谷子轉錄因子SiNF-YA5通過ABA非依賴途徑提高轉基因擬南芥耐鹽性
黃 鎖胡利芹徐東北李微微徐兆師李連城周永斌刁現民賈冠清馬有志陳 明,*
1中國農業科學院作物科學研究所 / 農作物基因資源與基因改良國家重大科學工程 / 農業部麥類生物學與遺傳育種重點實驗室, 北京100081;2西北農林科技大學農學院 / 旱區作物逆境生物學國家重點實驗室, 陜西楊凌 712100;3哈爾濱師范大學生命科學與技術學院 / 黑龍江省分子細胞遺傳與遺傳育種重點實驗室, 黑龍江哈爾濱 150025
谷子轉錄因子SiNF-YA5通過ABA非依賴途徑提高轉基因擬南芥耐鹽性
黃 鎖1,**胡利芹1,**徐東北1,2李微微1,3徐兆師1李連城1周永斌1,2刁現民1賈冠清1馬有志1陳 明1,*
1中國農業科學院作物科學研究所 / 農作物基因資源與基因改良國家重大科學工程 / 農業部麥類生物學與遺傳育種重點實驗室, 北京100081;2西北農林科技大學農學院 / 旱區作物逆境生物學國家重點實驗室, 陜西楊凌 712100;3哈爾濱師范大學生命科學與技術學院 / 黑龍江省分子細胞遺傳與遺傳育種重點實驗室, 黑龍江哈爾濱 150025
核轉錄因子Y (nuclear transcription factor Y, NF-Y)類轉錄因子在植物生長發育和非生物脅迫響應基因表達調控中發揮重要的作用, NF-Y由3種亞基(NF-YA、NF-YB、NF-YC)組成。本研究從抗逆性強的谷子品種龍谷25中克隆1個新的NF-Y類轉錄因子基因SiNF-YA5。該基因序列為924 bp, 編碼307個氨基酸, 分子量為33.76 kD, 等電點為9.19。SiNF-YA5在149~210位氨基酸之間含有CBF保守結構域。亞細胞定位分析表明, SiNF-YA5定位于細胞膜和細胞核。基因功能分析顯示, 在不同濃度高鹽處理下, 和野生型擬南芥(WT)相比SiNF-YA5轉基因擬南芥種子萌發率更高; 苗期SiNF-YA5轉基因擬南芥根表面積和植株鮮重顯著高于WT, 證明過表達SiNF-YA5基因可以顯著提高植物耐鹽性。基因表達分析結果顯示, 在SiNF-YA5轉基因擬南芥中參與鹽脅迫響應的基因NHX1和LEA7的表達量明顯高于WT。另一方面, SiNF-YA5轉基因擬南芥與WT相比對于ABA的敏感性差異不顯著, 以上結果證明SiNF-YA5主要通過ABA非依賴途徑提高轉基因植物對高鹽脅迫的耐性。
谷子; NF-Y類轉錄因子; 高鹽脅迫; ABA非依賴途徑
干旱、鹽堿、低溫等非生物脅迫嚴重影響作物的生長發育及產量[1]。植物在長期的進化過程中逐漸形成一套復雜的逆境應答機制, 以抵御不良環境對植物的損害[2-3]。大量研究證明, 植物細胞在染色體水平、轉錄水平以及轉錄后水平精確調控一系列脅迫應答基因的表達, 其中, 一些功能蛋白包括胚胎發育后期豐富蛋白(LEA蛋白)、滲透調節蛋白、離子區域化和水通道蛋白、脯氨酸及果聚糖合成酶及甜菜堿合成酶等直接發揮功能增強植物細胞抗逆性[4]。另外, 一些調控蛋白包括感應和傳導脅迫信號的蛋白激酶(例如 CDPK, cadium dependent phospholylation kinase; MAPK, mitogen-activated protein kinases)以及參與調控基因表達的轉錄因子(包括Bzip, basic leucine zipper類; NAC, nascent polypeptide-associated complex類; DREB, dehydration responsive element binding protein類; NF-Y, nuclear transcription factor Y類等)在植物脅迫應答過程中也發揮重要的基因表達調控作用[5]。近年來, 研究證明NF-Y類、NAC類、DREB類等轉錄因子參與多種逆境信號轉導途徑[6]。ABA (abscisic acid)是調控植物非生物脅迫響應的主要激素[7]。植物調控非生物脅迫響應的信號途徑主要被分為 ABA依賴性和ABA非依賴性信號途徑[2-3]。DREB1A轉錄因子主要參與 ABA非依賴的逆境信號途徑, 過表達DREB1A提高植物對低溫的抗性[8]; 而 MYBR/C (MYB/C recognition site)和ABRE (ABA responsive element)作為ABA依賴性信號途徑中的一類重要的順式作用元件, 能夠調節干旱響應基因的表達[9],過表達MYC類轉錄因子AtMYC2和MYB類轉錄因子AtMYB2基因可以提高植物對ABA的敏感性和對脫水逆境的耐受性[10]。
植物 NF-Y類轉錄因子是一類重要的逆境調節因子, 由NF-YA、NF-YB和NF-YC亞基組成。在行使功能時, NF-YB亞基和NF-YC亞基先在細胞質中形成異源二聚體, 然后遷移到細胞核中和NF-YA亞基結合形成有活性的異源三聚體[11-13]。NF-Y異源三聚體具有保守的 CCAAT-box位點結合特性, 而CCAAT-box順式調控元件存在于真核生物約25%基因的啟動子區域, 說明 NF-Y轉錄因子在調控真核生物細胞內基因表達方面具有重要作用[14-16]。NF-Y轉錄因子普遍存在于擬南芥[17]、水稻[18]、玉米[19]、大豆[20]、小麥[21-22]等作物中, 參與細胞增殖[23]、葉綠體形成[24]、胚胎發育、種子成熟[25]、光合作用[26-27]、固氮成分的合成[28-29]、花發育[30]等生長發育過程。