 g) 反復經200 ~ 300目硅膠,以氯仿-甲醇 (100∶0→6∶4) 梯度洗脫,再經Sephadex LH-20 (氯仿∶甲醇=1∶1)及Sephadex LH-20 (丙酮) 凝膠柱,最后使用半制備柱 (檢測波長為210 nm) 經甲醇∶水 (70%∶30%) (水和甲醇均加0.3%的甲酸) 洗脫50 min,分離得到化合物 4 (2.4 mg)。組分Fr. 7 (16.0 g) 反復經200 ~ 300目硅膠色譜柱,以氯仿-甲醇 (100∶0→0∶100) 梯度洗脫,再經Sephadex LH-20 (氯仿∶甲醇 = 1∶1) 及Sephadex LH-20 (丙酮) 凝膠柱得到化合物 5 (6.5 mg)。Fr. 7.5.3 (1.0 g) 經正相硅膠色譜柱及Sephadex LH-20 (丙酮) 得到化合物 6 (32.1 mg)。Fr. 7.5.5 (237.9 mg) 使用半制備柱 (檢測波長為210 nm) 經甲醇∶水 (65%∶35%) (水和甲醇均加0.3%的甲酸) 洗脫40 min分離得到化合物7 (6.7 mg)。組分Fr. 8 (1.1 g) 經反復GF254硅膠柱,以氯仿-甲醇 (100∶6→0∶100) 梯度洗脫,再使用半制備柱 (檢測波長為210 nm) 經甲醇∶水 (65%∶35%) (水和甲醇均加0.3%的甲酸) 洗脫40 min分離得到化合物 8 (30.8 mg)。
2 結果與分析
2.1 內生真菌的鑒定
利用NCBI里的BLAST,將Chaetomium sp. M336的測序結果與GenBank數據庫中的序列進行比對,結果顯示該序列與球毛殼屬 (Chaetomium) 的同源性達99%,因此Chaetomiumsp. M336鑒定為球毛殼屬真菌。
2.2 結構鑒定
化合物1C23H25O5Cl,黃色油狀,ESI-MSm/z: 417 [M + H]+;1H-NMR (acetone-d6, 600 MHz),δH: 8.77 (1H, s, H-1), 6.75 (1H, s, H-10), 6.68 (1H, dd,J=15.6, 8.4 Hz, H-4), 6.42 (1H, d,J=12.0, H-9), 3.46 (1H, m, H-19), 2.33 (1H, m, H-11), 1.70 (1H, s, H-15), 1.49 (1H, m, H-20), 1.39 (3H, m, H-12), 1.10 (1H, d,J= 6.4 Hz, H-22), 0.99 (1H, d,J= 6.8 Hz, H-14), 0.91 (3H, q,J= 6.8 Hz, H-13), 0.75 (1H, t,J= 6.0, H-21);13C-NMR (CDCl3, 150 MHz),δC: 201.1 (s,C-18), 183.8 (s, C-6), 168.6 (s, C-16), 164.8 (s, C-8), 158.0 (s, C-3), 152.4 (d, C-1), 147.8 (d, C-10), 140.6 (s, C-4a), 124.6 (s, C-17), 121.2 (d, C-9), 111.2 (s, C-8a), 109.2 (s, C-5), 110.4 (s,C-1), 106.0 (d, C-4), 88.2 (s, C-7), 45.6 (d, C-19), 39.6 (d, C-11), 29.8 (t, C-12), 26.0 (t, C-20), 25.9 (q, C-15), 19.6 (q, C-14), 14.8 (q, C-22), 11.9 (q, C-13), 11.6 (q, C-21)。上述數據與Phonkerd et al (2008) 報道基本一致,故鑒定該化合物為chaetoviridines F。
