Synthesis and Properties of Guanidinium Salts of 5-(3-Aminofurazan-4-yl)tetrazol-1-ol
ZHAI Lian-jie, WANG Bo-zhou, BI Fu-qiang, HUO Huan, LI Ya-nan, FAN Xue-zhong
(1.Xi′an Modern Chemistry Research Institute, Xi′an 710065, China;2.State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi′an 710065, China)
Synthesis and Properties of Guanidinium Salts of 5-(3-Aminofurazan-4-yl)tetrazol-1-ol
ZHAI Lian-jie1,2, WANG Bo-zhou1,2, BI Fu-qiang1,2, HUO Huan1,2, LI Ya-nan1,2, FAN Xue-zhong1
(1.Xi′an Modern Chemistry Research Institute, Xi′an 710065, China;2.State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi′an 710065, China)
The guanidinium (2), diaminoguanidinium (3), and guanidylguanidinium (4) salts of 5-(3-aminofurazan-4-yl)tetrazol-1-ol (1) were synthesized with a high yield using malononitrile as raw material, and their structures were characterized by IR, NMR, elemental analysis and thermal analysis. The single crystals of salts 2 and 3 were cultured and their structures were tested by X-ray single-crystal diffraction. The impact and friction sensitivities of three kinds of guanidinium salts were tested using the drop hammer method and friction sensitivity tester,and their thermal decomposition process was studied by DSC. The results show that three kinds of guanidinium salts exhibit the impact sensitivity greater than 24J and friction sensitivity greater than 360N and decomposition temperature between 266 and 277℃, revealing good thermal stability.
nitrogen-rich salts;furazan compound;guanidinium salt;azole compound;X-ray single-crystal diffraction
Introduction
The rational design of new energetic materials with high properties has been one of the most challenging tasks in the field of advanced materials[1]. Recent studies on C-C connected heterocycles based on 3- or 4-substituted furazans or furoxans revealed excellent characteristics regarding stability and detonation properties. Examples for these kinds of molecules are 4-amino-3-(5-tetrazole)furazan(furoxan)[2-3], 3,4-bis(1H-5-tetrazolyl)furazan(furoxan)[4-5], 3,4-bis(1-hydroxytetrazolyl)furazan(furoxan)[6], 3,3’-diamino-4,4’-bisfurazan(furoxan)[7-8], and recently published 3-nitroamino-4-(5-nitroamino-1,2,4-oxadiazole- 3-yl)furazan and 3,5-bis(4-nitroaminofurazan- 3-yl)-1,2,4-oxadiazole[9], etc. The C-C combination of 3- or 4-substituted furazans or furoxans with a tetrazole orN-hydroxy-tetrazole moiety benefits from the energetic tetrazole ring, the higher stability of the furazan ring, and the second carbon atom of the furazan or furoxan, which can be substituted with various energetic groups like amino, nitro, nitramino, or azido. Furthermore, the tetrazole or N-hydroxy-tetrazole moieties carry an acidic proton, thus enabling the formation of energetic salts by Brφnsted acid-base or salt metathesis reactions. The cations can be either nitrogen-rich bases like ammonia, hydrazine, and guanidines, or various metals, mainly alkali, alkaline earth.
In our continuing efforts to seek more powerful, less sensitive, eco-friendly and low-cost energetic materials, we were interested in some furazan-functionalized azolate-based (triazole, tetrazole and its N-oxides) salts that contain a high percentage of both oxygen and nitrogen. In this paper, we report our results on the synthesis, full analytical and spectroscopic characterization, energetic properties and X-ray structures of nitrogen-rich salts of the 5-(3-aminofurazan-4-yl)tetrazol-1-olate; the salts are less sensitive to impact and friction as well as display significant physical and energetic properties.
1 Experimental
1.1 Materials
Compounds 4-aminofurazan-3-carboxamidoxime and 4-aminofurazan-3-chloroxime were prepared with a high yield according to the literature procedure[10-11]. Other chemicals were analytically pure and obtained from commercial sources.
1.2 Synthesis
The synthetic pathway to the new energetic salts is shown in Fig.1.