除此之外, 一些NF-YA類轉錄因子在ABA信號途徑中發揮重要作用。基因芯片分析結果顯示, 誘導啟動NF-YA2、NFYA3、NF-YA7和NF-YA10基因的表達可以下調PYR1/PYL/RCAR、PP2C和SnRK2等ABA信號途徑相關基因的轉錄[31]。除此之外, 一些NF-YA類基因的擬南芥突變體和過表達植株在萌發期或者干旱條件下也表現與ABA有關的表型。擬南芥AtNF-YA5基因通過依賴ABA信號途徑調控干旱脅迫[32], 并且在萌發期, NF-YA5突變體也表現出對ABA高度敏感[33]。定量分析顯示, 過表達NF-YA1、NF-YA2、NF-YA3、NF-YA7、NF-YA9和NF-YA10均可導致擬南芥對ABA高度敏感[25]。這些研究結果都說明NF-Y轉錄因子調控植物抗逆反應依賴ABA信號途徑, 關于NF-Y轉錄因子通過ABA非依賴途徑調控植物抗逆反應未見報道。
谷子具有抗旱、耐貧瘠等特點, 是研究作物抗逆的理想材料[34]。本研究通過克隆并分析高鹽脅迫響應NF-Y類轉錄因子SiNF-YA5基因的特性和生物學功能, 旨在為 NF-Y類轉錄因子調控植物抗逆性的信號途徑提供證據, 同時也為作物耐鹽遺傳改良提供新的遺傳資源。
1 材料與方法
1.1 試驗材料
1.1.1 植物材料 擬南芥野生型(Columbia生態型, WT)由本實驗室保存, 谷子品種龍谷 25由中國農業科學院作物科學研究所刁現民課題組提供。
1.1.2 載體和菌株 大腸桿菌、農桿菌GV3101、pBI121載體、GFP載體都由本實驗室保存, pZero-Back載體購于北京天根公司。
1.1.3 試劑 限制性內切酶、T4 DNA連接酶購于Promega公司; in-Fusion克隆試劑盒購于TaRaKa公司; RT-PCR試劑盒購于全式金生物技術有限公司:質粒提取試劑盒、RNA提取試劑盒、DNA凝膠回收試劑盒、qRT-PCR試劑盒購于天根公司; 引物合成和測序由奧科生物技術科技有限公司完成; 其他化學藥品為國產分析純試劑。
1.2 谷子SiNF-YA5基因的生物信息學分析
谷子數據來源于Phytozome數據庫(http://www. phytozome.net/search.php), 利用 SMART 數據庫(http://smart.embl-heidelberg.de/)在線工具分析谷子SiNF-YA5蛋白的保守結構域。
1.3 SiNF-YA5基因的克隆及載體構建
根據谷子基因SiNF-YA5的CDS序列設計基因引物 A5-F1和A5-R1 (表 1), 用 RNA提取試劑盒(TIANGEN, 北京)提取龍谷 25植株總 RNA, 用TransScript II一步法反轉錄試劑盒(TransGen, 北京)反轉錄成cDNA, 以cDNA為模板擴增SiNF-YA5, 并將其回收純化, 采用in-Fusion試劑盒(TaKaRa)將其連接到pZeroBack載體上。以pZeroBack-SiNF-YA5質粒為模板, 引物為 A5-F2和 A5-R2 (表 1)擴增SiNF-YA5, BamH I酶切 GFP表達載體, 利用in-Fusion技術構建載體 16318hGFP-SiNF-YA5。同樣以 pZeroBack-SiNF-YA5質粒為模板, 引物為A5-F3和 A5-R3 (表 1)擴增 SiNF-YA5, Sma I酶切pBI121表達載體, 利用 in-Fusion技術構建載體pBI121-SiNF-YA5。
1.4 SiNF-YA5的亞細胞定位
參考 Yoo等[35]的方法制備谷子原生質體, 將融合表達的重組質粒 p16318hGFP-SiNF-YA5和 GFP空載體質粒作為對照分別轉化原生質體, 黑暗培養16 h以上, 并在激光共聚焦顯微鏡(Zeiss LSM700)下觀察定位情況。
1.5 擬南芥轉化
參考 Clough等[36]方法進行 SiNF-YA5基因的遺傳轉化, 將收獲的T0代種子種于含卡那霉素(50 mg L-1)的MS0培養基上, 篩選、擴繁獲得T3代的純合轉基因株系 OE1、OE2和 OE3, 進一步分析其功能。
1.6 RNA提取及SiNF-YA5的表達譜分析
將谷子幼苗在營養土中正常生長(溫度22℃、相對濕度65%、光照周期16 h光照/8 h黑暗) 3周后分別移至干旱(6% PEG-6000)、ABA (100 μmol L-1)、NaCl (100 mmol L-1)、低氮(0.2 mmol L-1)的水培營養液中脅迫處理, 于處理后0、1、6和24 h分別取樣,用 RNA提取試劑盒(TIANGEN)提取谷子植株總RNA。另外取NaCl (MS0+125 mmol L-1)處理的擬南芥轉基因和 WT植株, 提取總 RNA, -80℃保存備用。分別用4種脅迫下的谷子總RNA和NaCl處理下的擬南芥轉基因和WT植株RNA反轉錄產物作為模板, 以 TransScriptII一步法反轉錄試劑盒(TransGen, 北京)反轉錄成 cDNA, 以 SYBR Green染料法, 在 ABI Prism 7500上進行實時熒光定量PCR。RT-PCR反應體系含: 2×SuperReal PreMix Plus (含熒光染料)(TIANGEN) 12.5 μL、10 μmol L-1正向引物和反向引物各0.5 μL、50×ROX Reference DyeΔ 0.5 μL、RNase-free ddH2O 9.5 μL。反應條件為95℃預變性10 min; 95℃變性15 s, 60℃退火20 s, 72℃延伸30 s, 并收集熒光信號, 35個循環, 用2-ΔΔCt法計算該基因表達量。谷子SiNF-YA5基因qRT-PCR引物為 A5-F4和 A5-R4, 內參基因(Si001873m.g)引物為 SiActin-F和 SiActin-R (表 1); 擬南芥下游基因qRT-PCR引物為NHX1-F和NHX1-R、LEA7-F和LEA7-R, 內參基因(AT3G15260)引物為AtActin-F和AtActin-R (表1)。
