miR-34a通過Snail誘導肺癌EMT及促進其轉移的分子機制①
劉行仁 白義鳳 梁 良 馮 靜 鄧 菲
(四川省醫學科學院·四川省人民醫院呼吸科,成都610072)
·基礎免疫學·
miR-34a通過Snail誘導肺癌EMT及促進其轉移的分子機制①
劉行仁 白義鳳②梁 良②馮 靜③鄧 菲④
(四川省醫學科學院·四川省人民醫院呼吸科,成都610072)
目的:探討miR-34a在肺癌組織中的表達情況以及miR-34a在肺癌細胞侵襲和遷移過程中的作用及其機制。方法:qPCR檢測肺癌和正常肺組織中miR-34a的表達情況;使用miR-34a-mimic和miR-34a-inhibitor過表達和沉默miR-34a,qPCR檢測沉默和過表達效果;Western blot檢測沉默和過表達miR-34a后Snail蛋白的表達情況;熒光素酶報告基因檢測miR-34a與Snail的相互作用;Transwell侵襲實驗檢測miR-34a的表達對肺癌細胞侵襲能力的影響;劃痕實驗檢測miR-34a的表達對肺癌細胞遷移能力的影響;Western blot檢測E-Cadherin、Vimentin和Twist蛋白的表達情況。結果:與正常肺組織相比,肺癌組織中miR-34a表達明顯降低;且晚期、低分化和有淋巴結轉移的肺癌組織miR-34a表達明顯較早期、高分化和無淋巴結轉移的肺癌組織低;miR-34a-mimic和miR-34a-inhibitor可以有效抑制和過表達miR-34a的表達;miR-34a能與Snail的3′ UTR特異性結合;miR-34a可以調控肺癌H1650細胞的侵襲遷移能力;過表達miR-34a上調E-Cadherin,同時下調Vimentin和Twist蛋白的表達,沉默miR-34a則相反。結論:miR-34a在肺癌中表達明顯降低,且跟肺癌分期分級以及淋巴結轉移與否密切相關,同時miR-34a可以通過上皮間質轉化調節肺癌細胞侵襲和遷移能力。
miR-34a;肺癌;上皮間質轉化;E-Cadherin;Transwell;Snail
肺癌是世界上發病率最高的惡性腫瘤。肺癌也是我國發病率和死亡率最高的惡性腫瘤[1]。全球每年有超過160萬新發病例,占所有新發腫瘤的12.7%。同時肺癌也是死亡率最高的惡性腫瘤,每年有超過140萬肺癌患者死亡,占所有腫瘤死亡人數的19%[2]。雖然近年來肺癌治療手段不斷進步,但是肺癌患者尤其是晚期肺癌患者的五年生存率仍然很低。局部復發、淋巴結轉移以及遠處轉移是導致患者死亡的主要原因[3]。
腫瘤轉移是個多步驟,多階段的復雜過程,主要包括局部浸潤、浸入血管、隨血液循環系統轉移并在其中存活、移出血管和在新的部位定居并增殖,而腫瘤轉移的第一步是細胞與細胞間黏附的破裂[4,5]。Snail是近年發現的鋅指轉錄因子,是腫瘤進展過程中一個重要的調節子,可以促進腫瘤浸潤和轉移的因子[6]。Burton等[7]研究表明,Snail與乳腺癌組織分期和分級、淋巴結轉移的情況密切相關。Palma等[8]發現,Snail的高表達可以促進上皮間質轉化的發生,從而促進腫瘤細胞的侵襲和遷移。
miRNA是高度保守的非編碼RNA,通過調控相應基因的表達,參與細胞的增殖、凋亡、細胞分化等生物學行為,同時也可參與惡性腫瘤的侵襲、遷移、腫瘤微環境的調節以及腫瘤干細胞的調控等[9,10]。許多研究表明,miR-34a的缺失可能與肝癌、乳腺癌和結腸癌等腫瘤發生和發展密切相關[11-13]。但是miR-34a在肺癌發生發展過程中機制不甚明確。故本研究擬探討miR-34a在肺癌中的表達情況,以及在肺癌轉移過程中的作用及機制。
1 材料與方法
1.1 材料