1.2.1 5-(3-aminofurazan-4-yl)tetrazol-1-ol (1)
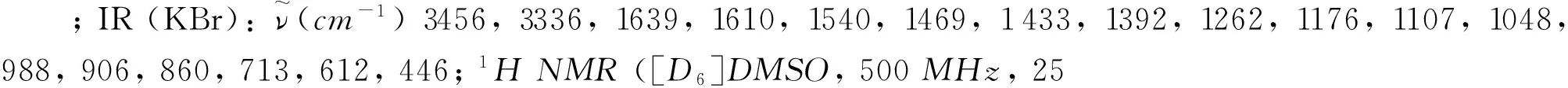
1.2.2Guanidinium5-(3-aminofurazan-4-yl)tetrazol-1-ol-ate(2)

1.2.3Diaminoguanidinium5-(3-aminofurazan-4-yl) -tetrazol-1-olate(3)

1.2.4Guanidylguanidinium5-(3-aminofurazan-4-yl) -tetrazol-1-olate(4)

1.3Determinationofperformance
Themeltinganddecompositionpoints(onsettemperature)wereobtainedonadifferentialscanningcalorimeter(TAInstrumentsCompany,ModelDSC-Q200)ataflowrateof50mL/min.About0.3mgofthesamplewassealedinaluminumpansforDSC.InfraredspectrawereobtainedfromKBrpelletsonaNicoletNEXUS870Infraredspectrometerintherangeof4000-400cm-1.Elementalanalyses(C,HandN)wereperformedonaVARI-El-3elementaryanalysisinstrument.ThesensitivitytoimpactstimuliwasdeterminedbyfallingHammerapparatus,applyingastandardmethodusinga2kgdropweight.ThefrictionsensitivityofthecompoundwasdeterminedusingaJuliusPetersapparatus.Thesamplemassusedforeachtestisabout20mg.
1.4CrystallographicMeasurements
Singlecrystalsfor2and3areobtainedfromtheaqueoussolutions,andX-raydiffractiondatawerecollectedwithaBrukerSMARTApexIICCDX-raydiffractometerequippedwithagraphite-monochromatizedMoKαradiation(λ= 0.071073nm).ThestructuresweresolvedeitherwithSHELXS-97[12],refinedwithSHELXL-97[13].Thefull-matrixleast-squaresrefinementonF2includedatomiccoordinatesandanisotropicthermalparametersforallnon-Hatoms.TheHatomswerefoundandrefined.CCDC-1444343 (for2),and-1444344 (for3)containthesupplementarycrystallographicdataforthispaper.
2 Results and Discussion
2.1Synthesisprocedure
4-Aminofurazan-3-carboxamidoximewasreadilysynthesizedthroughthereactionofmalononitrile,sodiumnitrite,andhydroxylamine,andwasthentreatedwithsodiumnitriteinaqueousHCltogivethechloroxime[10-11].ThechlorineatomsaresubstitutedbysodiumazideinDMFtoform4-aminofurazan-3-azidoglyoxime,whichisconvertedtotheneutralcompound1indiethylethercatalyzedbyHClgas[14].Inordertoovercometheproblemofisolatingthesensitive4-aminofurazan-3-azidoglyoximeandincreasetheyieldofcompound1,afacileone-potreactionisemployedtosafelyandeasilyperformthecyclizationreactionstartingfrom4-aminofurazan-3-carbohydroxamoylchlorideinvolvingthechloro-azidoexchangeinDMFandsubsequentcyclization,whereasthewholemixtureispouredontoicewater,extractedintodiethyletherandHClgasisbubbledthroughitaffordingtheneutralcompound1.Preparationofthecorrespondingsaltsof2-4waseasilyaccomplishedbydiluting1inmethanolandadditionofoneequivalentofthecorrespondingorganicbase.Thisstepbenefitsfromtheverypoorsolubilityoftheionictargetmolecules,contrarytotheneutralones,whichdissolvereadilyinmethanol.Precipitationofthedesiredioniccompoundsoccurredalmostquantitativeandledtohighpurities.