1.7 轉SiNF-YA5基因擬南芥的抗鹽性分析
將WT和轉SiNF-YA5基因株系OE1、OE2、OE3的種子經70%酒精處理3 min, 無菌水清洗3次, 每次1 min左右; 用0.5%~0.8%的次氯酸鈉處理15 min,無菌水清洗3次, 每次1 min; 4℃春化3 d, 再將種子分別點播至MS0、MS0+75 mmol L-1NaCl、MS0+100 mmol L-1NaCl和MS0+125 mmol L-1NaCl的培養基上, 每種材料64粒種子, 重復3次。在22℃、相對濕度65%、光照周期16 h光照/8 h黑暗條件下萌發種子, 統計萌發率, 從第1天開始統計, 連續統計4 d;同時, 將MS0培養基上正常生長7 d的擬南芥幼苗轉移至MS0、MS0+100 mmol L-1NaCl、MS0+125 mmol L-1NaCl和MS0+150 mmol L-1NaCl培養基上, 垂直培養7 d, 統計不同濃度處理下SiNF-YA5過表達株系和 WT植株鮮重, 使用根系掃描儀(WINRHIZO proLA2400)分析根長, 試驗重復 3次, 運用方差分析軟件分析轉基因株系與野生型之間的差異。

表1 SiNF-YA5基因克隆和Real-time PCR分析所用引物Table 1 Primers used for gene cloning and real-time PCR analysis
1.8 轉SiNF-YA5基因擬南芥對ABA敏感性分析
方法同 1.7。萌發試驗的 ABA處理濃度為 0.5 μmol L-1和1 μmol L-1, 苗期敏感性試驗的ABA處理濃度為30 μmol L-1和40 μmol L-1。
2 結果與分析
2.1 SiNF-YA5基因的特性分析
前期工作對谷子干旱脅迫轉錄組分析發現 1個在干旱處理下表達上調的 NF-YA類轉錄因子基因SiNF-YA5。在谷子基因組數據庫(http://www. phytozome.net/)搜索 SiNF-YA5全長序列, 發現SiNF-YA5基因編碼序列為924 bp, 有6個外顯子, 5個內含子, 編碼307個氨基酸, 分子量為33.76 kD。SiNF-YA5在149~210位氨基酸之間含有CBF保守域, 屬于CCAAT結合蛋白家族。
2.2 SiNF-YA5基因的表達模式分析
利用qRT-PCR分別檢測結果(圖1), 在NaCl處理下, SiNF-YA5的表達量逐漸上升并在24 h達到最大, 表達量是處理前的 13.0倍; 在 PEG處理下, SiNF-YA5的表達量逐漸上升, 在24 h達最高值, 表達量提高了4.0倍; 在低氮處理下, SiNF-YA5的表達量也呈上升趨勢, 在12 h達最高值, 表達量提升了6.0倍左右。在ABA處理下, SiNF-YA5的表達量在1 h有所上升, 但相比處理前僅提高1.5倍。
2.3 SiNF-YA5蛋白亞細胞定位分析
將融合表達的重組質粒p16318hGFP-SiNF-YA5和GFP空載體質粒作為對照分別轉化制備的原生質體, 激光共聚焦顯微鏡下觀察結果顯示, 對照 GFP蛋白在細胞核、細胞質、細胞膜中均有表達; 而轉入 16318hGFP-SiNF-YA5融合載體的原生質體在細胞核和細胞膜上都能觀察到綠色熒光信號, 表明SiNF-YA5定位在細胞膜和細胞核中(圖2)。

圖1 SiNF-YA5在不同處理下的表達模式Fig. 1 Expression patterns of the SiNF-YA5 gene under various treatments
2.4 高鹽條件下SiNF-YA5轉基因擬南芥種子萌發率分析
從第1天觀察WT和SiNF-YA5轉基因擬南芥株系OE-1、OE-2和OE-3, 連續4 d統計萌發率。結果顯示, 在MS0培養基中的SiNF-YA5轉基因擬南芥和WT種子萌發率基本保持一致, 在24 h以后萌發率維持在95%左右(圖3和圖4-A); 在75 mmol L-1NaCl的培養基中, 在24 h WT不萌發, SiNF-YA5轉基因擬南芥少量萌發, 在 48 h, 兩者的萌發率相差最大, WT為26.3%, SiNF-YA5轉基因擬南芥為84.5%, 72 h以后SiNF-YA5轉基因擬南芥萌發率接近100%,兩者差異逐漸減小(圖3和圖4-A); 在100 mmol L-1NaCl和125 mmol L-1NaCl的培養基中, 分別在36 h和48 h, SiNF-YA5轉基因擬南芥和WT的萌發率才開始出現差異, SiNF-YA5轉基因擬南芥的萌發率始終顯著高于WT (圖3和圖4-A)。以上結果表明, 高鹽處理條件下, SiNF-YA5轉基因擬南芥的萌發率明顯高于WT, 隨著NaCl濃度的增加, WT和SiNF-YA5轉基因擬南芥的萌發速率都減慢, 但SiNF-YA5轉基因擬南芥的萌發率始終顯著高于WT。另外, 在萌發第5天統計, 在100 mmol L-1和125 mmol L-1NaCl處理條件下, SiNF-YA5轉基因擬南芥綠苗數高于WT, 并達到極顯著水平(圖 4-B)。說明在擬南芥中過表達SiNF-YA5基因提高了擬南芥萌發期對高鹽脅迫的耐受性, SiNF-YA5正向調節植物對高鹽脅迫的耐性。