化合物2C23H23O5Cl,黃色油狀,ESI-MSm/z: 415 [M + H]+;1H-NMR (CDCl3, 600 MHz),δH: 8.04 (1H, s, H-1), 6.56 (1H, q,J= 7.6 Hz, H-20), 6.55 (1H, dd,J=15.6, 8.0 Hz, H-4), 6.53 (1H, s, H-10), 6.07 (1H, d,J= 15.6 Hz, H-9), 2.28 (1H, m, H-11), 1.90 (1H, d,J= 7.1 Hz, C-21), 1.86 (1H, s, H-22), 1.71 (1H, s, H-15), 1.43 (1H, q,J= 7.3 Hz, H-12), 1.09 (1H, d,J= 6.7 Hz, H-14), 0.89 (1H, t,J= 7.4);13C-NMR (CDCl3, 150 MHz),δC: 190.3 (s, C-18), 183.7 (s, C-6), 167.4 (s, C-16), 159.2 (s, C-8), 157.2 (s, C-3), 148.3 (d, C-1), 147.9 (d, C-10), 146.8 (d, C-20), 139.4 (s, C-4a), 137.7 (s, C-19), 126.1 (s, C-17), 119.8 (d, C-9), 110.8 (s, C-8a), 109.5 (s, C-5), 105.1 (d, C-4 ), 87.6 (s, C-7), 38.9 (d, C-11), 29.1 (t, C-12), 25.6 (q, C-15), 19.3 (q, C-14), 15.4 (q, C-21), 11.7 (q, C-13), 10.8 (q, C-22)。上述數據與Phonkerd et al(2008) 報道基本一致,故鑒定該化合物為chaetoviridines E。
化合物3C23H25O6Cl,黃色油狀,ESI-MSm/z: 433 [M + H]+;1H-NMR (acetone-d6, 600 MHz),δH: 8.66 (1H, s, H-1), 6.73 (s, H-4), 6.80 (1H, dd,J=15.6, 7.8 Hz, H-10), 6.42 (1H, d,J= 9.6 Hz, H-9), 3.73 (1H, m, H-5′), 3.56 (1H, m, H-4′), 2.33 (1H, m, C-11), 1.69 (3H, s, H-7), 1.47 (2H, m, H-12), 1.10 (3H, d,J= 6.7 Hz, H-6′), 1.08 (3H, d,J= 6.8 Hz, H-11), 1.07 (3H, d,J= 6.8 Hz, H-4′), 0.90 (3H, t,J= 7.1 Hz, H- 13);13C-NMR (acetone-d6, 150 MHz),δC: 206.1 (s, C-3′), 183.9 (s, C-6), 168.8 (s, C-1′), 161.6 (s, C-8), 158.1 (s, C-3), 151.6 (d, C-1), 147.8 (d, C-10), 147.8 (d, C-10), 140.7 (s, C-4a), 127.2 (s, C-2′), 121.2 (d, C-9), 111.4 (s, C-8a), 109.0 (s, C-5), 105.9 (d, C-4), 88.2 (s, C-7), 71.2 (d, C-5′), 51.8 (d, C-4′ ), 39.6 (d, C-11), 29.7 (t, C-12), 25.8 (q, 7-CH3), 21.7 (q, C-6′), 19.6 (q, C-11), 13.1 (q, C-4′), 11.9 (q, C-13)。上述數據與Borges et al(2011) 報道基本一致,故鑒定該化合物為5′-epichaetoviridin A。
化合物4C23H25O6Cl,黃色油狀,ESI-MSm/z: 433 [M + H]+;1H-NMR (acetone-d6, 600 MHz),δH: 8.66 (1H, s, H-1), 6.73 (1H,s, H-4), 6.80 (1H, dd,J=15.6 , 7.8 Hz, H-10), 6.42 (1H, d,J= 9.6 Hz, H-9), 3.73 (1H, m, H-5′), 3.56 (1H, m, H-4′), 2.33 (1H, m, C-11), 1.69 (3H, s, 7-CH3), 1.47 (2H, m, H-12), 1.10 (3H, d,J= 6.7 Hz, H-6′), 1.08 (3H, d,J= 6.8 Hz, 11-CH3), 1.07 (3H, d,J= 6.8 Hz, 4′-CH3), 0.90 (3H, t,J= 7.1 Hz, H-13);13C-NMR (acetone-d6, 150 