阿托伐他汀對慢性兔房顫模型心房電重構的影響
楊 倩, 史亞男, 李鑫檸, 宋學蓮, 齊曉勇
(河北省人民醫院 1心內科, 2腎內科, 河北 石家莊 050051)
阿托伐他汀對慢性兔房顫模型心房電重構的影響
楊 倩1△, 史亞男2, 李鑫檸1, 宋學蓮1, 齊曉勇1
(河北省人民醫院1心內科,2腎內科, 河北 石家莊 050051)
目的通過快速起搏心房3周建立慢性兔房顫模型,探討阿托伐他汀(ATO)對該模型心房電重構的影響及其可能機制。方法將24只新西蘭大白兔開胸,于左心房植入起搏和測試電極,隨機分為3組:模型(model)組和ATO組持續心房起搏3周,分別給予安慰劑和阿托伐他汀2.5 mg·kg-1·d-1灌胃;假手術(sham)組不起搏,不給藥。起搏前后用電生理刺激儀檢測心率、P波寬度、心房有效不應期(AERP)和房顫誘發率的變化;起搏后采用Western blot檢測心房Cav1.2、Kv4.3和髓過氧化物酶(MPO)的蛋白表達水平。結果Sham組、model組和ATO組分別有0、 5和4只兔誘發持續性房顫。起搏3周后,與sham組相比,model組和ATO組兔心率和P波寬度均增加,AERP縮短(P<0.05);ATO組與model組相比,AERP增加(P<0.05),心率和P波寬度無明顯變化。與sham組相比,model組和ATO組兔心房Cav1.2和Kv4.3的蛋白表達水平下降, MPO的蛋白表達水平升高(P<0.05);ATO組與model組相比,Cav1.2的表達增加,MPO的表達下降(P<0.05),Kv4.3無明顯變化。結論阿托伐他汀能夠通過抑制慢性兔房顫模型心房Cav1.2蛋白表達下降和AERP的縮短,抑制心房電重構,其潛在機制可能是阿托伐他汀抑制了心房MPO蛋白的高表達。
心房顫動; 電重構; 阿托伐他汀; 髓過氧化物酶
心房顫動(簡稱房顫,atrial fibrillation,AF)是臨床上最常見的心律失常之一,其發病率和致殘率高,嚴重影響著患者的生活質量[1]。但是,目前房顫的發病機制仍不清楚,治療措施尚不盡如人意。
近年來的研究表明,心房電重構在房顫的發生、復發和維持中發揮重要作用[2];炎癥和氧化應激可以通過下調離子通道的表達,引起心房有效不應期(atrial effective refractory period,AERP)縮短、房顫誘發率增加和誘發房顫的持續時間延長,從而導致心房電重構[3];降低炎癥和氧化應激水平則可通過抑制上述離子通道的表達下調,抑制心房電重構[4-5]。因此,抗炎和抗氧化應激治療可能成為房顫治療的新方法。
他汀類藥物除降脂外還具有抗炎和抗氧化應激作用[4]。研究表明,它不僅可以降低心臟術后患者房顫的發生率[6],還可以抑制急性房顫動物模型的心房電重構[7-8]。但是,他汀類藥物對于慢性房顫的影響尚不清楚。本研究通過快速心房起搏3周建立慢性兔房顫模型,探討阿托伐他汀(atorvastatin,ATO)對于該模型心房電生理重構的影響。
材 料 和 方 法
1動物和材料
選用24只新西蘭大白兔作為實驗對象,體重2.5~3.5 kg,由河北醫科大學動物實驗中心提供。動物心臟起搏器購自上海復旦旦華科技有限公司,起搏電壓5 V,起搏頻率為 600 次/min; 采用蘇州東方DF-5A型電生理刺激儀測定電生理指標。阿托伐他汀鈣片為輝瑞制藥有限公司產品; 抗L型鈣離子通道α1亞單位(Cav1.2)、瞬態外流鉀離子通道(Kv4.3)和髓過氧化物酶(myeloperoxidase,MPO)抗體均購自Santa Cruz;抗β-actin抗體購自Protein Tech。
2主要方法
