丹皮酚通過下調(diào)COX-2表達(dá)及PGE2合成抑制大腸癌細(xì)胞增殖及誘導(dǎo)細(xì)胞凋亡
李 明, 譚詩云
武漢大學(xué)人民醫(yī)院消化內(nèi)科,湖北 武漢430060
丹皮酚通過下調(diào)COX-2表達(dá)及PGE2合成抑制大腸癌細(xì)胞增殖及誘導(dǎo)細(xì)胞凋亡
李 明, 譚詩云
武漢大學(xué)人民醫(yī)院消化內(nèi)科,湖北 武漢430060
目的觀察丹皮酚對大腸癌細(xì)胞增殖活性和細(xì)胞凋亡的影響,并探討丹皮酚對大腸癌的抑制作用是否與其下調(diào)COX-2表達(dá)及PGE2合成有關(guān)。方法以大腸癌LoVo細(xì)胞株為研究對象,CCK-8比色法檢測不同濃度丹皮酚或PGE2或塞來昔布處理后細(xì)胞活力及丹皮酚對PGE2刺激細(xì)胞增殖的影響;流式細(xì)胞儀檢測細(xì)胞凋亡情況;Western blotting方法檢測COX-2和凋亡相關(guān)蛋白Bax、Bcl-2、Caspase-3、Caspase-9的表達(dá)情況;ELISA法檢測大腸癌細(xì)胞上清液中PGE2含量。結(jié)果丹皮酚處理后LoVo細(xì)胞活力顯著降低,呈明顯的時(shí)間和濃度依賴性;流式細(xì)胞儀檢測結(jié)果顯示丹皮酚可誘導(dǎo)LoVo細(xì)胞凋亡;丹皮酚可降低LoVo細(xì)胞COX-2表達(dá)及上清液中PGE2含量;經(jīng)PGE2處理后LoVo細(xì)胞活力明顯增強(qiáng),丹皮酚可抑制PGE2誘導(dǎo)的LoVo細(xì)胞活力增加;經(jīng)塞來昔布處理后,LoVo細(xì)胞活力顯著降低,且細(xì)胞凋亡率增加;經(jīng)丹皮酚處理后,LoVo細(xì)胞Bax蛋白表達(dá)上調(diào),Bcl-2蛋白表達(dá)下調(diào),Pro-Caspase-3、Pro-Caspase-9表達(dá)減弱,而Cleaved-Caspase-3、Cleaved-Caspase-9表達(dá)增強(qiáng),呈濃度依賴性。結(jié)論丹皮酚可抑制LoVo細(xì)胞增殖并誘導(dǎo)細(xì)胞凋亡,可能的機(jī)制是通過下調(diào)COX-2表達(dá)及PGE2合成進(jìn)而激活線粒體凋亡途徑。
丹皮酚;大腸癌;環(huán)氧合酶-2;前列腺素E2; 凋亡
大腸癌是常見消化道惡性腫瘤。全球大腸癌發(fā)病率位居惡性腫瘤的第3位,每年有超過120萬新增病例,并有超過60萬病例死亡[1]。歐美等發(fā)達(dá)國家和地區(qū)大腸癌發(fā)病率位居惡性腫瘤的第3位,病死率則高居惡性腫瘤相關(guān)死亡的第2位[2]。在我國,每年新發(fā)大腸癌病例約13萬,并以平均4%的增幅不斷攀升[3]。大腸癌已經(jīng)成為我國發(fā)病率增長速度最快,且發(fā)病年齡逐漸年輕化的惡性腫瘤,其病死率已位居我國惡性腫瘤的第4位[4]。目前研究認(rèn)為大腸癌的發(fā)生和進(jìn)展是一個多環(huán)節(jié)、多步驟的復(fù)雜過程。盡管Wnt信號通路的異常激活被認(rèn)為是大腸癌發(fā)生的始動因素,但是在絕大多數(shù)大腸癌中高表達(dá)的環(huán)氧合酶-2(cyclooxygenase-2, COX-2)被認(rèn)為在腫瘤進(jìn)展和侵襲轉(zhuǎn)移過程中起著關(guān)鍵作用。作為前列腺素E2(PGE2)合成的限速酶,過表達(dá)的COX-2又可大量產(chǎn)生PGE2。二者共同參與腫瘤發(fā)生與進(jìn)展[5-6]。因此,COX-2可能是抗腫瘤作用的一個靶點(diǎn)。