MHz),δC: 206.1 (s, C-3′), 183.9 (s, C-6), 168.8 (s, C-1′), 161.6 (s, C-8), 158.1 (s, C-3), 151.6 (d, C-1), 147.8 (d, C-10), 147.8 (d, C-10), 140.7 (s, C-4a), 127.2 (s, C-2′), 121.2 (d, C-9), 111.4 (s, C-8a), 109.0 (s, C-5), 105.9 (d, C-4), 88.2 (s, C-7), 71.2 (d, C-5′), 51.8 (d, C-4′), 39.6 (d, C-11), 29.7 (t, C-12), 25.8 (q, 7-CH3), 21.7 (q, C-6′), 19.6 (q, 11-CH3), 13.1 (q, 4′-CH3), 11.9 (q, C-13)。上述數據與Borges et al(2011) 報道基本一致,,故鑒定該化合物為5′-epichaetoviridin A。
化合物5C23H25O6Cl,黃色油狀,ESI-MSm/z: 573 [M + H]+;1H-NMR (CDCl3, 600 MHz),δH: 14.82 (1H, s, OH-8′), 13.94 (1H, s, OH-6), 11.96 (1H, s, OH-10), 11.71 (1H, s, OH-6′), 7.57 (1H, s, H-3′), 7.08 (1H, s, H-5′), 6.67 (1H, d,J= 8.3 Hz, H-13′), 6.48 (1H, t,J= 6.9 Hz, H-12′), 6.08 (1H, s, H-13), 4.79 (1H, d,J= 6.4 Hz, H-11′), 4.27 (1H, s, H-3), 3.69 (1H, s, H-16), 3.04 (1H, d,J= 17.7 Hz, H-15′), 2.88 (1H, d,J= 17.7 Hz, H-15′), 2.81 (1H, ddd,J= 18.7, 11.1, 7.1 Hz, H-5), 2.58 (1H, s, OH-3), 2.45 (3H, s, H-16′), 2.38 (1H, dd,J= 19.3, 6.5 Hz, H-5), 2.12 (1H, m, H-4), 1.92 (1H, m, H-4);13C-NMR (CDCl3, 150 MHz),δC: 195.6 (s, C-1′), 189.0 (s, C-10′), 186.7 (s, C-8′), 182.8 (s, C-8′), 179.8 (s, C-6), 171.0 (s, C-15), 161.4 (s, C-6′), 158.7 (s, C-10), 156.1 (s, C-14), 147.6 (s, C-4′), 146.5 (s, C-12), 132.7 (d, C-13′), 132.2 (s, C-2′), 131.4 (d, C-12′), 124.3 (d, C-5′), 121.0 (d, C-3′), 116.5 (s, C-11), 115.1 (s, C-7′), 110.4 (d, C-13), 106.5 (s, C-9′), 105.1(s,C-9), 100.1 (s, C-7), 83.9 (s, C-2), 66.8 (d, C-3), 53.5 (q, C-16), 37.8 (d, C-11′), 24.4 (t, C-5), 23.0 (t, C-4)。上述數據與Tabata et al(1993) 報道基本一致,故鑒定該化合物為xanthoquinodins A l。
化合物6C31H24O11,黃色油狀,ESI-MSm/z: 573 [M + H]+;1H-NMR (CDCl3, 600 MHz),δH: 14.93 (1H, s, OH-8′), 13.95 (1H, s, OH-6), 11.52 (1H, s, OH-6′), 11.09 (1H, s, OH-10), 7.57 (1H, s, H-3′), 7.08 (1H, s, H-5′), 6.69 (1H, d,J=7.6 Hz, H-13′), 6.54 (1H, t,J= 8.4 Hz, H-12′), 4.85 (1H, d,J= 6.0 Hz, H-11′), 4.46 (1H, d,J= 10.6 