2.1兔房顫模型建立 3%戊巴比妥鈉(1 mL/kg)經耳緣靜脈注射麻醉后,將兔仰臥位固定于動物手術臺上,備皮,于胸骨左緣第3、4肋間打開胸腔,暴露心臟,找到左心房,采用結扎左心耳的方法將起搏和測試電極固定于左心房,引出電極,關閉胸腔。電極沿皮下隧道移行至右側后背部,應用電生理刺激儀測試電極,保證裸露導線與心房肌接觸良好,且與胸壁絕緣。于兔右側背部脊柱旁制作起搏器囊袋,慶大霉素囊袋沖洗后,將動物起搏器與起搏電極連接固定,測試電極固定于皮下,留待電生理指標測定[8]。術后用青霉素抗感染治療,恢復1周。
2.2實驗分組 實驗兔隨機分為3組:模型組(model group,n=8)和阿托伐他汀組(ATO group,n=8):開胸,固定左心房電極,持續心房起搏3周,自起搏開始前3 d至起搏3周結束,每日分別給予生理鹽水10 mL或阿托伐他汀2.5 mg/kg溶解于10 mL生理鹽水中灌胃;假手術組(sham group,n=8):僅開胸和固定左心房電極,不起搏,不給藥。每日行心電圖檢查,保證起搏器正常工作。
2.3AERP測定 采用電生理刺激儀于起搏前后測定各組兔AERP。用S1S2程序遞減刺激法,以起搏閾值2倍為輸出電壓,測定150 ms基礎周長時的AERP,即AERP150。用S1S2間期遞減掃描,S1S2呈10∶1,步長為10 ms。當S2后不能引起心房激動時,將此時S2值增加10 ms,再次進行S1S2間期遞減掃描,步長為2 ms,以S2不能下傳心房的最長S1S2間期為AERP150,重復測量3次[9]。
2.4房顫誘發率測定 采用電生理刺激儀于起搏前后行心房Burst刺激,測定房顫誘發率。給予S1S1刺激,周長50 ms,電壓為2倍舒張期閾值電壓+0.5 V,重復8次(前4次每次6 s,后4次每次12 s)[10]。房顫誘發率(%)=誘發房顫成功兔只數/行Burst刺激兔總只數×100%。
2.5心房Cav1.2、Kv4.3和MPO的蛋白表達水平測定 取左心房心肌組織剪碎,用RIPA裂解液抽提心房肌組織總蛋白,Bardford比色法測定抽提蛋白的濃度。按照說明書進行操作,經電泳后采用半干轉的方法轉移到PVDF膜,經5% BSA封閉2 h,于經PBS稀釋的Ⅰ抗工作液中4 ℃反應過夜,再經1×PBST稀釋3 000倍的Ⅱ抗工作液中處理90 min,蛋白條帶通過顯影定影液顯色后,采用UVP分析儀器,對膠片進行掃描,然后括住每一個條帶,系統自動生成灰度值。
3統計學處理
運用SPSS 19.0軟件分析處理數據。計量資料采用均數±標準差(mean±SD)表示,3組間比較采用單因素方差分析。以P<0.05 為差異有統計學顯著性。
結 果
1動物一般情況
24只新西蘭大白兔中,模型組8只,術中死亡1只(死因為嚴重氣胸引起縱隔擺動),起搏過程中通過每日復查心電圖,發現起搏器未能奪獲心房1只(原因是起搏閾值異常增高),實驗結束時成功6只;阿托伐他汀組8只,術中術后均存活,起搏過程中通過每日復查心電圖,發現起搏器未能奪獲心房2只(原因是起搏閾值異常增高和起搏電極脫位),實驗結束時成功6只;假手術組8只,術中死亡2只(死因是嚴重氣胸引起縱隔擺動和心房破損引起大出血),實驗結束時行心房Burst刺激時,發現S1S1刺激(600次/分)均能奪獲心房,實驗結束時成功6只。
2心電圖各指標的變化
起搏前行心電圖檢查,3組兔均為竇性心律;起搏3周后,將模型組和阿托伐他汀組起搏器設置為關閉狀態,心電圖顯示3組兔仍為竇性心律,見圖1。

Figure 1. ECG recordings of sham group in II lead before and after RAP (paper speed was 50 mm/s).
圖1假手術組起搏前后II導聯心電圖
起搏前3組兔心率(heart rate)的差異無統計學顯著性;起搏3周后測得模型組和阿托伐他汀組心率較假手術組增加(P<0.05),但兩組間差異無統計學顯著性,見圖2。

Figure 2. Heart rate of the 3 groups after 3 weeks of pacing. Mean±SD.n=6.*P<0.05vssham group.
