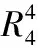Structural investigation, Hirshfeld surface analysis and quantum mechanical study of Piperazinium bis(4-chlorophenoxyacetate)
SU Ping, MENG Xianggao, XU Xingman
(College of Chemistry, Central China Normal University, Wuhan 430079)
?
Structural investigation, Hirshfeld surface analysis and quantum mechanical study of Piperazinium bis(4-chlorophenoxyacetate)
SU Ping, MENG Xianggao, XU Xingman*
(College of Chemistry, Central China Normal University, Wuhan 430079)

piperazine; 4-chlorophenoxyacetic acid; crystal structure; Hirshfeld surface analysis; DFT
1 Introduction
In recent years, a bundle of interests have been focused on the design of supra-molecular assemblies by controlling the formation of non-covalent interactions such as hydrogen bonds, electrostatic, C-H…π, and π…π[1-4]. From a medical view, co-crystals or molecular adducts are defined as a crystallized compound formed by more than one active pharmaceutical ingredient (API) by a specific stoichiometric ratio at a certain condition[5-9], in which non-covalent intermolecular interactions are the main driving forces[10-11]. A well designed pharmaceutical co-crystal or molecular adduct should be facilitating APIs’ chemical or physical properties such as hygroscopy, dissolution rate, saturability, and taste sense. Piperazine has been often used as a chemical agent for the control of nematode infestations, but their mode of action is still not clear[12]. 4-Chlorophenoxyacetic acid and its derivatives are selective systemic herbicide for the control of annual and perennial broad-leaved weeds. Its salts are readily absorbed by plant roots, and are translocated to the meristematic tissues of the roots and shoots[13]. In order to design some new types of multi-functional plant anti-stress agents, the titled organic salt, (C4H12N2)·(C8H6ClO3)2(I), was prepared by using 4-chlorophenoxyacetic acid and piperazine. In this work, its structural characteristic, Hirshfeld surface analysis and DFT calculation of frontier orbits were reported and discussed.
2 Experimental
2.1Materials and measurements
All chemicals were of reagent grade as received from commercial sources and used without further purification. C, H, N elemental analyses were performed on an Elementar Vario MICRO E III analyzer. IR spectra were recorded as KBr pellets on a Perkin Elmer spectrometer (4 000~400 cm-1). Quantum chemical calculation was performed from the crystal data with RB3LYP method at the 6-31G(d) level, and all the computations have been performed using Gaussian 03 program[14]on a Pentium IV computer.
2.2Synthesis
10.0 mL water solution of piperzaine (17.2 mg;0.2 mmol) was slowly added to 5.0 mL ethanol solution of 4-chlorophenoxyacetic acid (74.6 mg,0.4 mmol) and the resulting solution was stirred 30 minutes under 70°C. The final solution was filtered and the filtrate was kept at ambient temperature for one week. Colorless rod-like crystals suitable for X-ray diffraction were obtained. The products were filtered and washed three times with ca. 5.0 mL ethanol and ether, dried under infrared lamp. Yield: 72.0 mg (78.4%). Elemental analysis of C20H24Cl2N2O6: Calcd(%): C 52.30, H 5.27, N 6.10. Found (%): C 52.18, H 5.41, N 5.97.
2.3X-ray crystallography


Tab.1 Selected crystallographic data of compound (I)
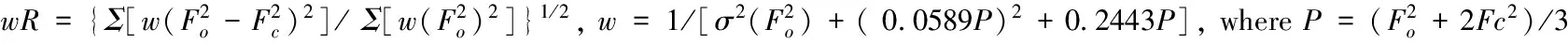

Tab.2 Selected bond distances (?) for compound (I)
Symmetry code: #1=2 -x,-y,-z.
3 Results and discussion
3.1Crystal structure description

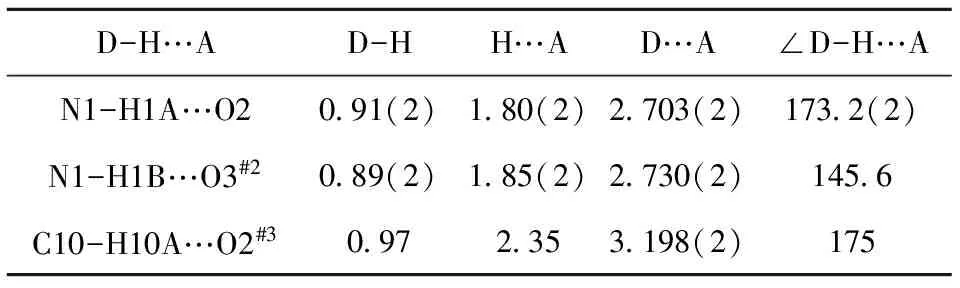
Tab.3 Hydrogen bond parameters in compound (I)(?,°)
Symmetry codes: #2=1+x,y,z; #3=2 -x,y,z.