Hz, H-3), 3.68 (3H, S, H-16), 3.02 (1H, d,J= 16.7 Hz, H-15), 2.96 (1H, d,J= 16.7 Hz, H-15), 2.70 (1H, d,J= 7.8 Hz, H-5), 2.45 (3H, s, H-16′), 2.36 (1H, m, H-5), 2.18 (1H, m, H-4), 2.07 (1H, m, H-4);13C-NMR (CDCl3, 150 MHz),δC: 195.8 (s, C-1′), 190.1 (s, C-10′), 186.9 (s, C-8), 181.8 (s, C-8′), 179.0 (s, C-6), 170.1 (s, C-15), 161.4 (s, C-6′), 160.4 (s, C-10), 155.2 (s, C-14), 147.9 (s, C-4′), 147.0 (d, C-12), 132.5 (s, C-2′), 132.4 (d, C-13′), 131.8 (d, C-12′), 124.6 (d, C-5′), 121.4 (d, C-3′), 115.0 (s, C-7′), 114.2 (s, C-13), 113.9 (d, C-11 ), 107.1 (s, C-9′), 101.8 (s, C-7), 85.7 (s, C-2), 71.9 (d, C-3), 53.6 (q, C-16), 39.5 (t, C-11′), 27.9 (t, C-5), 24.1 (t,C-4)。上述數據與Tabata et al(1993) 報道基本一致,故鑒定該化合物為xanthoquinodins B1。
化合物7 C31H24O11,黃色油狀,ESI-MSm/z: 571 [M - H]+;1H-NMR (CDCl3, 600 MHz),δH: 14.84 (1H, s, OH-8′), 13.83 (1H, s, OH-6), 11.82 (1H, s, OH-10), 11.72 (1H, s, OH-6′), 7.57 (1H, s, H-3′), 7.09 (1H, s, H-5′), 6.66 (1H, d,J= 8.2 Hz, H-13′), 6.49 (1H, dd,J= 8.3, 6.8 Hz , H-12′), 4.77 (1H, d,J= 6.6 Hz, H-11′), 4.25 (1H, dd,J= 12.4, 5.0 Hz, H-4), 3.69 (3H, s, H-16), 3.02 (1H, d,J= 18.0 Hz, H-15′), 2.94 (1H, d,J= 18.0 Hz, H-15′), 2.66 (2H, m, H-5), 2.45 (3H, s, H-15′), 2.14 (1H, m, H-4), 2.07 (1H, m, H-4);13C-NMR (CDCl3, 150 MHz),δC: 194.5 (s, 2C, C-6,C-6′), 192.9 (s, 2C, C-8,C-8′), 172.0 (s, 2C, C-15,C-15′), 156.3 (d, 2C, C-1,C-1′), 144.9 (s, 2C, C-4a,C-4a′), 116.7 (s, 2C, C-5,C-5′), 110.7 (s, 2C, C-8,C-8a′), 108.7 (s, 2C, C-3,C-3′), 86.0 (s, 2C, C-7, C-7′), 67.9 (d, 2C, C-4, C-4′), 39.0 (d, 2C, C-11, C-11′), 34.0 (d, 2C, C-9, C-9′), 23.7 (q, 2C, C-14, C-14′), 23.5 (q, 2C, C-12, C-12′), 22.7 (q, 2C, C-16, C-16′), 20.2 (q, 2C, C-10, C-10′)。上述數據與Tabata et al(1993) 報道基本一致,故鑒定該化合物為xanthoquinodins A2。