圖2起搏3周后3組兔心率變化
起搏前3組兔心電圖P波寬度(P-wave duration)的差異無統計學顯著性;起搏3周后測得模型組和阿托伐他汀組P波寬度較假手術組增加(P<0.05),但兩組間差異無統計學顯著性,見圖3。
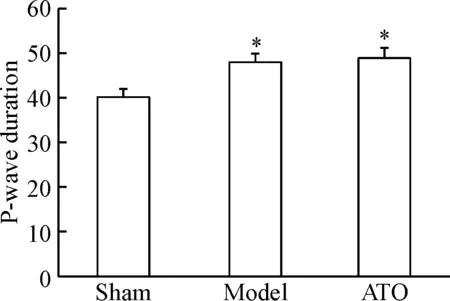
Figure 3. P-wave duration of the 3 groups after 3 weeks of pacing. Mean±SD.n=6.*P<0.05vssham group.
圖3起搏3周后3組兔P波寬度變化
3AERP的變化
起搏前3組兔AERP的差異無統計學顯著性;起搏3周后測得模型組和阿托伐他汀組的AERP較假手術組下降(P<0.05),但阿托伐他汀組經藥物治療后AERP較模型組增加(P<0.05),見圖4。

Figure 4. AERP of the 3 groups after 3 weeks of pacing. Mean±SD.n=6.*P<0.05vssham group;#P<0.05 vs model group.
圖4起搏3周后3組兔AERP變化
4房顫誘發率的變化
起搏前對3組兔經心房Burst刺激均未能誘發房顫。起搏3周后,經心房Burst刺激后,假手術組兔均未誘發房顫,模型組6只兔中有5只誘發持續性房顫(誘發率83%),阿托伐他汀組6只兔中有4只誘發持續性房顫(誘發率67%)。
5心房Cav1.2和Kv4.3蛋白表達水平的變化
起搏3周后,模型組和阿托伐他汀組Cav1.2和Kv4.3的蛋白表達水平較假手術組均明顯下降(P<0.05),但阿托伐他汀組經藥物治療后其Cav1.2的蛋白水平較模型組增加(P<0.05),Kv4.3的蛋白水平與對照組間的差異無統計學顯著性,見圖5。
6心房MPO蛋白表達水平的變化
起搏3周后,模型組和阿托伐他汀組MPO的蛋白表達水平較假手術組均明顯增加(P<0.05),阿托伐他汀組經藥物治療后其MPO的蛋白水平較模型組下降(P<0.05),見圖6。

Figure 5. The protein expression levels of Cav1.2 and Kv4.3 in the left atria. Mean±SD.n=6.*P<0.05vssham group;#P<0.05vsmodel group.
圖5左心房Cav1.2和Kv4.3的蛋白表達水平
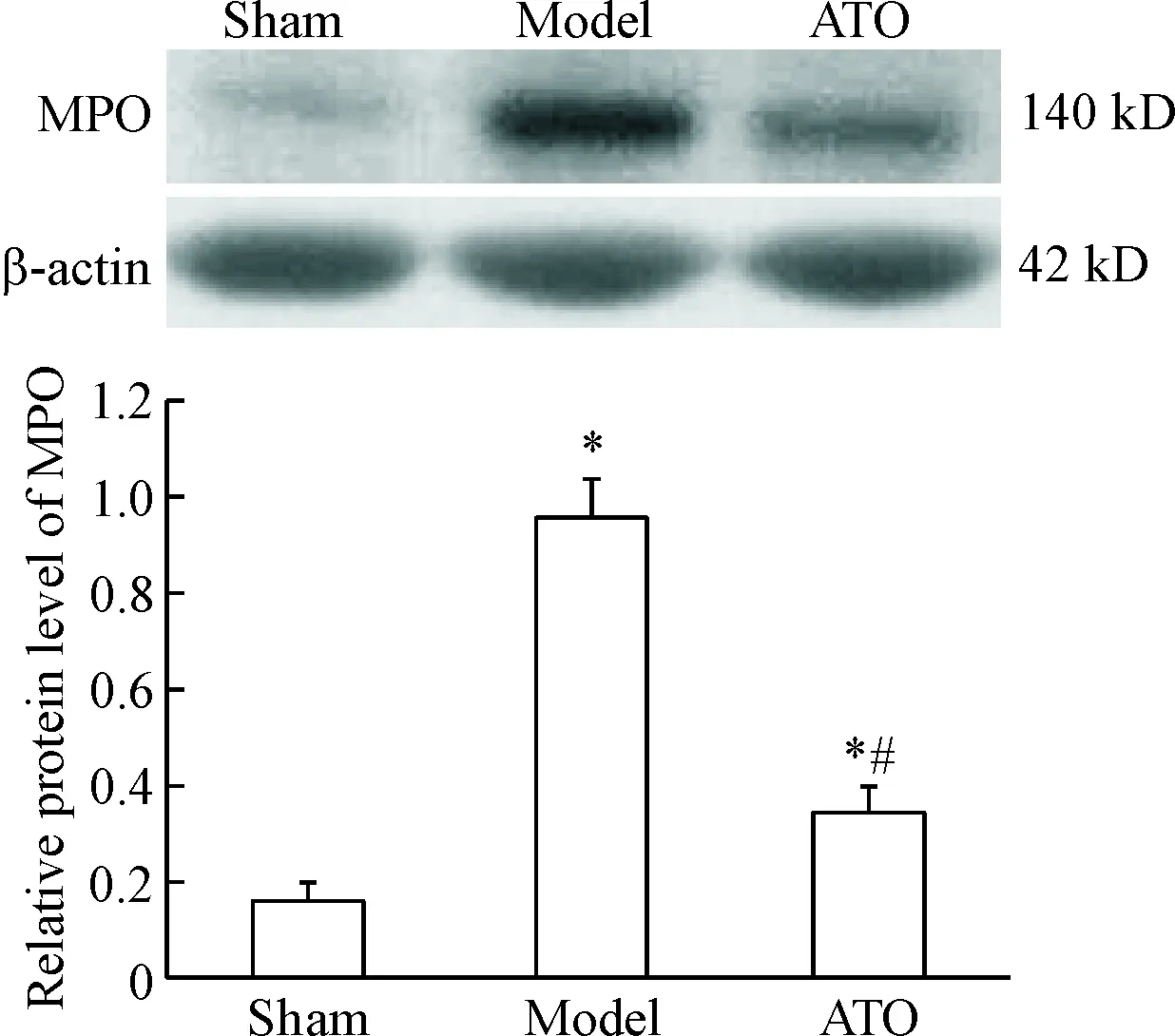
Figure 6. The protein expression levels of MPO in the left atria. Mean±SD.n=6.*P<0.05vssham group;#P<0.05vsmodel group.
圖6左心房MPO的蛋白表達水平
討 論
心房的電重構是指由于心房離子通道蛋白的表達異常,引起動作電位時程和AERP縮短、AERP頻率適應性降低、傳導速度減慢、空間不均一性增高和房內折返波縮短,導致心房促折返基質形成,從而誘發房顫[2]。臨床研究和動物實驗均證實了房顫時心房電重構的存在。Yue等[11]以400 min-1的頻率,分別起搏犬心房1 d、7 d和42 d,并將其與假手術組進行比較,結果顯示:隨著起搏時間延長,AERP逐漸縮短,AERP頻率適應性逐漸下降,誘發房顫的持續時間逐漸延長,這可能與心房 L型鈣離子通道和瞬態外流鉀離子通道密度的逐漸下調有關。Brundel等[12]研究發現,陣發性和持續性房顫患者與竇性心律者相比,AERP明顯縮短,AERP頻率適應性下降,心房L型鈣離子通道及5種鉀離子通道蛋白的表達明顯下降。