1.1.1 臨床標本采集及處理 收集2015年1月-2016年3月在我院收治的100例肺癌患者,其中男性65例,女性35例;年齡(61.24±3.61)歲。所有患者術前無化療或放療,全部患者術后病理分期均經兩名副高以上病理科醫師共同閱片確定,根據肺癌TNM分期標準,Ⅰ級39例、Ⅱ級18例、Ⅲ級37例、Ⅳ級6例。低分化28例,中分化32例,高分化40例。45例發生淋巴結轉移,55例未發生淋巴結轉移。同時取癌旁正常組織100例。腫瘤組織離體后分兩塊,一塊迅速投入RNA保存液中,另一塊用經焦碳酸二乙酯處理的冷磷酸緩沖液沖洗,去除血跡,迅速投入液氮凍存。
1.1.2 細胞株與主要試劑 人肺癌細胞株H1650購自ATCC。細胞培養條件:含10%胎牛血清的RPMI DMEM,37℃,5%CO2條件下培養。胎牛血清,RPMI DMEM培養基均購自Gibco公司。Snail、E-cadherin、Vimentin、Twist單克隆抗體購自CST。Transwell小室購自美國Millipore公司。Matrigel購自美國BD公司。脂質體LipofectamineTM2000、miR-34a mimic、miR-34a-inhibitor購自上海吉凱基因。Trizol購自美國Ambion公司。逆轉錄試劑盒(FSQ-101)購自日本TOYOBO公司。PCR試劑盒購自美國Kapa公司。熒光素酶活性檢測試劑盒購自Promega公司。熒光素酶報告載體由Promega公司合成。
1.2 方法
1.2.1 qPCR檢測miR-34a的表達 按照Trizol說明書提取組織中總RNA,超微量分光光度計測定RNA濃度,復能基因有限公司設計miR-34a引物,上游引物:5′-GTGCAGGGTCCGAGGT-3′;下游引物:5′-GCCGCTGGCAGTGTCTTAGCTG-3′。以100 ng總RNA為模板,逆轉錄cDNA,反應條件為:37℃ 15 min,98℃ 5 min。后根據Kapa PCR試劑盒說明書進行PCR反應。獲得數據以RQ=2-ΔΔCt計算mRNA表達量。實驗重復3次。
1.2.2 細胞轉染 取對數生長期的肺癌細胞H1650細胞,按照LipofectamineTM2000轉染試劑盒說明書將miR-34a-mimic、miR-34a-inhibitor和陰性對照轉染細胞。轉染后qPCR檢測miR-34a的表達情況。
1.2.3 Western blot檢測細胞轉染后中Snail、E-cadherin、Vimentin、Twist的表達 取轉染miR-34a-mimic和miR-34a-inhibitor 48 h的細胞蛋白,BCA法測定蛋白濃度,加入Loading buffer后變性蛋白。配制10%SDS-PAGE,每孔加入20 μg蛋白樣品。使用濕轉法電轉至PVDF膜,5%脫脂奶粉封閉2 h,1∶1 000 TBST稀釋一抗,4℃過夜;加入羊抗兔二抗1∶5 000 稀釋,室溫孵育2 h,ECL發光。重復3次。
1.2.4 Transwell侵襲實驗檢測miR-34a對肺癌細胞侵襲能力的影響 所有試劑及器材均于冰上預冷,將Transwell小室置于24孔板內,將Transwell小室內膜均勻涂抹Matrigel膠 50 μl (0.2 μg/μl),37℃孵育15 min,使膠凝固;消化、離心、計數細胞后,按照2.5×104個/ml用無血清培養基稀釋細胞,制成細胞懸液;按照每孔200 μl,將細胞懸液加入Transwell上室,同時在Transwell下室加入10%FBS+培養基500 μl,放入37℃孵箱培養;甲醛固定,結晶紫染色15 min,然后用棉簽輕輕擦拭內膜上的細胞。顯微鏡下技術,計數4個高倍視野(×40)下穿過濾膜的細胞數。實驗重復3次。