丹皮酚(Paeonol),又稱牡丹酚,是從中草藥牡丹、芍藥根皮和徐長卿干燥根或全草中提取的小分子酚類化合物。其具有抗炎、肝腎保護(hù)、降血糖、免疫調(diào)節(jié)、心血管保護(hù)、抗菌、解熱鎮(zhèn)痛、抗過敏、抑制血小板聚集和中樞抑制作用等廣泛的藥理學(xué)活性[7-11]。在腫瘤防治方面,丹皮酚對皮膚癌、乳腺癌及肝癌等多種腫瘤細(xì)胞均有抑制作用[12-15]。然而,目前關(guān)于丹皮酚抗腫瘤作用的分子機(jī)制知之甚少,對細(xì)胞因子、基因調(diào)控和信號轉(zhuǎn)導(dǎo)通路等方面的研究還有待進(jìn)一步深入研究。本實(shí)驗(yàn)擬觀察丹皮酚對大腸癌細(xì)胞增殖和凋亡的影響,并探討丹皮酚是否通過下調(diào)COX-2表達(dá)及PGE2合成發(fā)揮抗大腸癌細(xì)胞作用。
1 材料與方法
1.1 材料結(jié)腸癌LoVo細(xì)胞由中國科學(xué)院上海細(xì)胞庫提供;主要試劑:丹皮酚購自寧波天真藥業(yè)有限公司(純度>98%);胎牛血清購自GBICO;CCK-8試劑盒購自日本同仁化學(xué);培養(yǎng)板購自Corning公司;PGE2、塞來昔布購自sigma公司;凋亡試劑盒購自BD公司;PGE2ELISA試劑盒購自美國BPB公司;COX-2、Bax、Bcl-2、Pro-Caspase-3/9、Cleaved-Caspase-3/9及β-actin抗體購自Cell Signaling Technology公司。DMEM/F-12培養(yǎng)液、辣根酶標(biāo)記山羊抗兔或山羊抗鼠IgG購自碧云天生物技術(shù)研究所。
1.2 方法
1.2.1 細(xì)胞培養(yǎng):用10%胎牛血清的DMEM/F-12培養(yǎng)基培養(yǎng)LoVo細(xì)胞,將細(xì)胞培養(yǎng)瓶置于恒溫37 ℃,體積分?jǐn)?shù)5% CO2,飽和濕度的培養(yǎng)箱中。取對數(shù)期的細(xì)胞進(jìn)行后續(xù)實(shí)驗(yàn)。
1.2.2 CCK-8比色法檢測細(xì)胞活力:取對數(shù)生長期的細(xì)胞,消化、離心、洗滌后用培養(yǎng)基重懸,利用細(xì)胞計(jì)數(shù)板計(jì)數(shù),調(diào)整細(xì)胞濃度為5×104/ml;取96孔板3塊,分別標(biāo)記為d1、d2、d3,將細(xì)胞懸液以每孔200 μl接種至3塊96孔板中,放置培養(yǎng)箱中孵育過夜;細(xì)胞貼壁后,分為對照組和實(shí)驗(yàn)組,實(shí)驗(yàn)組分別加入實(shí)驗(yàn)設(shè)計(jì)好的處理藥物,對照組加入等量的細(xì)胞培養(yǎng)基,并設(shè)置空白組。每組設(shè)5個復(fù)孔,放置培養(yǎng)箱中分別培養(yǎng)24、48和72 h。每個處理時(shí)間截止前2 h取出培養(yǎng)板,每孔加入20 μl的CCK-8試劑,再置于細(xì)胞培養(yǎng)箱中繼續(xù)孵育2 h,后取出培養(yǎng)板,用酶標(biāo)儀測定各孔在450 nm處的吸光度值(A值),根據(jù)公式計(jì)算細(xì)胞活力=(處理組A值-空白組A值)/(對照組A值-空白組A值)×100%。