L型鈣離子通道與電重構密切相關,它是位于心肌細胞膜的異四聚體復合物,由α1亞單位(Cav1.2)、α2/δ亞單位及β亞單位組成。房顫時的快速心房率會引起心房細胞內鈣超載,從而引起L型鈣離子通道表達下調和AERP縮短,導致心房電重構[12-13]。近年來,對于Cav1.2的研究較多,結果顯示:快速的心房起搏能夠引起Cav1.2表達的明顯下調,而抑制Cav1.2的下調可以抑制心房電重構[14]。心肌鉀離子通道在心房存在5種亞型,其中Kv4.3在房顫的電重構中起重要作用。Kv4.3電流是動作電位早期復極電流,其促房顫作用目前尚不清楚,但臨床研究[14]和動物實驗[5]均顯示,房顫患者及房顫動物模型心房Kv4.3水平明顯下降,而抑制Kv4.3的下調可能抑制心房電重構。
近年來的研究顯示,炎癥和氧化應激在房顫的電重構過程中發揮重要作用[3,15],它們可以通過調節離子通道的表達和細胞內鈣穩態,降低心肌細胞動作電位時程和鈣瞬變,改變心房的傳導屬性,導致心房電重構[16-17]。而降低機體內炎癥及氧化應激水平可以抑制心房電重構,降低房顫的發生率[3-5]。他汀類藥物在房顫治療中可能發揮的作用逐漸引起人們的關注[4]。Shiroshita-Takeshita等[18]通過快速起搏心房1周建立犬房顫模型,并分別給予辛伐他汀、維生素C和維生素C聯合維生素E治療,結果顯示:辛伐他汀可以降低血漿C反應蛋白水平,抑制心房電重構,降低房顫發生率,而維生素C和維生素E則沒有此作用。Kumagai等[19]通過建立無菌性心包炎模型,亦證明:阿托伐他汀可以降低血漿C反應蛋白水平,抑制AERP縮短,減少房顫持續時間。
MPO可以介導多種活性氧和活性氮的生成,導致炎癥和氧化應激反應的加劇,在房顫中發揮重要作用[20]。對冠狀動脈搭橋術后患者進行右心耳MPO濃度測定后發現,房顫患者心房MPO沉積明顯高于非房顫患者[21];對于起搏器置入術后患者進行房顫發病率和血漿MPO濃度隨訪后發現,隨訪出現房顫的患者血漿MPO水平明顯高于未出現房顫患者[21];射頻消融術后患者高水平的MPO常可預示術后房顫的復發[22]。既往的研究證明,他汀類藥物能夠降低心血管患者血中MPO水平[23-24]。
既往探討他汀類藥物對房顫作用的動物實驗主要以犬為實驗動物,多為急性房顫動物模型(≤1周),具體機制尚不清楚。本研究通過快速心房起搏3周建立慢性兔房顫模型,對阿托伐他汀對該模型的影響和可能的機制進行了探討,結果顯示:快速心房起搏可以引起心房MPO蛋白表達的明顯增加,Cav1.2和Kv4.3蛋白表達的下降,以及AERP縮短;阿托伐他汀治療可以抑制MPO蛋白表達增加,Cav1.2蛋白表達的下降,以及AERP縮短,但是對Kv4.3蛋白表達的下降無影響。因此,阿托伐他汀可能通過抑制心房MPO水平發揮其對于心房電重構的抑制作用。
[1] Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study[J]. Circulation, 2014, 129(8):837-847.
[2] Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives[J]. J Am Coll Cardiol, 2014, 63(22):2335-2345.
[3] Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management[J]. Circ J, 2015, 79(3):495-502.
[4] Pinho-Gomes AC, Reilly S, Brandes RP, et al. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins[J]. Antioxid Redox Signal, 2014, 20(8):1268-1285.