1.2.5 劃痕實驗檢測miR-34a對肺癌細胞遷移能力的影響 劃痕實驗:將H1650細胞接種于6孔板,待細胞融合度生長在90%時,用200 μl消毒槍頭從上而下劃線,并在顯微鏡下觀察,測量劃痕的初始距離(0 time);在24、48、72 h后,分別測量劃痕的距離,并拍照,計算細胞的遷移率。實驗重復3次。
1.2.6 熒光素酶活性檢測 將熒光素酶報告載體與miR-34a-mimic共轉染H1650細胞。以轉染pRL-TK作為標準內質控。轉染36 h后,收獲細胞。按Promega公司熒光素酶活性檢測試劑盒說明書檢測H1650細胞熒光素酶活性。相對熒光素酶活性=螢火蟲熒光素酶活性值/海腎熒光素酶活性值。實驗重復3次。
2 結果
2.1 肺癌組織和正常肺組織中miR-34a mRNA的表達 qPCR結果表明:與癌旁正常組織相比,肺癌組織中miR-34a mRNA表達水平明顯降低[(2.51±0.31) vs (0.58±0.17),P<0.05],差異有統計學意義(圖1)。同時檢測miR-34a在肺癌H1650和H889細胞中的表達情況。qRCR結果表明:H1650肺癌細胞中miR-34a表達水平低,H889肺癌細胞中miR-34a表達水平高。
2.2 miR-34a表達與臨床病理資料的關系 統計分析表明:miR-34a的表達水平隨肺癌病理分期的增加而降低;隨分化程度的降低,miR-34a的表達水平逐漸降低;有淋巴結轉移的肺癌組織中,miR-34a的表達明顯降低;miR-34a的表達水平與年齡和性別無關。結果表明:miR-34a與肺癌病理分期分級以及淋巴結轉移與否有關,而與性別、年齡等無關(表1)。
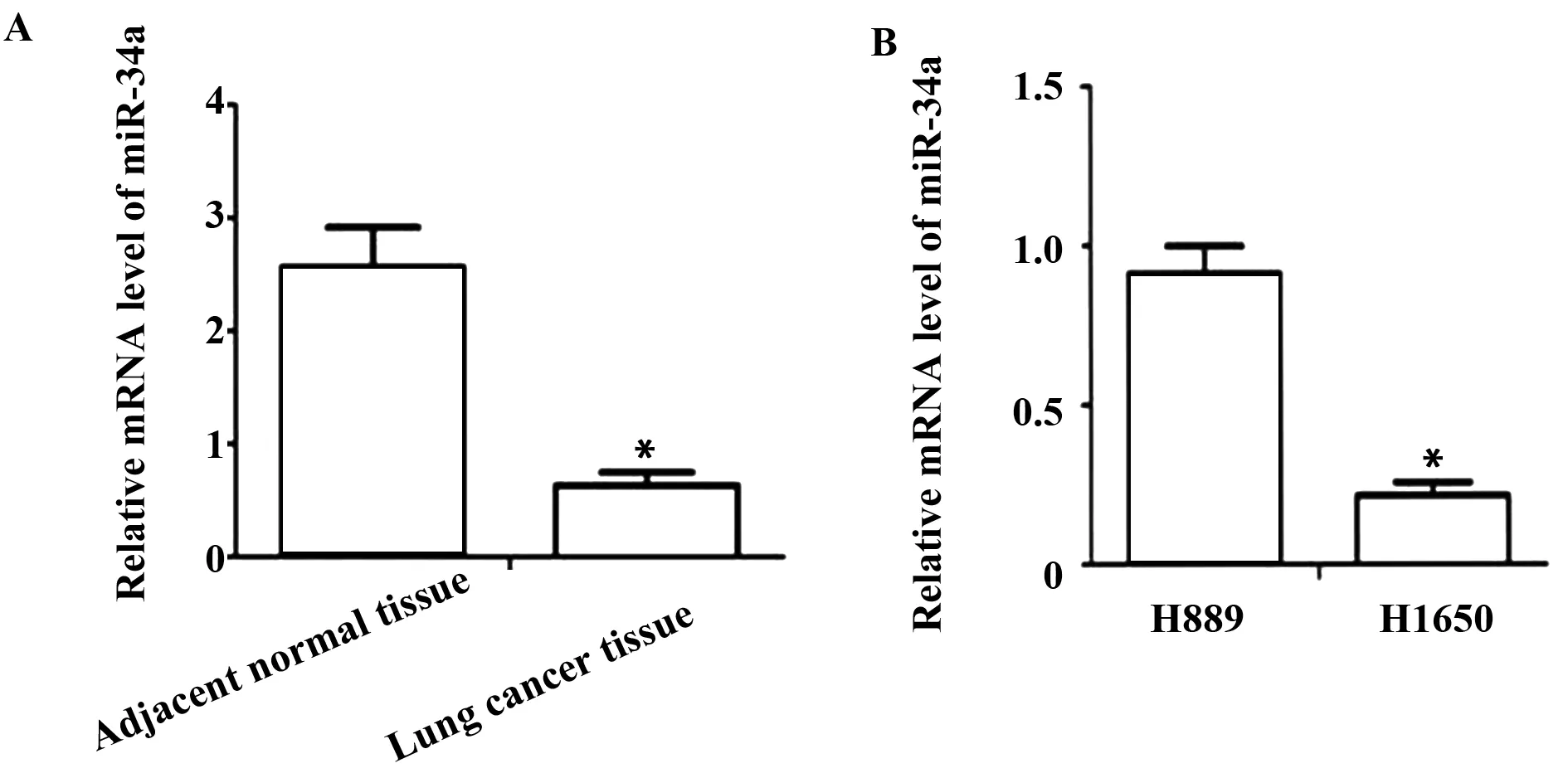
圖1 qPCR 檢測肺癌組織和細胞株中miR-34a 的表達情況Fig.1 qPCR was used to detect expression of miR-34a in lung cancer tissues and cell linesNote: *.P<0.05.
2.3 miR-34a-mimic和miR-34a-inhibitor轉染后顯著提高和降低miR-34a mRNA的表達 miR-34a-mimic轉染肺癌細胞H1650 48 h后,qPCR結果表明:與NC組相比,miR-34a-mimic組miR-34a mRNA水平明顯提高[ (0.22±0.03)vs(0.89±0.13),P<0.01],差異有統計學意義(圖2A);與NC組相比,miR-34a-inhibitor組miR-34a mRNA水平明顯提高[(0.22±0.03)vs(0.89±0.13),P<0.01],差異有統計學意義(圖2B)。結果表明,miR-34a-mimic可以顯著提高miR-34a表達水平;miR-34a-inhibitor可以顯著降低miR-34a表達水平。
2.4 miR-34a可以調節Snail的表達 Western blot結果顯示:與NC組相比,miR-34a-mimic組中Snail蛋白表達水平明顯下降(51.4±2.8) vs (13.7±0.8),P<0.05,而miR-34a-inhibitor組中Snail蛋白表達水平明顯提高[(72.4±3.1) vs (13.7±0.8),P<0.05],表明miR-34a可以調節Snail的表達(圖3)。