化合物8C31H30O6N6S4,黃色油狀,ESI-MSm/z: 711 [M + H]+;1H-NMR (CDCl3, 600 MHz),δH: 7.66 (1H, d,J= 6.8 Hz, H-14′), 7.34 (1H, d,J= 7.6 Hz, H-7), 7.30 (1H, t,J= 7.6 Hz, H-8), 7.30 (1H, m, H-11′), 7.22 (1H, m, H-12′), 7.22 (1H, m, H-13′), 7.19 (1H, s, H-9′), 6.94 (1H, t,J= 7.4 Hz , H-9), 6.80 (1H, d,J= 7.9 Hz, H-10), 6.21 (1H, s, H-5), 5.39 (1H, s, H-6), 4.42 (1H, d,J= 15.4 Hz, H-11), 4.36 (2H, d,J= 12.2Hz, H-3), 4.33 (2H, d,J= 12.2 Hz, H-3′), 4.29 (1H, d,J= 12.2 Hz, 3-CH2OH), 4.27 (1H, d,J= 12.2 Hz, 3′-CH2OH), 3.88 (1H, d,J= 15.4 Hz, H-7′), 3.71 (1H, d,J= 15.4 Hz, H-7′), 3.19 (3H, s, 2-CH3), 3.16 (3H, s, 2′-CH3), 3.10 (1H, d,J= 15.4 Hz, H-11), 2.96 (3H, s, 5′-CH3);13C-NMR (CDCl3, 150 MHz),δC: 166.8 (s, C-4′), 165.52 (s, C-1), 165.51 (s, C-1′), 163.2 (s, C-4), 148.3 (s, C-6a), 134.0 (s, C-10a′), 131.4 (d, C-8), 130.4 (s, C-14a′), 127.2 (d, C-9′), 126.5 (s, C-10a), 125.0 (d, C-7), 122.8 (d, C-13′), 120.6 (d, C-12′), 120.3 (d, C-9), 119.1 (d, C-14′), 111.4 (d, C-11′), 111.1 (d, C-10), 107.6 (s, C-8′), 80.1 (d, C-5), 76.5 (s, C-6′), 76.1 (s, C-3′), 74.8 (s, C-3), 73.7 (s, C-10b), 73.5 (s, C-11a), 61.2 (t, 3′-CH2OH), 60.5 (t, 3-CH2OH), 42.6 (t, C-11), 28.2 (q, 5′-CH3), 27.5 (q, 2-CH3), 27.4 (q, 2′-CH3), 27.1 (t, C-7′)。上述數據與Fujimoto et al(2004) 報道基本一致,故鑒定該化合物為chetomin。
3 討論與結論
由于植物內生真菌次生代謝產物具有豐富的結構及其廣泛的藥理活性和生物學功能,植物內生真菌代謝產物已經成為眾多學者的研究熱點 (Tan & Zhou, 2001)。近年來,植物內生真菌的次級代謝產物被認為是藥理活性分子的重要來源,隨著眾多研究人員對植物內生真菌進行藥物開發,從植物內生真菌的次級代謝產物中發現了大量具有顯著生物活性的化合物 (Leslie, 2006)。由于來自于植物各個組織的內生真菌種類繁多,所以內生真菌的研究有待于進一步的挖掘。
本研究從蛇足石衫內生真菌Chaetomiumsp. M336中分離純化并鑒定出8個化合物,依次為chaetoviridines F (1),chaetoviridines E (2),5′-epichaetoviridin A (3),5′-epichaetoviridin A (4),xanthoquinodins Al (5),xanthoquinodins A2 (6),xanthoquinodins B1 (7),毛殼菌素 (8)。McMullin et al(2013) 發現化合物3具有一定的抗惡臭假單胞菌和枯草芽孢桿菌活性,其他化合物根據相關文獻報道均具有一定的細胞毒活性。另外,化合物1、2、4、5、6、7、8具有細胞毒活性,表明它們可能是蛇足石衫內生真菌Chaetomiumsp. M336表現細胞毒的活性物質。本研究豐富了蛇足石杉內生真菌球毛殼屬 (Chaetomiumsp.) 的藥用價值,并對探索具有細胞毒活性化合物奠定了基礎。
BORGES WS,PUPO MT, MANCILLA G, et al, 2011. Azaphilones from the endophyteChaetomiumglobosum[J]. J Nat Prod, 74: 1 182-1 187.
