具有優異甲醇耐受性的Rh摻雜PdCu有序金屬間化合物納米粒子增強氧還原電催化
李蒙剛,夏仲泓,黃雅榮,陶璐,晁玉廣,尹坤,楊文秀,楊微微,*,于永生 ,*,郭少軍 ,3,*
1哈爾濱工業大學化工與化學學院,新能源轉化與儲存關鍵材料技術工信部重點實驗室,哈爾濱 150001
2北京大學工學院材料科學與工程系,北京 100871
3北京大學工學院工程科學與新興技術高精尖中心,北京 100871
1 Introduction
The rapidly rising energy demand and the expected exhaustion of environmentally unfriendly fossil energy carriers have strongly stimulated the exploration of highly efficient and alternative energy conversion devices1–3. Designing highperformance direct methanol fuel cells (DMFCs) has been regarded as an efficient and economical solution to achieve the conversion of sustainable andclean energy in a variety of applications ranging from portable electronics to electric vehicles4–6. However, even if the Pt or Pt-based alloys are applied for catalyzing the oxygen reduction reaction (ORR) in the cathode of DMFCs, intrinsically sluggish kinetics will still hinder their practical applications7–11. In addition, the extreme scarcity of Pt is also not sufficient to satisfy this requirement10,12,which calls for the exploration of new cathode catalysts with exciting activity and durability as alternatives to expensive Pt metals.
As one of the most promising candidates for replacing non-Pt multimetallic electrocatalysts, a series of advanced Pd-based nanocatalysts, such as PdAg, PdPb, PdMo and PdCu,etc.have received extensive attention to address the technical challenges towards unaffordable Pt usage issues, especially in the alkaline conditions5,12–18. Nevertheless, a critical issue at present is that the reported Pd-based multimetallic nanocatalysts cannot meet the demanding application environment during practical fuel cell operation due to the unsatisfactory activity and durability, which can be explained as a result of the limited electronic or strain effects originated from the alloying metals atoms and their instability of disordered structures13–15,19–21. The ordered intermetallic structure is a valuable research model for improving this unfavorable situation due to its lower negative entropy and stronger electronic interaction between Pd and other metal atoms (M, usually Cu, Zn, Fe, Pb,etc.)20–25, which cannot only enhance the activity and durability, but also greatly alleviate the effect of poisoning caused by the shuttle of methanol from anode to cathode26–28. In addition, heteroatomic incorporation into the host catalysts is also another effective tactic to enhance electrocatalytic activity by rationally modulating the local electronic structure or optimizing the adsorption/desorption of oxygen intermediates (O*)29–32. Inspired by the abovementioned analysis, Rh, which can provide a positive effect towards ORR performance5,33, is anticipated to achieve a remarkable nano-electrocatalyst by being introduced into ordered intermetallics.
Herein, a novel class of Rh-doped PdCu ordered intermetallic nanoparticles (NPs) was designed as highly active and stable electrocatalyts for ORR. Compared with the disordered PdCu NPs, ordered intermetallic PdCu NPs and disordered Rh-doped PdCu NPs, the optimal ordered intermetallic structure achieves the mass activityof as high as 0.96 A.mg?1at 0.9 V (vsRHE),7.4-fold higher than that of commercial Pt/C, and exhibits the excellent durability even after 20000 consecutive cycles. The significant role of the Rh atoms and the advantages of ordered intermetallics are perfectly manifested in this enhanced electrocatalytic system. Finally, we demonstrate the prominent methanol tolerance capacity for Rh-doped PdCu ordered intermetallics, indicating its favorable prospect as potential electrocatalysts towards the next-generation high-performance DMFCs.
2 Experimental
2.1 Chemicals
Palladium (II) acetylacetonate (Pd(acac)2, 99%) and rhodium(III) acetylacetonate (Rh(acac)3, 97%) were purchased from Sigma-Aldrich. Copper (II) acetylacetonate (Cu(acac)2, 97%)and Nafion (5% (w, mass fraction)) were obtained from Alfa Aesar. Iron (III) chloride hexahydrate (FeCl3.6H2O, AR.), L-ascorbic acid (AA, AR.), citric acid (CA, AR.) and potassium hydroxide (KOH, GR., 95%) were all purchased from Aladdin.Methanol (CH3OH, GR.) ethanol (C2H5OH, AR.), isopropanol(C3H8O, GR.) and cyclohexane (C6H12O6, AR.) were supplied by Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).The commercial carbon supported Pt catalyst (Pt/C, 20% (w))was provided by Johnson-Matthey Corp. All the reagents and chemicals were used directly without purification, and the ultrapure water (18.2 MΩ.cm?1) used in all experiments was prepared by an ultrapure purification system.