2.5 熒光素酶報告基因檢測Snail和miR-34a的作用關系 為明確miR-34a能否與Snail 3′UTR結合,將miR-34a-inhibitor與Snail-Wt、Snail-Mut共轉染到肺癌H1650細胞中。熒光素酶報告基因結果顯示:miR-34a-inhibitor可以明顯抑制Snail-Wt的熒光素酶活性;miR-34a-mimic對Snail-Mut的熒光素酶無明顯抑制作用。結果表明,miR-34a能與Snail的3′ UTR特異性結合(圖4)。
表1 miR-34a表達與肺癌臨床病理特征的關系
Tab.1 Relationship between miR-34a expression and clinicopathological features of lung cancer
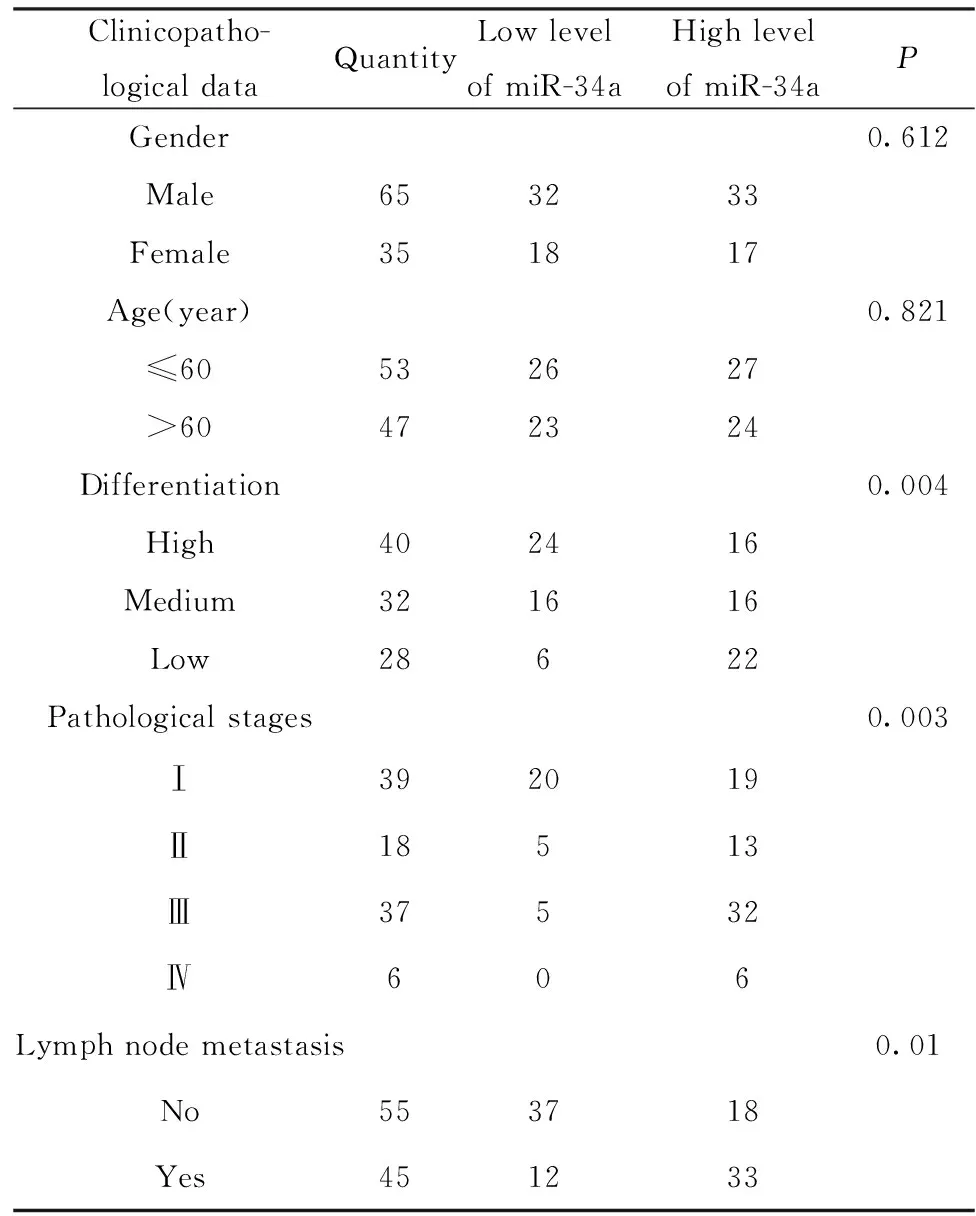
Clinicopatho-logicaldataQuantityLowlevelofmiR-34aHighlevelofmiR-34aPGender0.612Male653233Female351817Age(year)0.821≤60532627>60472324Differentiation0.004High402416Medium321616Low28622Pathologicalstages0.003Ⅰ392019Ⅱ18513Ⅲ37532Ⅳ606Lymphnodemetastasis0.01No553718Yes451233
2.6 miR-34a可以抑制人肺癌細胞H1650的侵襲能力 細胞侵襲穿過Matrigel的能力可以反映細胞的侵襲能力。Transwell結果顯示:NC組通過Matrigel基質膠的細胞數量為(346.57±28.70),明顯多于miR-34a-mimic組(131.92±13.80),差異有統計學意義(P<0.01)(圖5A);NC組通過Matrigel基質膠的細胞數量為(72.57±3.20),明顯少于miR-34a-mimic組(406.57±31.90),差異有統計學意義(P<0.01)(圖5B),表明miR-34a可以抑制人肺癌細胞H1650的侵襲能力。
2.7 miR-34a可以抑制人肺癌細胞H1650的遷移能力 在顯微鏡下分別測量0、24、48、72 h時,各組細胞任意三個部位的劃痕的寬度,遷移率=[D(t=24,48,72 h)-D(t=0 h)]/D(t=0 h)。劃痕實驗結果表明:與NC組相比,在24、48、72 h時,miR-34a-mimic組遷移率明顯降低[24 h (54.51±3.15)% vs (13.07±1.12)%,P<0.05;48 h (77.51±5.35)% vs (33.24±2.23)%,P<0.05;72 h (84.22±7.12)% vs (48.21±4.27)%,P<0.01],差異有統計學意義(圖6A);與NC組相比,在24、48、72 h時,miR-34a-inhibitor組遷移率明顯增高[24 h (55.83±6.25)% vs (14.02±1.29)%,P<0.05;48 h (78.52±5.18)% vs (32.55±2.18)%,P<0.05;72 h (86.52±8.49)% vs (38.23±4.42)%,P<0.01],差異有統計學意義(圖6B),表明miR-34a可以抑制人肺癌細胞H1650的遷移能力。
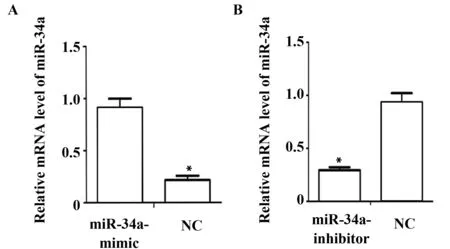
圖2 qPCR 檢測過表達和沉默miR-34a后的mRNA表達水平Fig.2 qPCR was used to detect mRNA expression levels after overexpression and silencing miR-34aNote: *.P<0.05.