[5] Nakatani Y, Nishida K, Sakabe M, et al. Tranilast prevents atrial remodeling and development of atrial fibrillation in a canine model of atrial tachycardia and left ventricular dysfunction[J]. J Am Coll Cardiol, 2013, 61(5):582-588.
[6] Kuhn EW, Liakopoulos OJ, Stange S, et al. Preoperative statin therapy in cardiac surgery: a meta-analysis of 90,000 patients[J]. Eur J Cardiothorac Surg, 2014, 45(1):17-26.
[7] Laszlo R, Menzel KA, Bentz K, et al. Atorvastatin treatment affects atrial ion currents and their tachycardia-induced remodeling in rabbits[J]. Life Sci, 2010, 87(15-16):507-513.
[8] 宋學蓮, 齊曉勇, 黨 懿, 等. 阿托伐他汀對快速起搏兔房顫模型心房電重構的影響[J]. 中國病理生理雜志, 2016, 32(4):623-627.
[9] Yu J, Li W, Li Y, et al. Activation of β(3)-adrenoceptor promotes rapid pacing-induced atrial electrical remodeling in rabbits[J]. Cell Physiol Biochem, 2011, 28(1):87-96.
[10] Zhao Y, Gu TX, Zhang GW, et al. Losartan affects the substrate for atrial fibrillation maintenance in a rabbit model[J]. Cardiovasc Pathol, 2013, 22(5): 383-388.
[11] Yue L, Feng J, Gaspo R, et al. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation[J]. Circ Res, 1997, 81(4):512-525.
[12] Brundel BJ, Van Gelder IC, Henning RH, et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation[J]. Circulation, 2001, 103(5):684-690.
[13] Christ T, Boknik P, W?hrl S, et al. L-type Ca2+current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases [J]. Circulation, 2004, 110(17):2651-2657.
[14] Brandt MC, Priebe L, B?hle T, et al. The ultrarapid and the transient outward K+current in human atrial fibrillation. Their possible role in postoperative atrial fibrillation[J]. J Mol Cell Cardiol, 2000, 32(10):1885-1896.