2.2 Preparation of disordered and ordered Rh-PdCu NPs
In the typical preparation of disordered Rh-PdCu NPs, 7.6 mg of Pd(acac)2, 6.5 mg of Rh(acac)3, 1 mg of Rh(acac)3, 5.4 mg of FeCl3, 35.6 mg of AA, 66 mg of CA and 5 mL of OAm were added into a 20 mL of vial. After being capped, the vial was sonicated until the reactants were completely dissolved. Then the resulting homogeneous solution was placed in a preheated oil bath of 200 °C for 15 h. As-obtained black colloids were separated by centrifugation and washed with the cyclohexane/ethanol mixture for three times.
As-synthesized disordered Rh-PdCu NPs were dispersed in cyclohexaneviasonicating, and then were dropwise added to another ethanol dispersion containing the carbon supports(Ketjen Black-300J). After being sonicated for 3 h, the products were collected by centrifugation and washed with ethanol for several times. The as-prepared black products were then dried at 60 °C under ambient condition overnight. In order to remove the organic surfactants around the surface of Rh-PdCu/C, the products were further annealed at 230 °C for 2 h. The cleaned Rh-PdCu/C was annealed under 5% H2/Ar atmosphere at 500 °C for 2 h with a heating rate of 2 °C.min?1to promote the phase transformation from disordered Rh-PdCu to ordered intermetallic NPs (denoted as Rh-PdCu/C-500).
2.3 Preparation of disordered and ordered PdCu NPs
The preparation process of disordered PdCu NPs was similar to that of disordered Rh-PdCu NPs, except the absence of Rh precursor. The ordered PdCu NPs were prepared by similar annealing method with the Rh-PdCu counterpart, except for changing the annealing temperature from 500 to 400 °C. The asobtained ordered PdCu NPs was denoted as PdCu/C-400.
2.4 Characterization
The morphology and structure were conducted by the transmission electron microscopy (TEM) (HITACHI H-7700 transmission electron microscopy with an accelerating voltage of 100 kV) and high resolution TEM (HRTEM) (FEI Tecnai-G2 F30 at an accelerating voltage of 300 kV) images. The composition and amount were determined by the scanning electron microscopy-energy dispersive X-ray spectroscopy(SEM-EDS) spectra (EOL JSM-6360 scanning electron microscope with an accelerating voltage of 200 kV) and inductively coupled plasma atomic emission spectrometry (ICPAES) analysis (Agilent 8800 instrument). Power X-ray diffraction (PXRD) patterns (PANalytical-XRD instrument with a CuKαradiation at 40 kV voltage and 30 mA current) were collected to study the crystalline phase of as-prepared products.
2.5 Electrochemical measurements
As-prepared products were dispersed in a mixed solvent containing isopropanol, ultrapure water and Nafion (the volume ratio is 4/6/0.005) to obtain a homogeneous ink with a concentration of 1 mg.mL–1. 10 μL of ink was dropped onto the polished glassy carbon rotating disk electrode (RDE, Pine Research Instrumentation, diameter of 5 mm, area of 0.196 cm2)to prepare the working electrode with the loading mass of Pd at 7.3 μg.cm?2. All the electrochemical experiments were performed using a three-electrode cell configuration on a CHI 760E (Chenhua, Shanghai) electrochemical workstation at room temperature. The leak-free saturated calomel electrode (SCE), a Pt plate (1.5 cm × 1 cm) and catalysts-modified RDE were used as the reference, counter and working electrodes, respectively. A 0.1 mol.L?1KOH solution was served as the electrolyte for ORR measurements, followed with 0.5 mol.L?1CH3OH was added to study the methanol tolerance performance. All the potentials mentioned in this work were convertedversus(vs) the reversible hydrogen electrode (RHE) according to the follow formula:Evs.RHE =EvsSCE + 0.059pH + 0.241. The positive-going ORR polarization curves were recorded in O2-saturated conditions at a scan rate of 20 mV.s?1and the cyclic voltammograms (CVs)curves were conducted in N2-saturated electrolyte with a sweep rate of 50 mV.s?1. The electrochemical accelerated durability tests (ADTs) were tested in O2-saturated 0.1 mol.L?1KOH solutions by performing the CVs between 0.6 V and 1.1 V (vsRHE) for 20000 cycles at a sweep rate of 200 mV.s?1.