Fig.1 ORTEP molecular structure of the titled compound shown as 30% thermal ellipsoid probability (symmetry code:#1=2-x,y,-z). N—H…O hydrogen bond are shown as green dashed lines
3.2Infrared spectrum
In comparison with free 4-chlorophenoxyacetic acid, there are some apparent differences between it and compound (I) (Figure 3). For instance, the strong C=O stretching vibration (1 735 cm-1) in free acid may indicate a formation of hydrogen-bonded dimer in contrast with a carboxylic monomer (1 750~1 710 cm-1). However, the C=O stretching vibration in compound (I) is significantly shifted to a low frequency at 1 593 cm-1. This is ascribed to the formation of two strong N-H…O hydrogen bonds around carboxylate O1 and O2 atom in compound (I), which weakening the energy of C=O stretching vibration. The vibration energy of C=O double bond is also delocalized to the C-O single bond, further resulting in its low-frequency shift. In a meanwhile, the other vibration frequencies of C—O, C—H, C=C, C—H bonds etc. in compound (I) also undergo a different degrees of low-frequency shift (Figure 3). these are caused by a formation of the hydrogen-bonded molecular adduct complex of compound(I).
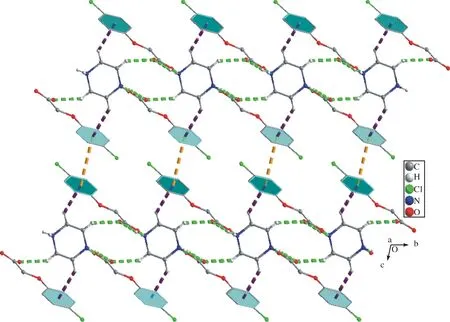
Fig.2 Part of the crystal packing in compound (I) with N-H…O/C-H…O hydrogen bonds, C-H…π and π…π shown as green, purple and yellow dashed lines, respectively
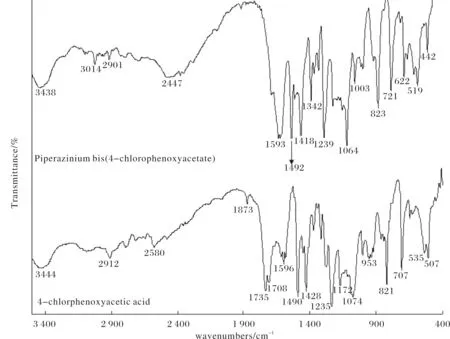
Fig.3 The Infrared spectrum of free 4-chlorophenoxy acetic acid (below) and compound (I) (above)
3.3Hirshfeld Surface
The Hirshfeld surfaces and fingerprint plots are able to be utilized to identify a type and region of intermolecular interactions, which are capable of being generated using Crystal-Explorer software[19]. Molecular Hirshfeld surface in a crystal structure is constructed based on the electron distribution. Its normalized contact distance (dnorm) based on bothde,diand van der Waals radii of a atom is listed in the following equation. Then, intermolecular contacts in the crystal are able to be analyzed by a combination ofdeanddiin the form of a 2D fingerprint plot as listed in the below equation[20].