1.2.3 PI/Annexin V檢測細(xì)胞凋亡率:取對數(shù)生長期細(xì)胞,消化、離心、洗滌后用培養(yǎng)基重懸,利用細(xì)胞計(jì)數(shù)板計(jì)數(shù),調(diào)整細(xì)胞濃度為1×105個/ml,將細(xì)胞懸液接種至6孔板內(nèi),每孔2 ml,放置培養(yǎng)箱中孵育過夜;細(xì)胞貼壁后,分別加入預(yù)設(shè)計(jì)藥物濃度處理48 h。取出6孔培養(yǎng)板,消化、洗滌、收集細(xì)胞,根據(jù)凋亡試劑盒操作說明進(jìn)行操作,每管加入緩沖液500 μl將各組細(xì)胞重懸,再根據(jù)要求加入PI及Annexin V試劑各5 μl,避光室溫孵育15 min,流式細(xì)胞儀檢測細(xì)胞凋亡率。
1.2.4 ELISA法檢測PGE2:按照預(yù)定分組處理24 h,吸取培養(yǎng)基離心后將上清液移至高壓消毒的EP管中備用。加入標(biāo)準(zhǔn)品至酶標(biāo)板中,設(shè)置梯度,分別取各組上清液樣品100 μl,加入至酶標(biāo)板中,再分別將每孔加入酶標(biāo)液50 μl,室溫放置90 min。具體操作步驟根據(jù)試劑盒要求進(jìn)行,根據(jù)標(biāo)準(zhǔn)品曲線計(jì)算樣品中PGE2濃度。
1.2.5 Western blotting檢測COX-2、Bax、Bcl-2、Pro-Caspase-3/9、Cleaved-Caspase-3/9蛋白表達(dá):取對數(shù)生長期細(xì)胞,消化、離心、洗滌后用培養(yǎng)基重懸,利用細(xì)胞計(jì)數(shù)板計(jì)數(shù),調(diào)整細(xì)胞濃度為1×105個/ml,將細(xì)胞懸液接種至細(xì)胞培養(yǎng)皿內(nèi),放置培養(yǎng)箱中孵育過夜,待細(xì)胞貼壁后,分別加入0、30、60、120 mg/L的丹皮酚處理48 h。消化、離心、洗滌、收集各組細(xì)胞,每管加入200 μl預(yù)冷蛋白裂解液,4 ℃條件下裂解30 min,再行超聲裂解;4 ℃條件下12 000 r/min離心10 min,將上清液小心移至經(jīng)高壓消毒的EP管內(nèi),分裝標(biāo)記后-80 ℃儲存。根據(jù)蛋白測定試劑盒說明書測定提取細(xì)胞總蛋白質(zhì);取40 μg樣品行SDS-PAGE電泳,然后轉(zhuǎn)膜至PVDF膜,5% BSA封閉1 h,根據(jù)蛋白分子量切割條帶,分別加入COX-2、Bax、Bcl-2、Pro-Caspase-3/9、Cleaved-Caspase-3/9一抗,4 ℃搖床上孵育過夜;加入TBST洗膜,再加入說明書要求稀釋的二抗,室溫下孵育2 h,再次TBST洗膜,ECL顯色、曝光、顯影、定影、拍照并分析數(shù)據(jù)。
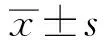
2 結(jié)果
2.1 丹皮酚對LoVo細(xì)胞活力的影響如圖1所示,15、30、60、120、240 mg/L的丹皮酚處理LoVo細(xì)胞后,與對照組(0 mg/L)比較,細(xì)胞存活率逐漸降低,呈時(shí)間和濃度依賴性,藥物濃度越高、作用時(shí)間越長,其細(xì)胞存活率越低。

注:與對照組(0 mg/L丹皮酚)比較,*P<0.05。
圖1 丹皮酚對LoVo細(xì)胞活力的影響
Fig 1 The effect of Paeonol on the cell survival in LoVo colorectal cancer cells
2.2 丹皮酚對LoVo細(xì)胞凋亡率的影響將30、60、120 mg/L的丹皮酚處理后的LoVo細(xì)胞用PI/Annexin V雙染標(biāo)記,再行流式細(xì)胞儀檢測。如圖2所示,丹皮酚處理后,細(xì)胞凋亡率從15.4%上升至38.6%,而對照組凋亡率僅為0.6%。

圖2 丹皮酚對LoVo細(xì)胞凋亡率的影響