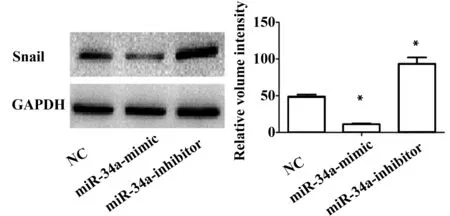
圖3 Western blot檢測細胞轉染后Snail蛋白的表達Fig.3 Expression of Snail were detected by Western blot after transfected with miR-34a-mimic and miR-34a-inhibitorNote: Error bars represent standard error.*.P<0.05.
2.8 miR-34a可以調節E-cadherin、Vimentin和Twist的表達 許多研究表明,上皮間質轉化在上皮性腫瘤的侵襲和遷移中激活,是上皮性腫瘤細胞獲得侵襲性的關鍵分子事件,在惡性腫瘤的侵襲和遷移中發揮重要的作用[14]。而E-Cadherin蛋白是上皮間質轉化過程中上皮性標志物,而Vimentin和Twist蛋白為上皮間質轉化過程中間質性標志物。
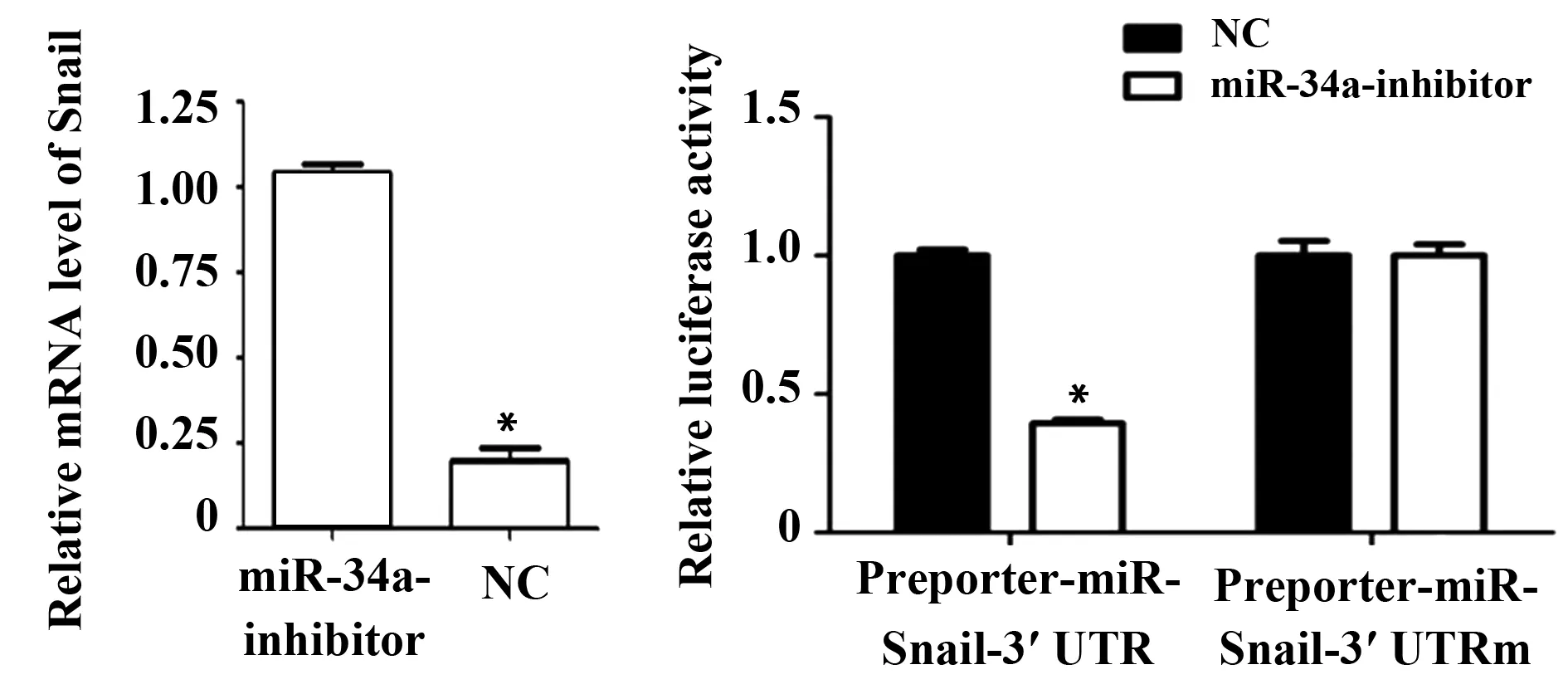
圖4 熒光素酶報告基因檢測Snail是miR-34a的直接靶點Fig.4 Luciferase report gene detects Snail direct target of miR-34aNote: *.P<0.05.