3 Results and discussion
TEM images were first used to characterize the structure of the Rh-PdCu NPs. The uniform near-spherical NPs with an average size of (6.5 ± 0.5) nm are observed (Fig. 1a and Fig. S1,see Supporting Information (SI)). The molar ratio of Rh/Pd/Cu in as-synthesized Rh-PdCu NPs is determined to be 5/48/47 by SEM-EDS, consistent with the corresponding ICP-AES result(Fig. 1b). Similarly, monodisperse PdCu NPs with the same size distribution can also be synthesizedviathe same approach,except without adding Rh precursor (Fig. S2, SI). Both NPs possess the typical fcc PdCu structure (JCPDS No. 48-1551),confirmed by PXRD patterns (Fig. 1c). In particular, the reflexes of Rh-PdCu NPs are shifted to lower 2θangles compared to those of PdCu NPs due to the incorporation of Rh atoms with a larger lattice constant (the inset of Fig 1c). The HRTEM and corresponding fast Fourier transformation (FFT) patterns of the Rh-PdCu NPs further indicate their fcc-phased structures, and the lattice spacings are measured to be 0.218 nm, which is slightly larger than that of the (111) facet of fcc PdCu (0.217 nm)(Fig. 1d).

Fig. 1 Structural and compositional characterization of disordered Rh-PdCu NPs. (a) Representative TEM image (inset is the corresponding size distribution) and (b) SEM-EDS spectra of disordered Rh-PdCu NPs. (c) PXRD patterns of disordered PdCu and Rh-PdCu NPs (inset is the expanded (111) peak patterns). (d) HRTEM image of disordered Rh-PdCu NP (inset is the corresponding FFT).
We then turned our attention to build a bridge between the disordered NPs with fcc structure and the ordered intermetallic NPs with bcc structureviaannealing under H2/Ar atmosphere.To avoid the undesired severe agglomeration of NPs, the asprepared NPs were deposited on carbon supports before the annealing treatment (Fig. S3, SI). For PdCu NPs, the ordered bcc-phased intermetallic NPs can be obtained by annealing at 400 °C (Fig. S4, SI). By contrast, the annealing temperature has to be elevated to 500 °C for Rh-PdCu NPs before they can be completely converted (Fig. 2a); otherwise, they can only be partially converted to bcc Rh-PdCu because the introduction of Rh atoms can inhibit the phase conversion of PdCu. As the annealing temperature increases, the monodispersity on carbon support of Rh-PdCu can be well maintained (Fig. 2b and Fig. S5,SI), accompanied with negligible particle agglomeration (Fig.S6, SI). Furthermore, the lattice spacing of 0.210 nm can be clearly obtained from HRTEM and corresponding FFT, which can be well assigned to (110) facet of bcc Rh-PdCu NPs. Based on the above-mentioned results, we can establish the structural models shown in Fig. 2d. Highly uniform disordered PdCu NPs can be first obtained by regulating the reaction conditions, and then Rh atoms are further substituted for Pd or Cu species to form disordered Rh-doped PdCu NPs, and thermal treatment is finally performed to achieve the ordered intermetallic NPs.
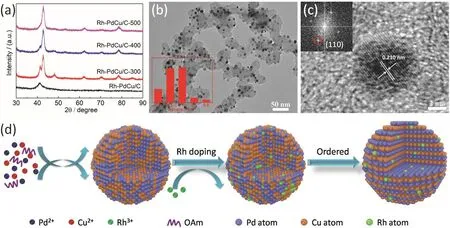
Fig. 2 Structural and compositional characterization of ordered intermetallic Rh-PdCu NPs. (a) PXRD patterns of Rh-PdCu/C with different annealing temperatures. (b) Representative TEM image of Rh-PdCu/C-500 (inset is the corresponding size distribution).(c) HRTEM image of Rh-PdCu/C-500 (inset is the corresponding FFT). (d) An illustration of the possible structural models of disordered PdCu NPs, disordered Rh-PdCu NPs and ordered Rh-PdCu NP.