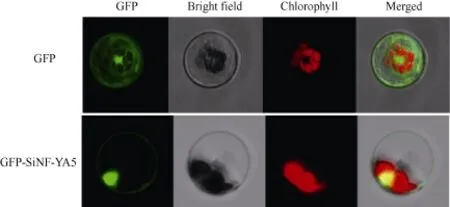
圖2 16318hGFP-SiNF-YA5蛋白的亞細胞定位分析結果Fig. 2 Subcellular localization of 16318hGFPSiNF-YA5 protein

圖3 高鹽處理下SiNF-YA5轉基因擬南芥和WT種子萌發情況Fig. 3 Seed germination situation of SiNF-YA5 transgenic Arabidopsis and WT under high salt stress condition
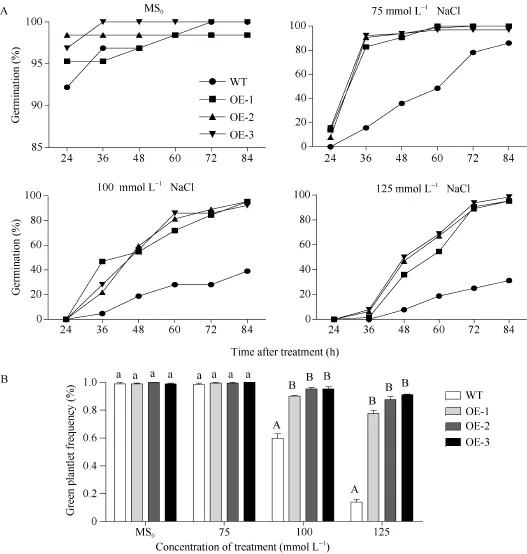
圖4 高鹽處理下SiNF-YA5轉基因擬南芥和WT種子的萌發率和綠苗率Fig. 4 Seed germination rates and green plantlet rates of SiNF-YA5 transgenic Arabidopsis and WT under high salt stressA: 高鹽處理下的種子萌發率; B: 高鹽處理下的綠苗率; 采用單因素方差分析法對數據進行統計分析, 柱上不同的小寫字母代表柱值在0.05水平上差異顯著, 不同大寫字母代表柱值在0.01水平上差異顯著。A: seed germination rate under high salt treatment; B: green plantlet frequency under high salt treatment; Data statically analysis was made by the means of one-way ANOVA. The values marked with different lowercase letters on the columns are significantly different at the 0.05 level; the values marked with different capital letters on the columns are significantly different at the 0.01 level.
2.5 SiNF-YA5轉基因擬南芥苗期耐鹽性鑒定
垂直培養 7 d后顯示, 在正常 MS0培養基上, SiNF-YA5轉基因擬南芥根表面積和植株鮮重與WT比較沒有明顯差別(圖 5和圖 6-A), 而在 100 mmol L-1和 125 mmol L-1NaCl脅迫處理下, 與WT相比, SiNF-YA5轉基因擬南芥的根表面積及植株鮮重增加, 在125 mmol L-1NaCl處理下的根表面積差異達到極顯著水平(圖 6-A), 轉基因株系的鮮重與WT相比差異達到顯著水平(圖6-B)。以上結果表明在植物中過表達SiNF-YA5基因可以顯著提高轉基因擬南芥苗期耐鹽性。在 150 mmol L-1NaCl處理條件下, 轉基因擬南芥及 WT都趨向于死亡, 差異不顯著(圖5)。
2.6 高鹽脅迫響應相關基因的表達分析
圖 7顯示, 在鹽脅迫下, 鹽脅迫響應相關基因Na+/H+轉運蛋白基因(NHX1)和種子胚胎發育后期富集的脫水保護蛋白基因(LEA7)在SiNF-YA5轉基因擬南芥中的表達明顯高于WT, 說明SiNF-YA5可能通過控制擬南芥中鹽脅迫相關基因NHX1和LEA7的表達來提高植物耐鹽性。
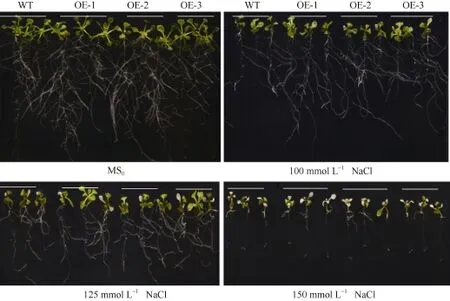
圖5 高鹽處理下SiNF-YA5轉基因擬南芥和WT苗期表型Fig. 5 Phenotype of SiNF-YA5 transgenic Arabidopsis and WT seedlings under high salt stress
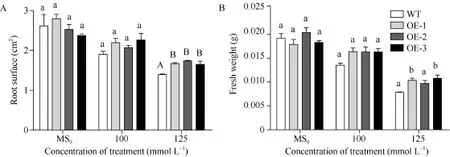
圖6 高鹽處理下SiNF-YA5轉基因擬南芥和WT苗期根表面積和鮮重Fig. 6 Root surfaces and fresh weights of SiNF-YA5 transgenic Arabidopsis and WT seedlings under high salt stressA: 高鹽處理下的根表面積; B: 高鹽處理下植株鮮重。采用單因素方差分析法對數據進行統計分析, 柱上不同的小寫字母代表柱值在0.05水平上差異顯著, 不同大寫字母代表柱值在0.01水平上差異顯著。A: root surface under high salt treatment; B: fresh weight under high salt treatment. Data statically analyzed by using method of one-way ANOVA. The values marked with different lowercase letters on the columns are significantly different at the 0.05 level; the values marked with different capital letters on the columns are significantly different at the 0.01 level.