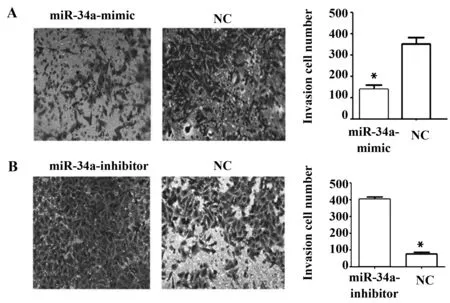
圖5 Transwell侵襲實驗檢測miR-34a對人肺癌細胞H1650侵襲能力的影響Fig.5 Effect of miR-34a on invasion ability of H1650 cells were detected by Transwell matrigel invasion assaysNote: Error bars represent standard error.*.P<0.05.
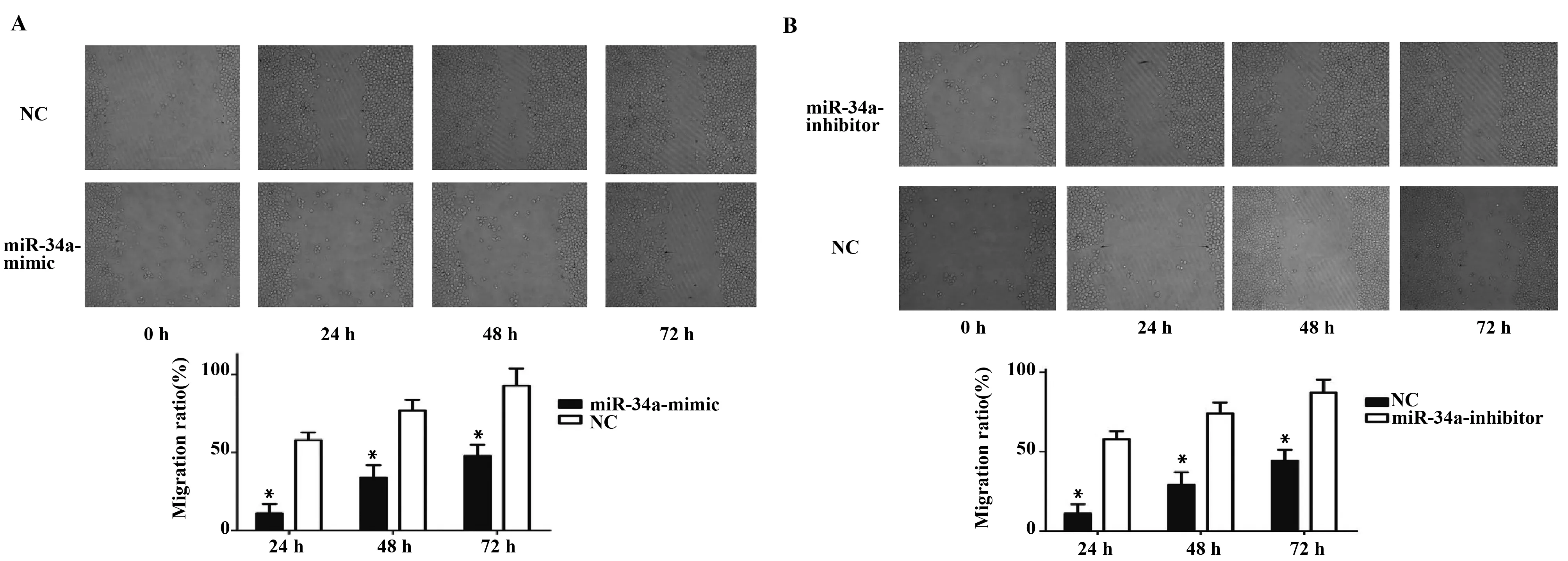
圖6 劃痕實驗檢測miR-34a-mimic和miR-34a-inhibitor對人肺癌細胞H1650遷移能力的影響Fig.6 Effeet of miR-34a on H1650 cell migration ability by wound healing a assaysNote: A.Effect of miR-34a-mimic on H1650 cells migration ability were detected by wound healing assays;B.Effect of miR-34a-inhibitor on H1650 cells migration ability were detected by wound healing assays.Error bars represent standard error.*.P<0.05.
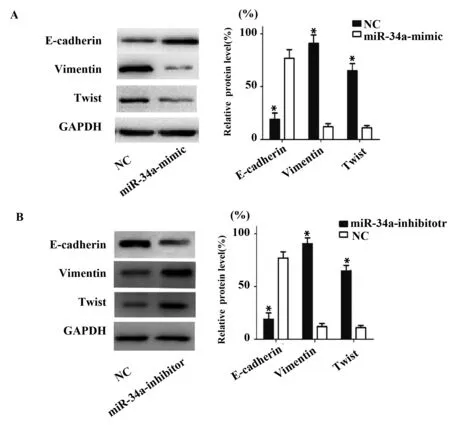
圖7 Western blot檢測過表達和沉默miR-34a后Ecadherin、Vimentin 和Twist 蛋白的表達水平Fig.7 Western blot was used to detect Ecadherin,Vimentin and Twist protein expression levels after overexpression and silencing miR-34a Note: Error bars represent standard error.*.P<0.05.