A:0 mg/L; B:30 mg/L; C:60 mg/L; D:120 mg/L
Fig 2 The effect of Paeonol on the cell apoptosis in LoVo colorectal cancer cells
2.3 丹皮酚對LoVo細(xì)胞COX-2表達(dá)及PGE2合成的影響如圖3A所示,30、60、120 mg/L的丹皮酚分別處理LoVo細(xì)胞48 h后,Western blotting 結(jié)果顯示隨著丹皮酚濃度的升高,COX-2蛋白表達(dá)量逐漸降低。濃度為30、60、120 mg/L的丹皮酚分別處理LoVo細(xì)胞48 h后對細(xì)胞上清液中PGE2濃度的影響,結(jié)果發(fā)現(xiàn)隨著藥物濃度的增加,細(xì)胞上清液中PGE2濃度明顯降低(見圖3B),與對照組比較,差異均有統(tǒng)計(jì)學(xué)意義(P<0.05)。

注:與對照組(0 mg/L丹皮酚)比較,#P<0.05。
圖3 丹皮酚對LoVo細(xì)胞COX-2表達(dá)及PGE2合成的影響
A:丹皮酚下調(diào)LoVo細(xì)胞COX-2表達(dá);B:丹皮酚降低LoVo細(xì)胞上清液中PGE2含量
Fig 3 The effect of Paeonol on the expression of COX-2 and synthesis of PGE2
2.4 PGE2對LoVo細(xì)胞增殖的影響不同濃度(5、10、20 μmol/L) PGE2處理LoVo細(xì)胞24 h后,隨著PGE2濃度的增加,LoVo細(xì)胞增殖作用也增加(見圖4A)。再選用10 μmol/L PGE2觀察PGE2作用不同時(shí)間對LoVo細(xì)胞增殖作用,發(fā)現(xiàn)PGE2作用48 h對LoVo細(xì)胞增殖促進(jìn)效果最佳(見圖4B)。
2.5 丹皮酚對PGE2促進(jìn)LoVo細(xì)胞增殖效應(yīng)的影響如圖5所示,10 μmol/L PGE2作用LoVo細(xì)胞48 h可明顯促進(jìn)細(xì)胞增殖,而給予60、120 mg/L丹皮酚后均可降低PGE2對LoVo細(xì)胞增殖的促進(jìn)效應(yīng),且120 mg/L丹皮酚抑制作用更為明顯。
2.6 COX-2特異性抑制劑塞來昔布對結(jié)直腸癌細(xì)胞增殖的影響如圖6所示,25、50、100 μmol/L的塞來昔布分別作用24、48、72 h對結(jié)直腸癌細(xì)胞系LoVo細(xì)胞增殖均有抑制作用,且呈現(xiàn)明顯的濃度依賴效應(yīng)及時(shí)間依賴效應(yīng)。
2.7 COX-2特異性抑制劑塞來昔布對LoVo細(xì)胞凋亡的影響將25、50、100 μmol/L的塞來昔布處理LoVo細(xì)胞48 h后,用PI/Annexin V雙染標(biāo)記,再行流式細(xì)胞儀檢測。如圖7所示,細(xì)胞凋亡率逐漸升高。

注:與對照組(0 μmol/L PGF2)比較,#P<0.05。
圖4 不同濃度PGE2處理不同時(shí)間對LoVo細(xì)胞增殖的影響
A:0、5、10、20 μmol/L PGE2分別處理LoVo細(xì)胞24 h后對細(xì)胞活力的影響;B:10 μmol/L PGE2處理LoVo細(xì)胞0、24、48、72 h后對細(xì)胞活力的影響
Fig 4 The effect of PGE2with different concentrations, different time on the cell survival in LoVo colorectal cancer cells