CHENG DH, DAI KM, 1992. Pharmacognosy identification ofHuperziaserrata[J]. Primary J Chin Trad Med, 6(4): 7-9. [程丹華, 戴克敏, 1992. 千層塔的生藥學鑒定 [J]. 基層中藥雜志, 6(4): 7-9.]
CHEN GD,YAO XS, CHEN Y, et al, 2013. Xanthoquinodins from the endolichenic fungal strainchaetomiumelatum[J]. J Nat Prod, 76: 702-709.
COOK KM,HILTON ST, MECINOVIC J, et al, 2009. Epidithiodiketopiperazines block the interaction between hypoxia-inducible Factor-1 (HIF-1) and p300 by a zinc ejection mechanism [J]. J Biol Chem, 284 (39): 26 831-26 838.
FUJIMOTO H, SUMINO M, OKUYAMA E, et al, 2004. Immunomodulatory constituents from an ascomycete,Chaetomiumseminudum[J]. J Nat Prod, 67: 98-102.
GU S, SHAO H, JIANG XH,et al, 2001. Potential applications of medicinal plants endophytic fungal diversity and its active ingredient [J]. Chin Pharm, 36 (1): 14-15. [谷蘇, 邵華, 蔣曉華, 等, 2001. 藥用植物內生真菌多樣性及其活性成分的潛在應用價值 [J]. 中國藥學雜志, 36 (1): 14-15.]
KUNGAL, ZABLUDOFF SD, FANCE DS, et al, 2004. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway [J]. Canc Cell, 6: 33-43.
LI X, QIN JC, TIAN Y, et al, 2013. Cytotoxic azaphilone alkaloids fromChaetomiumglobosumTY1 [J]. Bioorg Med Chem Lett, 23: 2 945-29 47.
LESLIE GUNATILAKA AA, 2006. Natural products from plant- associated microorganisms: distribution, structural diversity , bioactivity, and implications of their occurrence [J]. J Nat Prod, 69: 509-526.
MCMULLIN DR, MILLERA JD, SUMARAH MW, et al,2013. New azaphilones fromChaetomiumglobosumisolatedfrom the built environment [J]. Tetrahedron Lett, 54: 568-572.
PETRINI O,1991. Microbial ecology of leaves, Springer-Verlag New York: 179-197.
PHONKERDA N, KANOKMEDHAKULA S, KANOKMEDHAKUL K, et al, 2008. Bis-spiro-azaphilones and azaphilones from the fungiChaetomiumcochliodesVTh01 andC.cochliodesCTh05 [J]. Tetrahedron, 64: 9 636-9 645.
STAAB A, LOEFFLER J, SAID HM, et al, 2007. Effects of HIF-1 inhibition by chetomin on hypoxia-related transcription and radiosensitivity in HT 1080 human fibrosarcoma cells [J] . BMC Cancer, 7: 213-220.
TAN N, LIN YC, SHAO CL, et al, 2009. Identification and bioassay of three anthraquinone secondary metabolites of mangrove endophytic fungus #2240 from south China sea [J]. Chin J Appl Chem, 26 (3): 277-281. [譚倪, 林永成, 邵長倫, 等, 2009. 南海紅樹林內源真菌#2240的3個蒽醌類次級代謝產物的鑒別和藥理活性 [J]. 應用化學, 26 (3): 277-281.]
TAN RX, ZHOU WX, 2001. Endophytes: a rich source of functional metabolites [J]. Nat Prod. Rep, 18: 448-459 .
TABATA N, OMURA S, TOMODA H, et al, 1993. Structure and biosynthesis of xanthoquinodins, anticoccidial antibiotics [J]. J Am Chem Soc, 115: 8 558-8 564.
YU HY, SUN MY, YANG YJ, 2001. Advance in studies onHuperziaserrata[J]. Chin Trad Herb Drugs, 32 (3): 279-281. [余紅英, 孫遠明, 楊躍進, 2001. 草藥蛇足石杉的研究進展 [J]. 中草藥, 32 (3): 279-281.]