Western blot結果顯示:與NC組相比,miR-34a-mimic組中Vimentin、Twist蛋白表達水平明顯下降[Vimentin (0.12±0.01)% vs (0.82±0.06)%,Twist (0.11±0.02)% vs (0.72±0.06)%,P<0.05],但E-Cadherin表達水平明顯增高[(0.76±0.05)% vs (0.22±0.02)% ,P<0.05]。與NC組相比,miR-34a-inhibitor組中Vimentin、Twist蛋白表達水平明顯增高[Vimentin (0.13±0.01)% vs (0.92±0.08)%;Twist (0.12±0.02)% vs (0.68±0.06)%,P<0.05],但E-Cadherin表達水平明顯降低[(0.83±0.06)% vs (0.21±0.02)% ,P<0.05]。表明miR-34a可以調節E-cadherin、Vimentin和Twist的表達,表明miR-34a可以促進肺癌細胞的上皮間質轉化,見圖7。
3 討論
miRNA是一類內源性的、高度保守的非編碼單鏈RNA,廣泛存在于植物和多細胞動物的基因組中[15]。成熟的miRNA經過Drosha和Dicer酶剪切,可以與靶基因的3-UTR區結合降解或者抑制靶基因的翻譯[16]。miR-34a的編碼基因位于染色體1p36,研究表明,miR-34a受p53的直接調控,p54的低表達或者功能異常可以降低對miR-34a轉錄的刺激信號,從而降低miR-34a的表達,從而影響下游靶基因發揮生物學功能[17,18]。Garofalo等[19]使用miR-34a-mimic轉染非小細胞肺癌細胞系進行轉染,發現肺癌細胞的增殖能力明顯減弱。Gougelet等[20]對肝癌組織中miR-34a的表達分析發現,肝癌組織中miR-34a的表達水平明顯降低,且跟肝癌分期和患者不良預后密切相關。
本研究通過檢測肺癌和正常肺組織中miR-34a的表達發現,肺癌組織中miR-34a的表達明顯降低,miR-34a的表達水平隨肺癌病理分期的增加而降低;隨分化程度的降低,miR-34a的表達水平逐漸降低;有淋巴結轉移的肺癌組織中,miR-34a的表達明顯降低,表明miR-34a在肺癌組織中起抑癌作用。
上皮間質轉化發生于多種生理和病理過程中,表現為上皮標記基因的表達與細胞間黏附力的喪失,間質標記基因、細胞遷移與運動力的獲得和細胞形態的改變[21]。E-cadherin是維持上皮細胞結構極性和完整性的糖蛋白,對于維持細胞與細胞間的黏附能力非常重要。許多研究表明,E-cadherin在許多惡性腫瘤中表達低下,且E-cadherin的低表達與腫瘤侵襲、遷移和不良預后密切相關。而Snail是E-cadherin的抑制子,可以負性調節E-cadherin的表達[22]。Chen等[23]在肝癌中的研究發現,Snail高表達的腫瘤組織中,E-cadherin表達常常下降,同時與腫瘤轉移密切相關。
本研究通過使用miR-34a-mimic和miR-34a-inhibitor后,Snail表達明顯降低和提高,使用熒光素酶報告基因檢測miR-34a和Snail相互作用發現,miR-34a能與Snail的3′ UTR特異性結合。Transwell和劃痕實驗表明,miR-34a可以調控肺癌細胞的侵襲和遷移能力。Western blot檢測E-cadherin、Vimentin和Twist蛋白的表達水平發現,E-cadherin表達水平與Snail相反,但Vimentin和Twist蛋白的表達水平與Snail表達趨勢一致。
本研究表明miR-34a在肺癌組織中表達明顯降低,而且與腫瘤分期分級以及淋巴結轉移密切相關,同時發現miR-34a可以通過誘導上皮間質轉化來促進肺癌細胞的侵襲和遷移。提示miR-34a可能參與肺癌的發展過程,有可能成為預測肺癌進展和預后,以至可能成為肺癌治療的靶標。
[1] Vargas AJ,Harris CC.Biomarker development in the precision medicine era:lung cancer as a case study[J].Nat Rev Cancer,2016,16(8):525-537.
[2] Siegel RL,Miller KD,Jemal A.Cancer statistics,2015[J].CA,2015,65(1):5-29.
[3] Silva GT,Bergmann A,Thuler LCS.Incidence,associated factors,and survival in metastatic spinal cord compression secondary to lung cancer[J].Spine J,2015,15(6):1263-1269.
[4] Condamine T,Ramachandran I.Regulation of tumor metastasis by myeloid-derived suppressor cells[J].Annual Rev Med,2015,66:97.
[5] Zhao D,Besser AH,Wander SA,etal.Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation[J].Oncogene,2015,34(43):5447-5459.
[6] Heerboth S,Housman G,Leary M,etal.EMT and tumor metastasis[J].Clin Translat Med,2015,4(1):1.
[7] Burton LJ,Smith BA,Smith BN,etal.Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion,migration and osteoclastogenesis in prostate and breast cancer cells[J].Carcinogenesis,2015,36(9):1019-1027.
[8] Palma C,Grassi ML,Thomé CH,etal.Proteomic analysis of epithelial to mesenchymal transition (EMT) reveals cross-talk between SNAIL and HDAC1 proteins in breast cancer cells[J].Mole Cell Proteomics,2016,15(3):906-917.
[9] Pipan V,Zorc M,Kunej T.MicroRNA polymorphisms in cancer:a literature analysis[J].Cancers,2015,7(3):1806-1814.