注:與空白組比較,*P<0.05;與單獨(dú)PGE2組比較,#P<0.05。
圖5 丹皮酚抑制PGE2誘導(dǎo)的LoVo細(xì)胞增殖效應(yīng)
Fig 5 Paeonol could inhibit PGE2-induced proliferation of LoVo cells
2.8 丹皮酚對LoVo細(xì)胞凋亡相關(guān)蛋白Bax、Bcl-2、Caspase-3/9表達(dá)的影響Western blotting結(jié)果顯示(見圖8),丹皮酚作用48 h后,LoVo細(xì)胞Bax蛋白表達(dá)隨著藥物濃度的升高也逐漸增加,而Bcl-2蛋白表達(dá)量則逐漸降低,Pro-Caspase-3、Pro-Caspase-9表達(dá)減弱,而Cleaved-Caspase-3、Cleaved-Caspase-9表達(dá)增強(qiáng),呈濃度依賴性。

注:與對照組(0 μmol/L賽來昔布)比較,#P<0.05。
圖6 COX-2特異性抑制劑塞來昔布對LoVo細(xì)胞增殖的影響
Fig 6 The effect of Celecoxib on the cell proliferation in LoVo colorectal cancer cells

圖7 塞來昔布對細(xì)胞LoVo細(xì)胞凋亡的影響
A: 0 μmol/L; B: 25 μmol/L; C: 50 μmol/L; D:100 μmol/L
Fig 7 The effect of Celecoxib on the apoptosis in LoVo colorectal cancer cells
3 討論
前期研究發(fā)現(xiàn),丹皮酚對肝癌等多種腫瘤細(xì)胞均有抗腫瘤作用。Chou等[16]研究發(fā)現(xiàn)丹皮酚可抑制角叉菜聚糖誘導(dǎo)的大鼠熱覺過敏反應(yīng),減少大鼠爪部皮膚滲出液中中性粒細(xì)胞浸潤和TNF-α、IL-β、IL-10、COX-2、PGE2的含量。Chae等[17]研究發(fā)現(xiàn)丹皮酚可顯著拮抗脂多糖誘導(dǎo)的炎癥反應(yīng)。但是,丹皮酚對于腫瘤中特別是大腸癌中COX-2的作用及其意義并未報(bào)道,因此,本研究以COX-2及其產(chǎn)物PGE2為切入點(diǎn),對丹皮酚在大腸癌中增殖和凋亡中的作用及其作用機(jī)制進(jìn)行探討。

圖8 丹皮酚對LoVo細(xì)胞凋亡相關(guān)蛋白Bax、Bcl-2、Caspase-3、Caspase-9表達(dá)的影響
Fig 8 The effect of Paeonol on the expressions of Bax, Bcl-2, Caspase-3 and Caspase-9 in LoVo colorectal cancer cells
本實(shí)驗(yàn)CCK-8細(xì)胞活性實(shí)驗(yàn)結(jié)果顯示,丹皮酚處理可降低細(xì)胞活力,呈時(shí)間和濃度依賴性,藥物濃度越高、作用時(shí)間越長,其細(xì)胞存活率越低。丹皮酚處理LoVo細(xì)胞48 h后,可顯著促進(jìn)細(xì)胞凋亡,呈劑量依賴性。這些結(jié)果提示丹皮酚可能通過抑制細(xì)胞增殖并誘導(dǎo)細(xì)胞凋亡發(fā)揮抗腫瘤作用。細(xì)胞凋亡是程序化、多基因調(diào)控的細(xì)胞死亡過程,細(xì)胞凋亡機(jī)制在維持機(jī)體內(nèi)環(huán)境穩(wěn)定中扮演著重要角色,是重要生理調(diào)控機(jī)制[18-19];細(xì)胞過度增殖和凋亡不足在腫瘤發(fā)生、發(fā)展中具有重要意義[20],因此通過誘導(dǎo)細(xì)胞凋亡可能是腫瘤治療的有效途徑之一。