ZHANG JC, WANG ZM, ZHANG HY, et al, 2010. The summarize about recent research process on fern endophyte [J]. Chin Agric Sci Bull, 26 (20): 70-72. [張君誠, 王錚敏, 張杭穎,等, 2010. 蕨類植物內生菌研究進展 [J]. 中國農學通報,26 (20): 70-72.]ZOU WX, TAN RX, 2001. Recent advances on endophyte research [J]. Chin Bull Bot, 43 (9): 881-892. [鄒文欣, 譚仁祥, 等, 2001. 植物內生菌研究新進展 [J]. 植物學報, 43 (9): 881-892.]
Isolation and identification of secondary metabolites from a new endophyteHuperziaserrata
YU Fei-Xue1,2, YANG Yin-He2, ZHAO Pei-Ji2, CHEN Yao1*
( 1.KunmingUniversityofScienceandTechnology, Kunming 650500, China; 2.StateKeyLaboratoryofPhytochemistryandPlantResoursesinWestChina,KunmingInstituteofBotany,ChineseAcademyofScience, Kunming 650201, China )
Many kinds of endophytic fungi are widely associated with various plants and a lot of systemic researches focusing on secondary metabolites of endophytes had been conducted. However, the research on endophyte inHuperziaserratais rare, which is a traditional Chinese medicinal fernQianCengTa, belongs toHuperziaof Huperziaceae. Finding active compounds from endophytes inH.serratacan provide a new way for further exploitation resources of this medicinal plant. The purpose of this study was to search cytotoxic active compounds of endophytes fromH.serrata. We isolated the chemical constitution ofChaetomiumsp. M336 which was an endophytic fungus isolated in the roots ofHuperziaserrata. TheChaetomiumsp. M336 was fermented on solid PDA media. The fungus containing with medium was extracted with ethyl acetate∶methanol∶acetic acid = (80∶15∶5, V/V/V) mixed organic solvent, and the secondary metabolites of endophytic fungi were obtained. The compounds of the fermented extracts were separated and purified by column chromatography involving normal-phase silica gel, Sephadex LH-20 and reversed-phase semi-preparative HPLC. Their structure of the compounds were elucidated by their physical and chemical properties, including nuclear magnetic resonance (NMR) spectroscopy, and electrospray ionization mass spectrometry (ESI-MS) and according to the reported literatures. They results were as follows: eight compounds were isolated and purified from the ethyl acetate extracts of the fungusChaetomiumsp. M336. They were identified as chaetoviridines F, chaetoviridines E, 5′-epichaetoviridin A, 5′-epichaetoviridin A, xanthoquinodins Al, xanthoquinodins A2, xanthoquinodins B1, chetomin. In conclusion, eight compounds were isolated from M336. Compound 3 showed some certain anti-bacterial activity, others exhibit cytotoxicity. This preliminary work will provide information for further search on natural cytotoxic activity products of endophytic fungi fromHuperziaserrata.
Chaetomiumsp., endophytic fungi, structure identification, cytotoxicity
10.11931/guihaia.gxzw201509025
2015-09-28
2015-01-25
國家重點基礎研究發展“973”計劃項目 (2013CB127505) [Supported by the National Basic Research Program of China “973” Program, (2013CB127505)]。
于飛雪 (1987-),女,吉林長春人,碩士研究生,研究方向為天然藥物化學,(E-mail)15025152330@139.com。
陳瑤,教授,研究方向為藥物基因組學,(E-mail)Chyhdd@sina.com。
Q946.91
A
1000-3142(2016)09-1112-07
于飛雪, 楊銀河, 趙沛基, 等. 蛇足石杉中的新內生真菌及其代謝產物的分離與鑒定 [J]. 廣西植物, 2016, 36(9):1112-1118
YU FX, YANG YH, ZHAO PJ, et al. Isolation and identification of secondary metabolites from a new endophyteHuperziaserrata[J]. Guihaia, 2016, 36(9):1112-1118