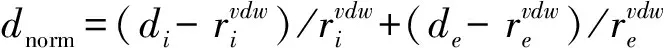
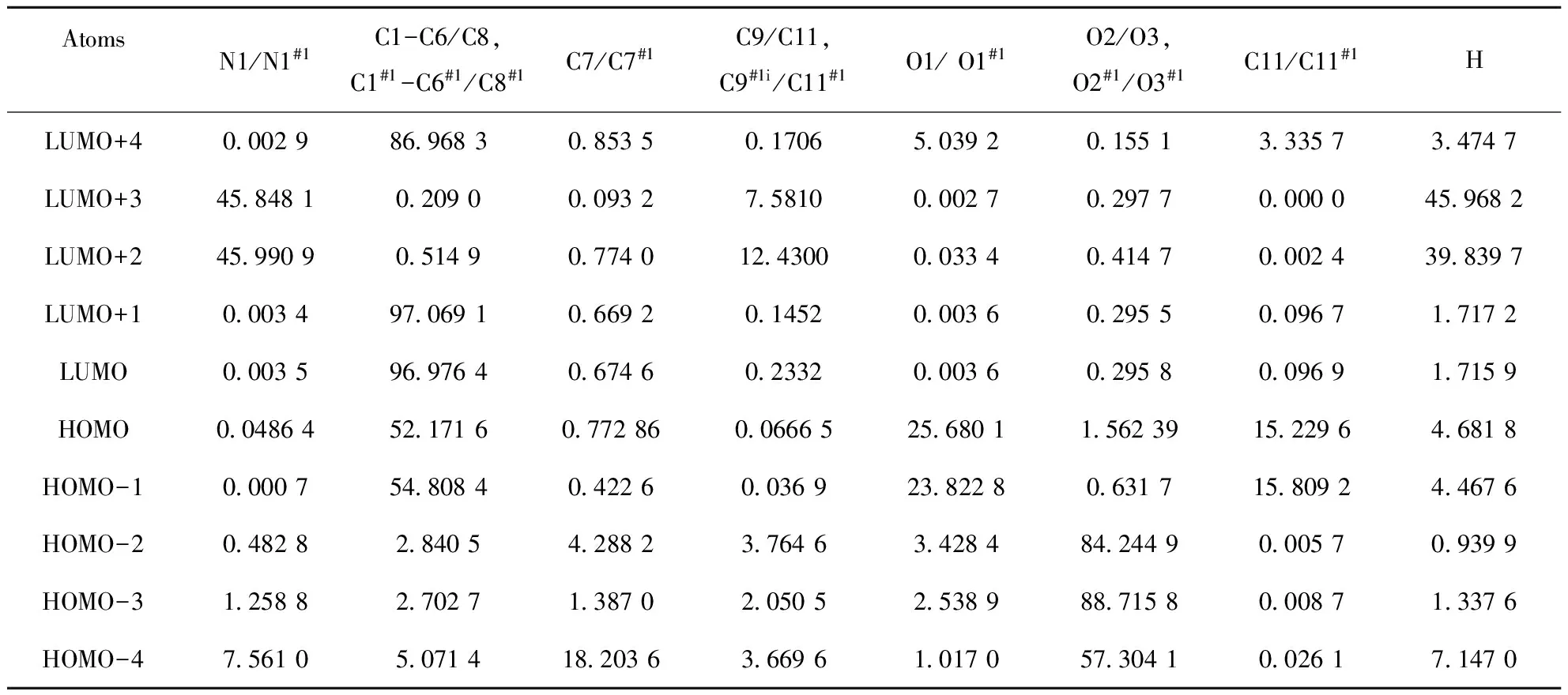
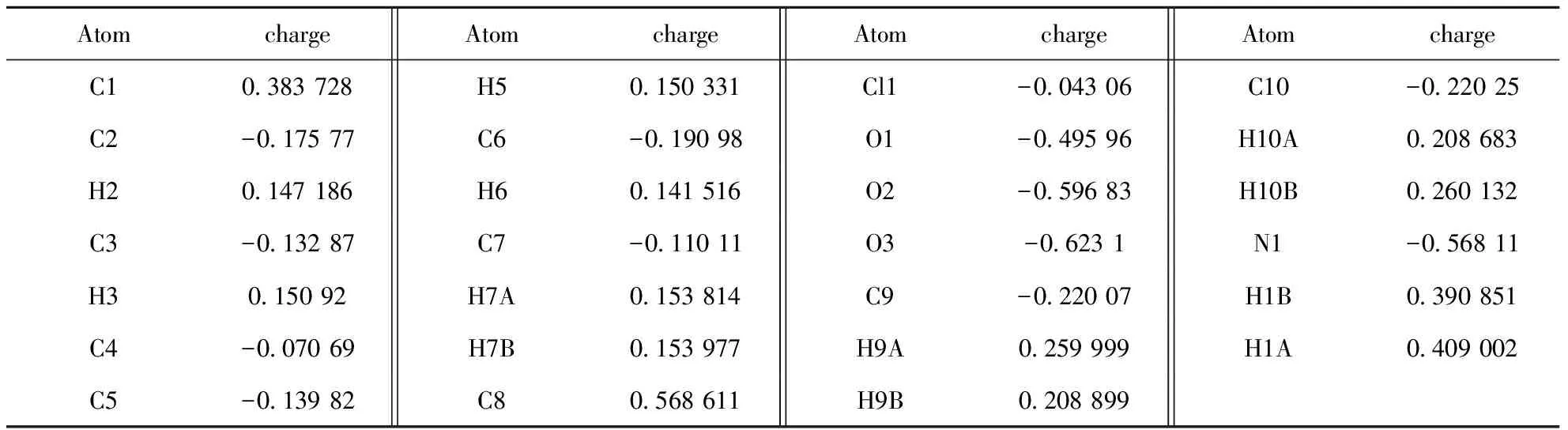
The Hirshfeld surfaces of compound (I) were mapped over dnorm(Figure 4a) and shape index (Figure 4b). The intermolecular interactions between piperazinium nitrogen atom and carboxylate group (Table 2) were shown in the Hirshfeld surface as the bright red areas in Figure 4a due to the N-H…O hydrogen bond, and the light red spots are corresponding to C-H…O and C-H…π interactions. The O…H/H…O intermolecular interactions appear as distinct spikes in the 2D fingerprint plot (Figure 4d). Complementary regions are visible in the fingerprint plots where one molecule acts as a donor (de>di) and the other as an acceptor (de Fig.4 Hirshfeld surfaces mapped with (a) dnorm; (b) Shape index, (c) full fingerprint plot; (d) H…O contacts (N-H…O and C-H…O); (e) H…C contacts (C-H…π) and (f) C…C contacts (π…π) 3.4Quantum mechanical study 3.4.1Stability The optimization of compound (I) within the Cipoint group is carried out with N1-H1A distance being fixed. The final outcome gives a better convergence criterion and no imaginary frequency has been observed. The total energy for compound (I) is -2257.75486797 a.u. and the energies of HOMO and LUMO orbits are -0.20945 and -0.00346 a.u., respectively. The HOMO-LUMO gap is 0.20599 a.u., which means compound (I) has a better stability. 3.4.2Frontier molecular orbital composition In order to ascertain the structure and bond characteristics in (I), these highest occupied and lowest unoccupied frontier molecular orbital populations were analyzed by us. The atomic orbital composition from different type of atoms in the frontier molecular orbits were expressed as the atomic orbital coefficient square sum in the type of atomic orbits and corrected by normalizing the specific molecule orbital[22]. These atoms in (I) are classified into eight groups according to their hybridized type and coordination environment: 1) nitrogen N1 from piperazium cation; 2) aromatic carbon C1-C6; 3) methylene carbon C7; 4) methylene carbon C9 and C10; 5) phenolic oxygen O1; 6) carboxylate O2/O3; 7) chloride C11; 8) all hydrogen atoms. According to an orbital contribution analysis (Table 4), it is suggested that the compositions of HOMO come mainly from orbits of symmetry-related C1-C6 atoms