[15] Hu YF, Chen YJ, Lin YJ, et al. Inflammation and the pathogenesis of atrial fibrillation[J]. Nat Rev Cardiol, 2015, 12(4): 230-243.
[16] Kao YH, Chen YC, Cheng CC, et al. Tumor necrosis factor-α decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes [J]. Crit Care Med, 2010, 38(1):217-222.
[17] Musa H, Kaur K, O’Connell R, et al. Inhibition of platelet-derived growth factor-AB signaling prevents electromechanical remodeling of adult atrial myocytes that contact myofibroblasts[J]. Heart Rhythm, 2013, 10(7):1044-1051.
[18] Shiroshita-Takeshita A, Schram G, Lavoie J, et al. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs[J]. Circulation, 2004, 110(16):2313-2319.
[19] Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model[J]. Cardiovasc Res, 2004, 62(1):105-111.
[20] Friedrichs K, Baldus S, Klinke A. Fibrosis in atrial fibrillation-role of reactive species and MPO[J]. Front Physiol, 2012, 3:214.
[21] Rudolph V, Andrié RP, Rudolph TK, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation[J]. Nat Med, 2010, 16(4):470-474.
[22] Li SB, Yang F, Jing L, et al. Myeloperoxidase and risk of recurrence of atrial fibrillation after catheter ablation[J]. J Investig Med, 2013, 61(4):722-727.
[23] Ndrepepa G, Braun S, Sch?mig A, et al. Impact of therapy with statins, β-blockers and angiotensin-converting enzyme inhibitors on plasma myeloperoxidase in patients with coronary artery disease[J]. Clin Res Cardiol, 2011, 100(4):327-333.
[24] Andreou I, Tousoulis D, Miliou A, et al. Effects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: a randomized placebo-controlled study[J]. Atherosclerosis, 2010, 210(1):194-198.
(責任編輯: 陳妙玲, 宋延君)
Effects of atorvastatin on atrial electrical remodeling in a rabbit model of chronic atrial fibrillation
YANG Qian1, SHI Ya-nan2, LI Xin-ning1, SONG Xue-lian1, QI Xiao-yong1
(1DepartmentofCardiology,2DepartmentofNephrology,HebeiGeneralHospital,Shijiazhuang050051,China.E-mail:yangqian8411@163.com)
AIM: To evaluate the effects of atorvastatin (ATO) on atrial electrical remodeling in a rabbit mo-del of chronic atrial fibrillation (AF) produced by 3 weeks of rapid atrial pacing (RAP).METHODSThe sternotomy was performed and the pacing and testing electrodes were fixed to the left atria of 24 New Zealand white rabbits. The animals were randomly divided into 3 groups. The rabbits in model group and ATO group were subjected to RAP for 3 weeks, and then were treated with placebo and ATO (2.5 mg·kg-1·d-1), respectively. The rabbits in sham group did not receive RAP and drugs. Electrophysiological examination was performed to test heart rate, P-wave duration, atrial effective refractory period (AERP) and AF inducibility. The protein expression levels of Cav1.2, Kv4.3 and myeloperoxidase (MPO) were detected by Western blot.RESULTSSustained AF was induced in 5 and 4 rabbilts in model group and atorvastatin group and no rabbits in sham group was found. After 3 weeks of RAP, compared with sham group, heart rate and P-wave duration were increased and AERP was shortened in model group and ATO group (P<0.05). Compared with model group, AERP was increased in ATO group (P<0.05), while heart rate and P-wave duration had no difference between these 2 groups. Compared with sham group, the protein levels of Cav1.2 and Kv4.3 were decreased, and protein level of MPO was increased in model group and ATO group (P<0.05). Compared with model group, Cav1.2 was increased and MPO was decreased in ATO group (P<0.05), while Kv4.3 had no difference between these 2 groups.CONCLUSIONAtorvastatin suppresses the down-regulation of atrial Cav1.2 protein level and the shortening of AERP, thus preventing atrial electrical remodeling in a rabbit model of chronic AF. The effect of atrovastatin on reducing atrial MPO level may be the potential mechanism.
Atrial fibrillation; Electrical remodeling; Atorvastatin; Myeloperoxidase
1000- 4718(2017)11- 1975- 05
2017- 05- 10
2017- 06- 22
△通訊作者 Tel: 0311-85988263; E-mail: yangqian8411@163.com
R541.7; R363
A
10.3969/j.issn.1000- 4718.2017.11.009