本研究進(jìn)一步探討了丹皮酚對細(xì)胞凋亡線粒體途徑的影響,丹皮酚處理48 h后,Western blotting檢測結(jié)果顯示LoVo細(xì)胞的Bcl-2表達(dá)下調(diào),Bax表達(dá)上調(diào),Pro-Caspase-3、Pro-Caspase-9表達(dá)減弱,而Cleaved-Caspase-3、Cleaved-Caspase-9表達(dá)增強(qiáng),且呈劑量依賴性。
COX-2在包括大腸癌在內(nèi)的多種腫瘤細(xì)胞中均呈過表達(dá)狀態(tài)。而作為PGE2合成的限速酶,過表達(dá)的COX-2又可產(chǎn)生大量PGE2。二者共同參與腫瘤的發(fā)生與發(fā)展。下調(diào)COX-2及PGE2表達(dá)后可促進(jìn)細(xì)胞凋亡、抑制血管新生、促進(jìn)細(xì)胞免疫而發(fā)揮抗腫瘤作用。因此,COX-2可能是抗腫瘤作用的一個靶點(diǎn)。流行病學(xué)研究已經(jīng)證實(shí),非甾體類抗炎藥(nonsteroidal anti-inflammatory drugs, NSAIDs)或COX-2特異性抑制劑在一定程度上可預(yù)防胃腸道腫瘤[21]。三項(xiàng)隨機(jī)對照研究(PreSAP、APC、Approve)評估了COX-2特異性抑制劑在散發(fā)結(jié)腸腺瘤患者復(fù)發(fā)中的預(yù)防作用及安全性,結(jié)果發(fā)現(xiàn)三項(xiàng)研究中均可有效降低結(jié)腸腺瘤的復(fù)發(fā),特別是降低了進(jìn)展期結(jié)腸腺瘤(advanced adenomas)的復(fù)發(fā)率[22-24]。然而,長期應(yīng)用此類藥物可導(dǎo)致胃腸道甚至一系列心血管毒性等不良反應(yīng)發(fā)生,限制了其在健康人群中的應(yīng)用,臨床推薦僅對特殊家族性腺瘤息肉病等特殊人群使用。因此,尋找毒性小,而又能有效抑制COX-2的藥物可為大腸癌治療提供更好的選擇,也可能為大腸癌的預(yù)防和治療提供更好的策略。研究證實(shí)過表達(dá)的COX-2可通過減弱TGF-β的抑制細(xì)胞增殖效應(yīng)促進(jìn)細(xì)胞增殖[25];同時(shí),COX-2可調(diào)控PI3K信號通路及Bcl-2表達(dá)抑制細(xì)胞凋亡,也可通過誘導(dǎo)P53基因突變及調(diào)控Fas蛋白減弱凋亡信號發(fā)揮抗細(xì)胞凋亡作用[26-27]。本實(shí)驗(yàn)中,丹皮酚能抑制LoVo細(xì)胞COX-2表達(dá)及PGE2合成,PGE2處理后可促進(jìn)LoVo細(xì)胞的增殖效應(yīng),且丹皮酚可抑制PGE2促進(jìn)LoVo細(xì)胞的增殖效應(yīng)。此外,COX-2特異性抑制劑處理后也可觀察到細(xì)胞增殖抑制及細(xì)胞凋亡增加。這些研究結(jié)果提示丹皮酚可能通過抑制COX-2及PGE2合成抑制LoVo細(xì)胞增殖并誘導(dǎo)細(xì)胞凋亡。
綜上所述,本研究發(fā)現(xiàn),丹皮酚在體外能抑制LoVo細(xì)胞增殖并誘導(dǎo)其凋亡,可能的機(jī)制是抑制COX-2表達(dá)和PGE2合成,進(jìn)而激活線粒體凋亡途徑。
[1]Jemal A, Bray F, Center MM, et al. Global cancer statistics [J]. CA Cancer J Clin, 2011, 61(2): 69-90.