In order to verify the advantages of ordered intermetallic Rhdoped PdCu NPs, the electrocatalytic ORR activities of PdCu/C,PdCu/C-400, Rh-PdCu/C and Rh-PdCu/C-500 were investigated in an O2-saturated 0.1 mol.L?1KOH solution at 1600 r?min?1with a scan rate of 20 mV.s?1. The commercial Pt/C served as a benchmark for comparing the electrocatalytic properties. Fig. 3a shows the positive-going ORR polarization curves of different catalysts, from which we can observe that both PdCu/C-400 and Rh-PdCu/C-500 have a positive shift compared with unannealed counterparts, respectively, indicative of the enhanced ORR activities of ordered intermetallic NPs. Moreover, for Rh-doped PdCu electrocatalysts, both the disordered and the ordered samples exhibit the decreased ORR overpotentials compared to the PdCu counterparts due to the positive potential shifts. The Rh-PdCu/C-500 possesses the highest half-wave potential (E1/2)of 0.909 V, which is 41, 13, 19 mV higher than that of PdCu/C,PdCu/C-400 and Rh-PdCu/C, respectively, and even 61 mV more positive than that of the commercial Pt/C, revealing the most remarkable electrocatalytic activity of Rh-doped ordered intermetallic NPs for ORR (Fig. S7, SI). The ORR kinetic currents were further normalized against the amount of noble metals (Pt or Pd) to quantify the intrinsic ORR mass activities of different catalysts. Among them, the Rh-PdCu/C-500 is found to exhibit the highest mass activity of 0.96 A.mg?1at 0.9 V (vsRHE), 7.4 times higher than that of commercial Pt/C (Fig. 3b).In addition, the mass activities of PdCu/C, PdCu/C-400 and Rh-PdCu/C are only 2.3, 5.4 and 4.5 fold higher than that of commercial Pt/C, respectively, indicating that the doping of Rh atoms and further ordering possess a significant effect in terms of enhanced ORR activities of PdCu NPs. Furthermore, the similar trend has been observed at 0.875 V (vsRHE), in which the Rh-PdCu/C-500 shows the most superior mass activity (1.72 A.mg?1), 6.3 times better than that of commercial Pt/C (Fig. 3c).Rh-PdCu NPs undergo an incomplete conversion when the annealing temperature cannot reach 500 °C (Fig. 2a), which results in decreased ORR activities (Fig. S8, SI). More excitingly, the ordered intermetallic Rh-doped PdCu NPs have far exceeded most of the state-of-the-art PdCu-based ORR electrocatalysts mentioned in previous reports with respect to ORR catalytic activity in alkaline condition (Table S1, SI).
The poor durability has severely limited the practical application of cathode ORR catalysts in fuel cells, and therefore become an indispensable parameter for evaluating the ORR electrocatalytic performance. The electrochemical durability of different catalysts in alkaline solution was measured by conducting the ADTs with a scan rate of 200 mV.s?1between 0.6 V to 1.0 V (vsRHE). There is negatively-shiftedE1/2of only 6 mV in the ORR polarization curves of Rh-PdCu/C-500 before and after 20000 consecutive scanning cycles (Fig. 3d), while theE1/2of the commercial Pt/C, PdCu/C, PdCu/C-400 and Rh-PdCu/C negatively shifted by 40, 20, 16 and 8 mV, respectively(Fig. S9, SI). More distinctly, the Rh-PdCu/C-500 showed only 13.9% loss in mass activity, whereas the commercial Pt/C,PdCu/C, PdCu/C and Rh-PdCu/C declined by 69.2%, 31.4%,28.8% and 17.0%, respectively, indicating the superior durability of Rh-doped ordered intermetallic NPs (Fig. 3e). The excellent durability of Rh-PdCu/C-500 can further be evidenced by the structural and compositional changes before and after ADTs(Fig. S10, SI). There is negligible morphology change and size change and no obvious composition change (from 5/48/47 to 6/50/44 for Rh/Pd/Cu) after 20000 cycles.

Fig. 3 ORR performance of different catalysts in 0.1 mol·L–1 KOH. (a) ORR polarization curves, (b) the mass activities and enhancement factors(vs the commercial Pt/C) at 0.9 V (vs RHE) and (c) the mass activities and enhancement factors (vs the commercial Pt/C) at 0.875 V (vs RHE) of different catalysts. (d) ORR polarization curves of Rh-PdCu/C-500 before and after different potential cycles. (e) The normalized mass activity changes of different catalysts at 0.9 V (vs RHE) before and after different potential cycles.