2.2X-Raycrystallography
Compound2·H2Ocouldonlybeobtainedinthecrystallineformwithinclusionofonecrystalwatermolecule.ItcrystallizesincolorlessplateletsinthetriclinicspacegroupP-1withtwoformulaunitsintheunitcellandadensityof1.598g/cm3at293K.AsshowninFig.2,the1-hydroxytetrazoleringandthefuroxanringarenearlyplanarwithadihedralanglebetweenthemof1.6°.TheC-NandN-NsingleanddoublebondswithintheazoleringsareallintherangeofformalC-NandN-Nsingleanddoublebonds(C-N: 0.147nm, 0.122nm;N-N: 0.148nm, 0.120nm)[15].TheN-Obondlength[O2-N7: 0.1312(22)nm]oftheN-hydroxygroupissilghtlyshorterthantheN-Odistancesinthefurazanring[O1-N3: 0.1374nm,O1-N2: 0.1403nm].ThetransferoftheprotonfromtheN-hydroxytetrazoletoaminewasconfirmedbythecrystaldata.Sincetheaminoisexcellenthydrogen-bondingdonors,thediscrete2arelinkedintoa2Ddoublelayerbythehydrogen-bondinginteractionsbetweenguanidiniumcationsand1anions[N1-H1A…O2i: 0.2913nm;N9-H9B…N3ii: 0.3249nm.Symmetrycodes:i: -x-1, -y+1, -z+1;] (Fig.2).The3Dnetworkofthestructureisformedfurtherbythehydrogen-bondinginteractionsfromwatermoleculesandadjacentlayers.
Diaminoguanidinium salt 3 crystallizes in the monoclinic space group Cc with four formula units in the unit cell. The calculated density of 3 (1.643 g/cm3) is significantly higher than that found for 2·H2O (1.598 g/cm3). As can be seen from Fig.3, the asymmetric unit contains one independent 1 anion and one diaminoguanidinium cation, in which proton transfer from theN-hydroxy tetrazole to ammonia was also confirmed. The coordination geometry of a single anion consists of interactions with four diaminoguanidinium cations and three 1 anions.
2.3 Thermal behavior
The thermal behaviors of 2-4 are determined by DSC at a heating rate of 10℃/min and their DSC curves are depicted in Fig.4.
All salts melt prior to decomposition with melting points ranging from 163℃ for 3 to 223℃ for 4. Moreover, the salts exhibit excellent thermal stabilities with thermal decomposition temperatures (Td) in the range of 266 to 277℃, which are higher than that of 1,3,5-trinitro-1,3,5-triazinane (RDX) (Td=210℃). Guanidylguanidinium salt 4 is the most thermally stable (Td=277℃), which most likely arises from four strong hydrogen bonds. For guanidinium salt 2, its DSC curve shows a small endothermic peak at 95℃, corresponding to the loss of crystal water.
2.4 Sensitivities
The nitrogen-rich salts are quite insensitive explosives. The impact sensitivities of 2-4 are more than 24J, possibly due to the extensive hydrogen-bonding interactions between the cations and anions after salt formation. Friction sensitivity is also quite low (>360 N) for all salts.
3 Conclusions
(1) The guanidinium, diaminoguanidinium, and guanidylguanidinium salts of 5-(3-aminofurazan-4-yl) tetrazol-1-ol were synthesized and characterized.
(2) The structures of 2 and 3 were confirmed by X-ray single-crystal diffraction, which show that there are extensive hydrogen-bonding interactions between the cations and anions in these salts. It is the intramolecular and intermolecular hydrogen bonds that play a pivotal role in molecular density, thermal stability and sensitivity.
(3) All the nitrogen-rich salts show good thermal stabilities (decomposition temperature between 266 and 277℃), low impact sensitivities (24J), revealing that salts 2-4 can be used as insensitive energetic materials with excellent thermal stabilities.
[1] Badgujar D M, Talawar M B, Asthana S N, et al. Advances in science and technology of modern energetic materials: An overview[J]. Journal of Hazardous Materials, 2008, 151(2): 289-305.
[2] Wang Rui-hu, Guo Yong, Zeng Zhuo, et al. Furazan-functionalized tetrazolate-based salts: a new family of insensitive energetic materials[J]. Chemistry - A European Journal, 2009, 15(11): 2625-2634.
[3] Liang Li-xuan, Wang Kai, Bian Cheng-ming, et al. 4-Nitro-3-(5-tetrazole)furoxan and its salts: synthesis, characterization, and energetic properties[J]. Chemistry - A European Journal, 2013, 19(44): 14902-14910.
[4] Huang Hai-feng, Zhou Zhi-ming, Liang Li-xuan, et al. Nitrogen-rich energetic monoanionic salts of 3,4-bis(1H-5-tetrazolyl)furoxan[J]. Chemistry - An Asian Journal, 2012, 7(4): 707-714.