圖7 NaCl處理下SiNF-YA5轉基因擬南芥中鹽脅迫相關基因表達水平Fig. 7 Expression levels of two stress-tolerant genes in SiNF-YA5 transgenic Arabidopsis under NaCl treatment
2.7 SiNF-YA5轉基因植株對ABA敏感性分析
在ABA處理下, WT和SiNF-YA5轉基因株系萌發率無差異(圖8-A和圖9)。取上述MS0培養基上正常生長7 d的WT和SiNF-YA5轉基因擬南芥幼苗, 分別轉接到正常MS0和含有30 μmol L-1和40 μmol L-1ABA的MS0培養基上照光, 垂直培養7 d, 結果顯示, WT和轉基因擬南芥在地上部分和地下部分無顯著差異(圖8-B)。說明無論在萌發期還是苗期, SiNF-YA5轉基因擬南芥與 WT相比對ABA 敏感性沒有差異, 證明 SiNF-YA5不參與ABA信號途徑, 它通過ABA非依賴途徑調控植物的耐鹽性。

圖8 SiNF-YA5轉基因擬南芥和WT對ABA敏感性Fig. 8 Sensitivity analysis of SiNF-YA5 transgenic Arabidopsis and WT under ABA treatmentA: SiNF-YA5轉基因擬南芥和WT種子在含有0.5 μmol L-1和1.0 μmol L-1ABA的培養基上萌發情況; B: SiNF-YA5轉基因擬南芥幼苗和WT幼苗在含有30 μmol L-1和40 μmol L-1ABA的培養基上對ABA敏感性。A: seed germination situation of SiNF-YA5 transgenic Arabidopsis and WT under 0.5 μmol L-1and 1.0 μmol L-1ABA treatment; B: sensitivity analysis of SiNF-YA5 transgenic Arabidopsis seedlings under 30 μmol L-1and 40 μmol L-1ABA treatment.
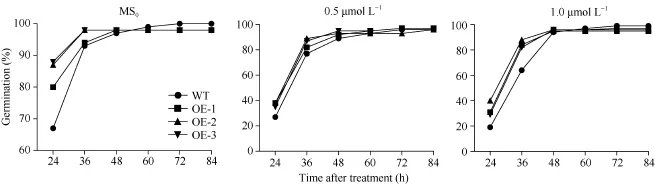
圖9 ABA處理下SiNF-YA5轉基因擬南芥和WT種子的萌發率Fig. 9 Seed germination rates of SiNF-YA5 transgenic Arabidopsis and WT under ABA treatment
3 討論
NF-Y轉錄因子是一類重要的逆境調控因子, 它由3類亞基構成, NF-YA亞基可進一步被分為7個亞族。本研究從谷子中克隆 1個 NF-YA類基因SiNF-YA5, 根據已經發表的谷子NF-YA類轉錄因子進化樹分析結果顯示 SiNF-YA5屬于第 III亞族[37],與水稻NF-YA蛋白(OsHAP2E)進化關系最近, 而與擬南芥NF-YA蛋白進化關系較遠。目前, 已經報道許多 NF-Y類轉錄因子依賴 ABA信號途徑參與干旱、耐鹽脅迫反應。大豆GmNF-YA3受ABA和NaCl脅迫誘導表達, 在擬南芥中過表達 GmNF-YA3能夠提高植物的抗旱性。在正常條件下, 在 GmNF-YA3過表達擬南芥中, ABA合成及信號傳導相關基因和脅迫相關基因轉錄水平提高[20]。擬南芥 AtNF-YA1基因依賴 ABA信號途徑負向調控植物鹽脅迫耐性,抑制幼苗的生長。在苗期, 過表達AtNF-YA1基因的轉基因擬南芥提高了植物對鹽和ABA的敏感性, 當ABA抑制劑存在時, 過表達 AtNF-YA1擬南芥對鹽敏感的表型恢復[38]。谷子 SiNF-YA1 (Si037045m)和SiNF-YB8 (Si032469m)基因通過ABA信號途徑激活脅迫相關基因表達, 改善植物生理特性從而正向調節植物耐鹽性和耐旱性[37]。同時發現SiNF-YA5與擬南芥AtNF-YA1、谷子SiNF-YA1和SiNF-YB8均不在同一亞族, 推測SiNF-YA5可能通過與上述基因不同的其他途徑調控耐鹽和抗旱性。本文研究結果表明,在高鹽處理條件下, SiNF-YA5轉基因擬南芥在萌發期的萌發率顯著高于WT (圖4); 在苗期, SiNF-YA5轉基因擬南芥的根系比WT發達, 鮮重顯著大于WT (圖 6)。然而, 與 SiNF-YA1和 SiNF-YB8基因不同, SiNF-YA5轉基因擬南芥在萌發期和苗期對 ABA均不敏感(圖8), 所以推測SiNF-YA5通過ABA非依賴途徑提高植物對 NaCl脅迫的耐性。Chamindika創建擬南芥NF-YA類轉錄因子的10個過表達材料, 觀察ABA調節的種子萌發和植物生長發育的情況, 結果發現所有的材料生長發育受到抑制, 但萌發期對ABA敏感性存在差異。在ABA不敏感的過表達材料中進行基因表達檢測發現 ABA信號途徑相關基因下調[39]。同樣, NF-YC類轉錄因子在種子萌發期對ABA的反應也不完全相同, 種子萌發期NF-YC4突變體對ABA敏感, 而NF-YC3 和NF-YC9突變體則不敏感[40]。這些研究結果都表明 NF-Y類轉錄因子調控耐鹽的途徑存在差異, 同時存在著ABA依賴型和ABA非依賴型的耐鹽信號調控途徑。
在SiNF-YA5轉基因株系中, 我們檢測到參與鹽脅迫響應基因NHX1和LEA7的表達量較WT都顯著提高(圖7)。同時, 分別分析NHX1和LEA7基因的啟動子區域, 發現兩者均有4個CAATT-box結構域(NF-Y類轉錄因子結合元件), 因此推測SiNF-YA5可能主要通過 ABA非依賴途徑直接激活下游基因NHX1和 LEA7基因表達完成植物的耐鹽調控。NHX1是第1個在擬南芥中發現的Na+/H+轉運蛋白,能夠促進鈉離子在液泡中的積累[41]。鹽脅迫環境下構建及分析 SOS 轉錄調控網絡時發現擬南芥 bZIP類轉錄因子At5g65210首先通過ABA非依賴途徑接收細胞膜上感受器傳遞的外界 Na+信號, 然后調控液泡膜上的 Na+/H+轉運基因 NHX1[42]。NHX1主要利用液泡膜的H+-ATPase和液泡膜H+-PPase產生的跨膜質子梯度將胞質中的 Na+逆濃度梯度運入液泡中降低 Na+對植物細胞的毒害作用[43]。LEA蛋白是一類參與細胞抗逆保護的蛋白質, 廣泛存在于植物的種子中, 能夠在干旱脅迫時保護膜系統以及生物大分子免受脫水傷害。植物遭受干旱、鹽漬及低溫等脅迫時, 體內LEA基因的表達會增加。已有研究報道LEA基因可以增加轉基因水稻耐鹽和抗旱性[44]。關于SiNF-YA5提高轉基因植物對高鹽脅迫耐性的信號途徑還需要進一步分析, 本研究初步闡明了谷子SiNF-YA5在調節高鹽脅迫響應中的 ABA非依賴型信號途徑, 為進一步了解谷子抗逆機制提供了新的依據。
4 結論
從谷子中分離出NF-Y類轉錄因子基因SiNF-YA5。SiNF-YA5被定位于細胞核及細胞膜。SiNF-YA5受低氮、干旱、高鹽等脅迫的誘導表達。在植物中過表達SiNF-YA5可以顯著提高植物在萌發期及苗期的耐鹽性。SiNF-YA5轉基因植物對ABA的敏感性與WT差異不顯著, 證明SiNF-YA5通過ABA非依賴途徑調控植物的耐鹽性。
[1] Boyer J S. Plant productivity and environment. Science, 1982, 218: 443-448
[2] Xiong L M, Schumaker K S, Zhu J K. Cell signaling during cold, drought, and salt stress. Plant Cell, 2002, 14: S165-S183
[3] Zhu J K. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol, 2002, 53: 247-273
[4] The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 2000, 408: 796-815
[5] Riechmann J L, Ratcliffe O J. A genomic perspective on plant transcription factors. Curr Opin Plant Biol, 2000, 3: 423-434