of two benzene ring atoms (52.17%) of 4-chlorophenoxycarboxylate anion, phenolic oxygen O1 atom (25.68%) and chloride atom C11 (15.23%) and some hydrogen atoms (4.68%). In contrast, the components of LUMO come overwhelmingly from the orbits of phenyl carbon atoms C1-C6 (96.98%), 1.72% from hydrogen atoms, and only a little (<1.0%) from the other atoms. These results here demonstrate that the electrons are mainly delocalized into two benzene rings and compound (I) may be involved into some intermolecular interactions (i.e., C-H…π and π…π) via benzene ring. Additionally, the 4-piperazium cation is easily to form hydrogen bonds with an electron donor via hydrogen H1A and H1B atoms as they possess the highest positive charge (Table 5). This is also verified by the experimental crystal structure result. Tab.4 Frontier molecular orbital (MO) compositions (%) from different type of atoms in (I) Symmetry code: #1=2-x,y,-z. Tab.5 Mulliken charges in the optimized structure of compound (I) (a.u.) Fig.5 Molecular frontier orbits from HOMO-4 to LUMO+4 in compound (I) In summary, we have reported a new organic salt complex (I), piperazinium bis(4-chlorophenoxyacetate), in which the component ions are linked into a 3D framework by a combination of N-H…O hydrogen bonds, C-H…O, C-H…π and π…π interactions. Hirshfeld surface analysis indicates that N-H…O hydrogen bonds consist of 25.7% of the total surface. Natural Bond Orbital analysis performed by using RB3LYP/6-31G(d) method shows these HOMO and LUMO orbits are mainly distributed over the phenyl rings which can facilitate the formation of C-H… π and π…π interactions. [1] SHERRINGTON D C, TASKINEN K A. Self-assembly in synthetic macromolecular systems multiple hydrogen bonding interactions [J]. Chem Soc Rev, 2001, 30: 83-93. [2] SETH S K, MANNA P, SINGH N J,et al. Molecular architecture using novel types of non-covalent π-interactions involving aromatic neutrals, aromatic cations and π-anions [J]. Cryst Eng Comm, 2013, 15: 1285-1288. [3] SINGH S K, DAS A. The n→π* interaction: a rapidly emerging non-covalent interaction[J]. Phys Chem Chem Phys, 2015, 17: 9596-9612. [4] MAHADEVI A S, SASTRY G N. Cooperativity in Noncovalent Interactions[J]. Chem Rev, 2016, 116: 2775-2825. [5] MCNAMARA D P, CHILDS S L, GIORDANO J. Use of a Glutaric acid cocrystal to improve oral bioavailability