[2]Siegel R, Naishadham D, Jemal A.Cancer statistics, 2013 [J]. CA Cancer J Clin, 2013, 63(1): 11-30.
[3]Center MM, Jemal A,Ward E. International Trends in Colorectal Cancer Incidence Rates [J]. Cancer Epidemiol Biomarkers Prev, 2009, 18(6):1688-1694.
[4]Ganapathi S, Kumar D, Katsoulas N, et al. Colorectal cancer in the young: trends,charactistics and outcome [J]. Int J Colorectal, 2011, 26(7): 927-934.
[5]Wang D, Wang H, Brown J, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer [J]. J Exp Med, 2006, 203(4): 941-951.
[6]Chell S, Kaidi A, Williams AC, et al. Mediators of PGE2synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer [J]. Biochim Biophys Acta, 2006, 1766(1): 104-119.
[7]Park J, Kim HY, Lee SM. Protective effects of moutan cortex radicis against acute hepatotoxicity [J]. Afr J Tradit Complement Altern Med, 2011, 8(5): 220-225.
[8]Hu S, Shen G, Zhao WG, et al. Paeonol, the main active principles of Paeonol moutan,ameliorates alcoholic steatohepatitis in mice [J]. J Ethnopharmacol, 2010, 128(1): 100-106.
[9]Lee H, Lee G, Kim H, et al. Paeonol, a major compound of Moutan Cortex, attenuates cisplatin-induced nephrotoxicity in mice [J]. Evid-Based ComplAlt, 2013, 2013: 310989.
[10]Fu PK, Wu CL, Tsai TH, et al. Anti-inflammatory and anticoagulative effects of Paeonol on LPS-induced acute lung injury in rats [J].Evid-Based ComplAlt, 2012, 2012: 837513.
[11]ThiHa D, Trung TN, HienIT, et al. Selected compounds derived from Moutan Cortex stimulated glucose uptake and glycogen synthesis via AMPK activation in human Hep G2 cells [J]. J Ethnopharmacol, 2010, 131(2): 417-424.
[12]Horng CT, Shieh PC, Tan TW, et al. Paeonol suppresses chondrosarcoma metastasis through up-regulation of miR-141 by modulating PKCδ and c-Src signaling pathway [J]. Int J Mol Sci, 2014, 15(7): 11760-11772.
[13]Peng LH, Xu SY, Shan YH, et al. Sequential release of salidroside and paeonol from a nanosphere-hydrogel system inhibits ultraviolet B-induced melanogenesis in guinea pig skin [J]. Int J Nanomed, 2014, 9: 1897-908.
[14]Lin X, Yi Z, Diao J, Shao M, et al. ShaoYao decoction ameliorates colitis-associated colorectal cancer by downregulating proinflammatory cytokines and promoting epithelial-mesenchymal transition [J]. J Transl Med, 2014, 12: 105.
[15]Cai J, Chen S, Zhang W, et al. Paeonol reverses paclitaxel resistance in human breast cancer cells by regulating the expression of transgelin 2 [J]. Phytomedicine, 2014, 21(7): 984-991.
[16]Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia [J]. Br J Pharmacol, 2003, 139(6): 1146-52.
[17]Chae HS, Kang OH, Lee YS, et al. Inhibition of LPS-induced iNOS, COX-2 and inflammatory mediator expression by paeonol through the MAPKs inactivation in RAW 264.7 cells [J]. Am J Chin Med, 2009, 37(1): 181-194.
[18]Modjtahedi H, Cho BC, Michel MC, et al. A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer [J]. Naunyn Schmiedebergs Arch Pharmacol, 2014, 387(6): 505-21.