[6] Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol, 1999, 17: 287-291
[7] Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol, 2006, 57: 781-803
[8] Jiang C, Iu B, Singh J. Requirement of a CCGAC cis-acting element for cold induction of the BN115 gene from winter Brassica napus. Plant Mol Biol, 1996, 30: 679-684
[9] Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA, 2000, 97: 11632-11637
[10] Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB)function as transcriptional activators in abscisic acid signaling. Plant Cell, 2003, 15: 63-78
[11] Mantovani R, Li X Y, Pessara U, Hooft van Huisjduijnen R, Benoist C, Mathis D. Dominant negative analogs of NF-YA. J Biol Chem, 1994, 32: 20340-20346
[12] Frontini M, Imbriano C, Manni I, Mantovani R. Cell cycle regulation of NF-YC nuclear localization. Cell Cycle, 2004, 3:217-222
[13] Kahle J, Baake M, Doenecke D, Albig W. Subunits of the heterotrimeric transcription factor NF-Y are imported into the nucleus by distinct pathways involving importin beta and importin 13. Mol Cell Biol, 2005, 25: 5339-5354
[14] Steidl S, Tuncher A, Goda H, Guder C, Papadopoulou N, Kobayashi T, Tsukagoshi N, Kato M, Brakhage A. A single subunit of a heterotrimeric CCAAT-binding complex carries a nuclear localization signal: piggy back transport of the pre-assembled complex to the nucleus. J Mol Biol, 2004, 342: 515-524
[15] Ceribelli M, Dolfini, D, Merico D, Gatta R, Vigano A M., Pavesi G, Mantovani R. The histone-like NF-Y is a bifunctional transcription factor. Mol Cell Biol, 2008, 28: 2047-2058
[16] Maity S N, de Crombrugghe B. Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci, 1998, 23:174-178
[17] Siefers N, Dang K K, Kumimoto R W, Bynum W E, Tayrose G, Holt B F. Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol, 2009, 149: 625-641
[18] Thirumurugan T, Ito Y, Kubo T, Serizawa A, Kurata N. Identification, characterization and interaction of HAP family genes in rice. Mol Genet Genomics, 2008, 279: 279-289
[19] Nelson D E, Repetti P P, Adams T R, Creelman R A, Wu J, Warner D C, Anstrom D C, Bensen R J, Castiglioni P P, Donnarummo M G, Hinchey B S, Kumimoto R W, Maszle D R, Canales R D, Krolikowski K A, Dotson S B, Gutterson N, Ratcliffe O J, Heard J E. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA, 2007, 104: 16450-16455
[20] Ni, Z Y, Hu Z, Jiang Q Y, Zhang H. GmNFYA3, a target gene of miR169, is a positive regulator of plant tolerance to drought stress. Plant Mol Biol, 2013, 82: 113-129
[21] Qu B, He X, Wang J, Zhao Y, Teng W, Shao A, Zhao X, Ma W, Li B, Li Z, Tong Y. A wheat CCAAT box-binding transcription factor increases the grain yield of wheat with less fertilizer input. Plant Physiol, 2014; 167: 411-423
[22] Stephenson T J, McIntyre C L, Collet C, Xue G P. Genome-wide identification and expression analysis of the NF-Y family of transcription factors in Triticum aestivum. Plant Mol Biol, 2007, 65: 77-92
[23] Sun X C, Ling S, Lu Z H, Ouyang Y D, Liu S S, Yao J. OsNF-YB1, a rice endosperm-specific gene, is essential for cell proliferation in endosperm development. Gene, 2014, 551: 214-221
[24] Miyoshi K, Ito Y, Serizawa A, Kurata N. OsHAP3 genes regulate chloroplast biogenesis in rice. Plant J, 2003, 36: 532-540
[25] Mu J, Tan H, Hong S, Liang Y, Zuo J. Arabidopsis transcription factor genes NF-YA1, 5, 6, and 9 play redundant roles in male gametogenesis, embryogenesis, and seed development. Mol Plant, 2013, 6: 188-201
[26] Alam M M, Tanaka T, Nakamura H, Ichikawa H, Kobayashi K, Yaeno T, Yamaoka N, Shimomoto K, Takayama K, Nishina H, Nishiguchi M. Overexpression of a rice heme activator protein gene (OsHAP2E) confers resistance to pathogens, salinity and drought, and increases photosynthesis and tiller number. Plant Biotechnol J, 2015, 13: 85-96