[5] Godovikova T I, Vorontsova S K, Konyushkin L D, et al. Synthesis of 5-(1,2,5-oxadiazol-3-yl)-1H-etrazoles from 3-cyano-1,2,5-oxadiazoles[J]. Russian Chemical Bulletin International Edition, 2009, 58(2): 406-409.
[6] Fischer D, Klap?tke T M, Reymann M, et al. Energetic alliance of tetrazole-1-oxides and 1,2,5-oxadiazoles[J]. New Journal Chemistry,2015, 39, 1619-1627.
[7] Fischer D, Klap?tke T M, Reymann M, et al. Dense energetic nitraminofurazanes[J]. Chemistry - A European Journal, 2014, 20(21): 6401-6411.
[8] Fischer D, Klap?tke T M, Stierstorfer J. Synthesis and characterization of diaminobisfuroxane[J]. European Journal of Inorganic Chemistry, 2014, 2014(29):5808-5811.
[9] WEI Hao, HE Chun-lin, ZHANG Jia-heng, et al. Combination of 1,2,4-oxadiazole and 1,2,5oxadiazole moieties for the generation of high-performance energetic materials[J]. Angewandte Chemie International Edition, 2015, 54(32): 9367-9371.
[10] Martinez H, Zheng Z Y, Dolbier Jr R D, et al. Energetic materials containing fluorine. Design, synthesis and testing of furazan-containing energetic materials bearing a pentafluorosulfanyl group[J]. Journal of Fluorine Chemistry, 2012, 143:112-122.
[11] Tsyshevsky R, Pagoria P, ZHANG Mao-xi, et al. Searching for low-sensitivity cast-melt high-energy-density materials: synthesis, characterization, and decomposition kinetics of 3,4-bis(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazole-2-oxide[J]. Journal of Physical Chemistry C, 2015, 119(7): 3509-3521.
[12] Sheldrick G M. SHELXS-97, Program for X-ray crystal structure determination[CP/CD]. G?ttingen: University of G?ttingen, 1997.
[13] Sheldrick G M. SHELXL-97, Program for the refinement of crystal structures[CP/CD]. G?ttingen: University of G?ttingen 1997.
[14] Tselinskii I V, Mel’nikova S F, Romanova T V, et al. Synthesis and reactivity of carbohydroximoyl azides: II. 4-substituted 1,2,5-oxadiazole-3-carbohydroximoyl azides and 1-hydroxy-5-(4-R-1,2,5-oxadiazol-3-yl)tetrazoles[J]. Russian Journal of Organic Chemistry, 2001, 37(11): 1638-1642.
[15] Allen F H, Kennard O, Watson D G, et al. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds[J]. Journal of the Chemical Society, Perkin Transactions 2, 1987, 1(12):1-19.
5-(3-氨基呋咱-4-基)-1-羥基四唑胍鹽的合成及性能研究
翟連杰1,2, 王伯周1,2, 畢福強1,2, 霍 歡1,2, 李亞南1,2, 樊學忠1
(1. 西安近代化學研究所, 陜西 西安 710065;2. 氟氮化工資源高效開發與利用國家重點實驗室,陜西 西安 710065)
以丙二腈為原料,高收率合成了5-(3-氨基呋咱-4-基)-1-羥基四唑(1)的胍鹽(2)、二氨基胍(3)以及聯胍鹽(4)。采用紅外光譜、核磁共振、元素分析、熱分析等對其結構進行了表征;培養了2和3的單晶,并采用X射線單晶衍射測試了其晶體結構;通過落錘法和摩擦感度儀測試了3種胍鹽的撞擊感度和摩擦感度;采用DSC研究了3種胍鹽的熱分解過程。結果表明,3種胍鹽的撞擊感度均大于24J,摩擦感度均大于360N,分解點介于266~277℃,顯示出良好的熱穩定性。
富氮鹽;呋咱化合物;胍鹽;唑類化合物;X射線單晶衍射
10.14077/j.issn.1007-7812.2017.03.002
date:2016-06-17; Revised date:2016-09-11
TJ55;O65 Document Code:A Article ID:1007-7812(2017)03-0017-04
Foundation:Natural Science Foundation of China (No.21243007)
Biography:ZHAI Lian-jie(1988-), male, doctoral candidate. Research field: Synthesis and properties of energetic materials. E-mail: trihever0210@126.com