[19]Moran AE, Hunt DH, Javid SH, et al. Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6JMin/+ mice [J]. J Biol Chem, 2004, 279(41):43261-43272.
[20]Roberts RB, Min L, Washington MK, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis [J]. Proc Natl Acad Sci U S A, 2002, 99(3): 1521-1526.
[21]Marnett LJ, DuBois RN. COX-2: atarget for colon cancer prevention [J]. Annu Rev Pharmacol Toxicol, 2002, 42: 55-80.
[22]McLean MH, Murray GI, Fyfe N, et al. COX-2 expression in sporadic colorectal adenomatous polyps is linked to adenoma characteristics [J]. Histopathology, 2008, 52(7): 806-815.
[23]Ogino S, Kirkner GJ, Nosho K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer [J]. Clin Cancer Res, 2008, 14(24): 8221-8227.
[24]Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2 [J]. Annu Rev Pharmacol Toxicol, 1998, 38: 97-120.
[25]Milella M, Gelibter A, Di Cosimo S, et al. Pilot study of celecoxib and infusional 5-fluorouracil as second-line treatment for advanced pancreatic carcinoma [J]. Cancer, 2004, 101(1): 133-138.
[26]Young LE, Sanduja S, Bemis-Standoli K, et al. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis [J]. Gastroenterology, 2009, 136(5):1669-1679.
[27]Daikoku T, Hirota Y, Tranguch S, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice [J]. Cancer Res, 2008, 68(14): 5619-5627.
(責(zé)任編輯:馬 軍)
Paeonol inhibits cell proliferation and induces cell apoptosis through down regulating the expression of COX-2 and PGE2synthesis in LoVo colorectal cancer cells
LI Ming, TAN Shiyun
Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan 430060, China
Objective To observe the effect of Paeonol on cell proliferation and apoptosis, and investigate whether its antitumor effect is associated with down regulation of COX-2 expression and PGE2synthesis in colorectal cancer cells. Methods The LoVo colorectal cancer cells served as the experimental subjects. After treatment with Paeonol at different concentrations respectively at different time, the cell survival of LoVo was determined by the CCK-8 method. LoVo cells were treated with PGE2and with or without Paeonol for different time and the cell survival was determined. After treatment with Paeonol at different concentrations for 48 hours, cell apoptosis was detected by flow cytometry. Western blotting was used to detect espressions of COX-2, Bax, Bcl-2, Caspase-3 and Caspase-9 proteins. The expression of PGE2in the culture medium was detected by ELISA. Results After treatment with Paeonol, the proliferation of LoVo cells was significantly inhibited, which showed a dose-and time-dependent manner. Flow cytometry assays demonstrated that Paeonol significantly induced apoptosis of LoVo cells. Paeonol reduced cyclooxygenase-2 (COX-2) expression and prostaglandin E2(PGE2) synthesis in LoVo cells. PGE2promoted the cell proliferation and Paeonol inhibited PGE2-induced proliferation of LoVo cells. Celecoxib inhibited cell proliferation and induced apoptosis of LoVo cells. Increasing doses of Paeonol increased expression of Bax and decreased expression of anti-apoptotic Bcl-2 attenuated expressions of Pro-Caspase-3 and Pro-Caspase-9, strengthened expressions of Cleaved-Caspase-3 and Cleaved-Caspase-9. Conclusion Paeonol can inhibit cell proliferation and induce apoptosis of colorectal cancer cells, which is associated with down requlation of COX-2 espression and PGE2synthsis.
Paeonol; Colorectal cancer; Cyclooxygenase-2; Prostaglandin E2; Apoptosis
10.3969/j.issn.1006-5709.2017.02.003
李明,主治醫(yī)師,博士,研究方向:消化道腫瘤的防治。E-mail:liming870221@sina.com
譚詩云,醫(yī)學(xué)博士,主任醫(yī)師,教授,博士研究生導(dǎo)師,研究方向:消化道腫瘤的防治。E-mail:tanshiyun1962@163.com
R735.3+4
A 文章編號:1006-5709(2017)02-0128-06
2016-07-31