[27] Stephenson T J, McIntyre C L, Collet C, Xue G P. TaNF-YB3 is involved in the regulation of photosynthesis genes in Triticum aestivum. Funct Integr Genomics, 2011, 11: 327-340
[28] Combier J P, Frugier F, de Billy F, Boualem A, El-Yahyaoui F, Moreau S, Vernie T, Ott T, Gamas P, Crespi M, Niebel A. MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes Dev, 2006, 20: 3084-3088
[29] Zanetti M E, Blanco F A, Beker M P, Battaglia M, Aguilar O M. A C subunit of the plant nuclear factor NF-Y required for rhizobial infection and nodule development affects partner selection in the common bean-Rhizobium etli symbiosis. Plant Cell, 2010, 22:4142-4157
[30] Hackenberg D, Keetman U, Grimm B. Homologous NF-YC2 subunit from Arabidopsis and tobacco is activated by photooxidative stress and induces flowering. Int J Mol Sci, 2012, 13:3458-3477
[31] Leyva-Gonzalez M A, Ibarra-Laclette E, Cruz-Ramirez A, Herrera-Estrella L. Functional and transcriptome analysis reveals an acclimatization strategy for abiotic stress tolerance mediated by Arabidopsis NF-YA family members. PLoS One, 2012, 7:e48138
[32] Li W X, Oono Y, Zhu J, He X J, Wu J M, Iida K, Lu X Y, Cui X P, Jin H L, Zhu J K. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell, 2008, 20: 2238-2251
[33] Warpeha K M, Upadhyay S, Yeh J, Adamiak J, Hawkins S I, Lapik Y R, Anderson M B, Kaufman L S. The GCR1, GPA1, PRN1, NFY signal chain mediates both blue light and abscisic acid responses in Arabidopsis. Plant Physiol, 2007, 143:1590-1600
[34] 劉敬科, 刁現民. 我國谷子產業現狀與加工發展方向. 農業工程技術: 農產品加工業, 2013, (12): 15-17
Liu J K, Diao X M. Foxtail millet processing industry status and development trend in our country. Agric Eng Technol (Agric Prod Process), 2013, (12): 15-17 (in Chinese)
[35] Yoo S D, Cho Y H, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc, 2007, 2: 1565-1572
[36] Beehtold N, Ellis J, Pelletier G. In plant Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. Life Sci, 1993, 316: 1194-1199
[37] Feng Z J, He G H, Zheng W J, Lu P P, Chen M, Gong Y M, Ma Y Z, Xu Z S. Foxtail millet NF-Y families: genome-wide survey and evolution analyses identified two functional genes important in abiotic stresses. Front Plant Sci, 2015, 6: 1142
[38] Li Y J, Fang Y, Fu Y R, Huang J G, Wu C A, Zheng C C. NFYA1 is involved in regulation of postgermination growth arrest under salt stress in Arabidopsis. PLoS One, 2013, 8(4): e61289
[39] Siriwardana C L, Kumimoto R W, Jones D S, Holt B F. Gene Family Analysis of the Arabidopsis NF-YA transcription factors reveals opposing abscisic acid responses during seed germination. Plant Mol Biol Rep, 2014, 32: 971-986
[40] Kumimoto R W, Siriwardana C L, Gayler K K, Risinger J R, Siefers N, Holt B F. NUCLEAR FACTOR Y transcription factors have both opposing and additive roles in ABA-mediated seed germination. PLoS One, 2013, 8: e59481
[41] Gaxiola R A, Rao R, Sherman A, Grisafi P, Alper S L, Fink G R. The Arabidopsis thaliana proton transporters, AtNHX1 and Avpl, can function in cation detoxification in yeast. Proc Natl Acad Sci USA, 1999, 96: 1480-1485
[42] 謝崇波, 金谷雷, 徐海明, 朱軍. 擬南芥在鹽脅迫環境下 SOS轉錄調控網絡的構建及分析. 遺傳, 2010, 6: 639-646
Xie C B, Jin G L, Xu H M, Zhu J. Construction and analysis of SOS pathway-related transcription a regulatory network underlying salt stress response in Arabidopsis. Hereditas (Beijing), 2010, 6: 639-646 (in Chinese with English abstract)