[10] Wong HKA,El Fatimy R,Onodera C,etal.The cancer genome atlas analysis predicts MicroRNA for targeting cancer growth and vascularization in glioblastoma[J].Mole Ther,2015,23(7):1234-1247.
[11] Rokavec M,?ner MG,Li H,etal.IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis[J].J Clin Investiga,2015,125(3):1362-1362.
[12] Xia C,Liu Y,Lou G,etal.DNA-damaging compound 0404 effectively inhibits hepatocellular carcinoma by upregulation of miR-34a and miR-200c expression[J].Cancer Res,2015,75(15 Supplement):1645-1645.
[13] Adams BD,Wali VB,Cheng CJ,etal.MiR-34a silences c-SRC to attenuate tumor growth in triple-negative breast cancer[J].Cancer Res,2016,76(4):927-939.
[14] Katsuno Y,Lamouille S,Derynck R.TGF-β signaling and epithelial-mesenchymal transition in cancer progression[J].Curr Opin Oncol,2013,25(1):76-84.
[15] Hausser J,Zavolan M.Identification and consequences of miRNA-target interactions [mdash] beyond repression of gene expression[J].Nat Rev Genet,2014,15(9):599-612.
[16] Hausser J,Syed AP.Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation[J].Genome Res,2013,23(4):604-615.
[17] Kim HR,Roe JS,Lee JE,etal.p53 regulates glucose metabolism by miR-34a[J].Biochem Biophysical Res Communicat,2013,437(2):225-231.
[18] Chang TC,Wentzel EA,Kent OA,etal.Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis[J].Molecular Cell,2007,26(5):745-752.
[19] Garofalo M,Jeon YJ,Nuovo GJ,etal.MiR-34a/c-dependent PDGFR-α/β downregulation inhibits tumorigenesis and enhances TRAIL-induced apoptosis in lung cancer[J].PLoS One,2013,8(6):e67581.
[20] Gougelet A,Sartor C,Bachelot L,etal.Antitumour activity of an inhibitor of miR-34a in liver cancer with β-catenin-mutations[J].Gut,2015:gutjnl-2014-308969.
[21] Biddle A,Mackenzie IC.Cancer stem cells and EMT in carcinoma[J].Cancer Metastasis Rev,2012,31(1-2):285-293.
[22] De Craene B,Berx G.Regulatory networks defining EMT during cancer initiation and progression[J].Nat Rev Cancer,2013,13(2):97-110.
[23] Chen J,Chan AWH.SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3β/β-catenin signaling[J].Hepatology,2013,57(6):2287-2298.
[收稿2016-09-28 修回2017-04-20]
(編輯 許四平)
Mechanism of miR-34a on invasion and migration ability of human lung carcinoma by Snail induced EMT
LIUXing-Ren,BAIYI-Feng,LIANGLiang,FENGJing,DENGFei.
DepartmentofRespiration,SichuanProvincialPeople′sHospital,SichuanAcademyofMedicalSciences,Chengdu610072,China
Objective:To investigate the exression of miR-34a on lung cancer and normal lung tissues,and the effect and mechanism of miR-34a in lung cancer cell invasion and migration.Methods: qPCR was used to detect the expression of miR-34a on lung cancer.miR-34a-mimic and miR-34a-inhibitor were used to overexpress and knockdown miR-34a.qPCR was used to detect the effectiveness.Western blot was used to detect the expression of Snail after induced with miR-34a-mimic and miR-34a-inhibitor.Luciferase reporter gene was used to detect interaction between miR-34a and Snail.Transwell invasion assay was used to detect invasion ability after induced with miR-34a-mimic and miR-34a-inhibitor.Scratch assay was used to detect migration ability after induced with miR-34a-mimic and miR-34a-inhibitor.The expression of E-cadherin,Vimentin and Twist were detected by Western blot.Results: miR-34a expression was significantly reduced in lung cancer.With the stage of lung cancer progression,the expression of miR-34a reduced.With the differentiation of lung cancer progression,the expression of miR-34a decreased.Decreasing of miR-34a was associated with lung cancer lymph node metastasis.miR-34a-mimic and miR-34a-inhibitor could overexpress and knockdown miR-34a.miR-34a could regulate expression of Snail.Snail was the direct target of miR-34a;miR-34a could regulate the invasion ability of human lung carcinoma H1650 cells;miR-34a could regulate the migration of human lung carcinoma H1650 cells;miR-34a could regulate the expression of E-cadherin,Vimentin and Twist.Conclusion: miR-34a plays the role of tumor suppressor factor in lung cancer.miR-34a can regulate the invasion and migration ability of lung carcinoma H1650 cells by Snail induced EMT.
miR-34a;Lung cancer;EMT;E-Cadherin;Transwell;Snail
10.3969/j.issn.1000-484X.2017.05.002
①本文受國家自然科學基金(No.81301910)和四川省衛計委科研課題(No.110137)資助。
劉行仁(1980年-),男,主治醫師,主要從事肺部感染及肺部腫瘤治療方面的研究,E-mail:syyliudoc@sina.com。
R541.6 R332
A
1000-484X(2017)05-0646-06
②四川省醫學科學院·四川省人民醫院腫瘤科,成都610072。
③四川省醫學科學院·四川省人民醫院中醫科,成都610072。
④通訊作者,四川省醫學科學院·四川省人民醫院腎內科,成都610072,E-mail:dengfei_here@163.com。