of a low solubility API [J]. Pharm Res, 2006, 23: 1888-1897. [6] TRASK A V. An overview of pharmaceutical cocrystals as intellectual property [J]. Mol Pharm, 2007, 4: 301-309. [7] AAKER?Y C B, FASULO M E, DESPER J. Cocrystal or salt: Does it really matter? [J]. Mol Pharm, 2007, 4: 317-322. [8] CAIRA M R. Sulfa drugs as model cocrystal formers [J]. Mol Pharm, 2007, 4: 310-316. [10] AAKEROY C B, FORBES S, DESPER J. Altering physical properties of pharmaceutical co-crystals in a systematic manner [J]. Cryst Eng Comm, 2014, 16: 5870-5877. [11] PERLOVICH G L, MANIN A N. Design of pharmaceutical cocrystals for drug solubility improvement [J]. Russ J Gen Chem, 2014, 84: 407-414. [12] MARTIN R J. Electrophysiological effects of piperazine and diethylcarbamazine on Ascaris suum somatic muscle [J]. British J Pharmacol, 1982, 77: 255-265. [13] TOMLIN C. The Pesticide Manual[M]. 10th edn. London: British Crop Protection Council/Royal Society of Chemistry, 1994. [14] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 03, Revision B.01[P]. Pittsburgh PA : Gaussian, Inc., 2003. [15] SHELDRICK G M. SADABS, Program for crystal data correction[P]. Germany:University of G?tingen, 1996. [16] SHELDRICH G M. SHELXTL-97, Program for crystal structure refinement[P]. Germany: University of G?ttinggen, 1997. [17] SHELDRICK G M. A short history of SHELX [J]. Acta Cryst A,2008, 64:112-122. [18] SPEK A. Single-crystal structure validation with the program PLATON [J]. J Appl Cryst, 2003, 36: 7-13. [19] SPACKMAN M A, MCKINNON J J. Fingerprinting intermolecular interactions in molecular crystals [J]. Cryst Eng Comm,2002, 4: 378-392. [20] SPACKMAN M A, JAYATILAKA D. Hirshfeld surface analysis [J]. Cryst Eng Comm, 2009, 11: 19-32. [21] MCKINNON J J, SPACKMAN M A, MITCHELL A S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals [J]. Acta Cryst B, 2004, 60: 627-668. [22] ZHANG J, YANG B Q, ZHU H Y, et al. Synthesis, characterization, crystal structure and quantum chemical calculation of novel compound 1,3-dimethyl-2-ferrocenylmethylbenzimidazolium iodide [J]. Chin J Chem, 2006, 24: 637-641. 2016-04-22. 中央高校基本科研業務費資助項目(20205010031). 1000-1190(2016)04-0571-08 二(4-氯苯氧乙酸)哌嗪鹽的結構、Hirshfeld表面分析和量化計算研究 蘇 萍, 孟祥高, 徐星滿 (華中師范大學 化學學院, 武漢 430079) 哌嗪; 4-氯苯氧乙酸; 晶體結構; Hirshfeld表面分析; DFT O626.4 A *通訊聯系人. E-mail: xingmanxu@mail.ccnu.edu.cn.

4 Conclusion