[43] Wu Y Y, Chen Q J, Chen M, Chen J, Wang X C. Salt-tolerant transgenic perennial ryegrass (Lolium perenne L.) obtained by Agrobacterium tumefaciens-mediated transformation of the vacuolar Na+/H+antiporter gene. Plant Sci, 2005, 169: 65-73
[44] Babu R C, Zhang J X, Blum A, Ho T H D, Wu R, Nguyen H T. HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. Plant Sci, 2004, 166: 855-862
Transcription Factor SiNF-YA5 from Foxtail Millet (Setaria italica) Conferred Tolerance to High-salt Stress through ABA-independent Pathway in Transgenic Arabidopsis
HUANG Suo1,**, HU Li-Qin1,**, XU Dong-Bei1,2, LI Wei-Wei1,3, XU Zhao-Shi1, LI Lian-Cheng1, ZHOU Yong-Bin1,2, DIAO Xian-Min1, JIA Guan-Qing1, MA You-Zhi1, and CHEN Ming1,*
1Institute of Crop Science, Chinese Academy of Agricultural Sciences / National Key Facility For Crop Gene Resource and Genetic Improvement / Key Laboratory of Biology and Genetic Improvement of Triticeae Crop, Ministry of Agriculture, Beijing 100081, China;2College of Agronomy, Northwest A&F University / State Key Laboratory of Arid Region Crop Adversity Biology, Yangling 712100, China;3College of Life Science and Technology, Harbin Normal University / Key Laboratory of Molecular Cytogenetics and Genetic Breeding of Heilongjiang Province, Harbin 150025, China
Nuclear transcription factor Y (NF-Y) consisting of three subunits, NF-YA, NF-YB, and NF-YC, plays an essential role in many biologic processes, including growth, development, and abiotic stress response. In this study, an NF-Y like transcription factor gene SiNF-YA5 was isolated from foxtail millet variety Longgu 25. The full-length sequence of SiNF-YA5 gene is 924 bp, encoding 307 amino acids. Molecular weight and isoelectric point of SiNF-YA5 protein are 33.76 kD and 9.19, respectively. There is a conserved CBF domain from the 149th to the 210th amino acids of SiNF-YA5. According to the subcellular localization analysis, SiNF-YA5 was mainly localized and expressed on the plasma membrane and nucleus in plant cell. Gene functionalanalysis showed that under different NaCl concentration treatments, the germination rate of SiNF-YA5 transgenic Arabidopsis was significantly higher than that of wild-type (WT) Arabidopsis during seed germination stage; root surface area and fresh weight of SiNF-YA5 transgenic Arabidopsis remarkably increased compared with WT during seedling stage. Those results indicated that the overexpression of SiNF-YA5 in transgenic plants could enhance tolerance to high salt. Gene expression analysis showed that the expressions of two salt stress related genes, namely NHX1 and LEA7, increased significantly in SiNF-YA5 transgenic plants. On the other hand, there was no obvious difference in sensitivity to ABA between SiNF-YA5 transgenic Arabidopsis and WT showed during seed germination and seedling stages indicating that SiNF-YA5 could enhance salt tolerance through ABA-independent pathway in transgenic plants.
Foxtail millet (Setaria italic); NF-Y like transcription factor; High salt stress; ABA independent signaling pathway
10.3724/SP.J.1006.2016.01787
本研究由國家轉基因新品種生物培育科技重大專項(2016ZX08002-002)和中國農業科學院創新工程資助。
This work was funded by the National Majar Project for Developing New GM Crops (2014ZX08002-002) and the Innovation Project of Chinese Academy of Agricultural Sciences.
*通訊作者(Corresponding author): 陳明, E-mail: chenming02@caas.cn, Tel: 13683360891**同等貢獻(Contributed equally to this work)
聯系方式: E-mail: hnndhs@126.com, Tel: 17701300735
稿日期): 2016-03-06; Accepted(接受日期): 2016-06-20; Published online(
日期): 2016-07-04.
URL: http://www.cnki.net/kcms/detail/11.1809.S.20160704.0826.014.html