By summarizing the ORR electrocatalytic performance of different catalysts in terms of both mass activities and durability,two crucial conclusions can be drawn as follows: (a) doping Rh atoms into PdCu NPs contributes to enhance the activity and durability efficiently toward ORR electrocatalysis; (b) further an ordered intermetallic counterpart can also promote an enhanced ORR activity and durability. We can attribute the superior ORR performance of the Rh-PdCu/C-500 to the electronic and stabilizing effect caused by the trace Rh-doping, as well as the optimized stronger atomic interaction due to the ordered intermetallic structure. Firstly, it is widely accepted that the doping of heteroatoms tends to cause the charge transfer from the dopants to Pt/Pd atoms34,35, thus the rearrangement induced bythe partial substitution of Pd or Cu atoms by Rh atoms would result in a strong electron transfer of Pd atoms and the change of the surface electronic structure of PdCu NPs, which shifts the center of thed-band downward and gives rise to the reduced affinity of Pd to oxygen-containing intermediates21,36–38. This is of great benefit for improving the ORR performance of PdCu NPs. Furthermore, Rh has been considered as a positive element to enhance ORR performance in terms of both activity and durability due to the stabilizing effect of Rh atoms for structure and composition and suppression of the loss of transition metal atoms5,29,33, which can be further confirmed by the abovementioned TEM image and SEM-EDS spectra after ADTs (Fig.S10, SI). Finally, by rationally modulating the ordered intermetallic structure, stronger geometric and electronic effect will function in combination compared with the disordered Rh-PdCu/C, which changes the surface coordination of the active site and therefore causes the shift of d-band center and the change of adsorption strength towards oxygenated species21,22,25.In addition, a stronger atomic interaction and higher mixing enthalpy of ordered intermetallic structure also improve the chemical and structural stability, thereby higher activities and more superior durability towards ORR can be achieved24,39–41.
A common phenomenon in DMFCs is that the methanol molecules can crossover from anode to cathode through the proton exchange membranes, which dramatically decreases the power efficiency and stability of devices due to the strong adsorption of the CO-like species related to the interpenetrating methanol on catalysts surface, thus raising a challenge for the anti-poisoning properties of the cathode catalysts20,42–44. For this purpose, the methanol tolerance capacities of commercial Pt/C and Rh-PdCu/C-500 were further evaluated to highlight the preponderance on anti-poison of Rh-doped ordered intermetallic PdCu NPs. A typical methanol oxidation peak was clearly observed at 0.88 V (vsRHE) for commercial Pt/C in N2-saterated 0.1 mol.L–1KOH + 0.5 mol.L–1CH3OH (Fig. 4a), while this peak was hardly generated and CV curves underwent almost no variation in shape upon the addition of CH3OH for the Rh-PdCu/C-500 catalysts (Fig. 4b), indicating the lower electrocatalytic oxidation activity towards methanol of Rh-PdCu/C-500. The ORR polarization curves of commercial Pt/C exhibited a weak anti-methanol capacity as theE1/2negatively shifted by 235 mV and a distinct oxidation peak was observed(Fig. 4c). On the contrary, the shape of ORR polarization curve of Rh-PdCu/C-500 was almost unchanged, and theE1/2decreased by only 5 mV after adding 0.5 mol.L?1methanol into the electrolyte (Fig. 4d), revealing the better methanol antiinterference ability of Rh-PdCu/C-500 under ORR-tested conditions. The key factor to excellent methanol tolerance is that the CO poisoning generated during the diffusion of methanol molecules can be greatly relieved with the presence of Rh atoms45–47, which will significantly facilitate the application of ordered intermetallic Rh-doped PdCu NPs in practical devices.

Fig. 4 The methanol tolerance capacities of commercial Pt/C and Rh-PdCu/C-500. CV curves of (a) commercial Pt/C and(b) Rh-PdCu/C-500 recorded in N2-saturated 0.1 mol·L?1 KOH and 0.1 mol·L–1 KOH + 0.5 mol·L?1 CH3OH. ORR polarization curves of(c) commercial Pt/C and (d) Rh-PdCu/C-500 recorded in O2-saturated 0.1 mol·L?1 KOH and 0.1 mol·L–1 KOH + 0.5 mol·L?1 CH3OH.
4 Conclusions
To summarize, we have successfully demonstrated the strategy of combining Rh doping and ordering to noticeably enhance the ORR performance of PdCu NPs. The complete phase transition temperature of NPs was increased from 400 °C to 500 °C due to the introduction of Rh atoms. The as-obtained ordered intermetallic Rh-doped PdCu NPs exhibit the highest mass activity at 0.9 V (vsRHE) for ORR in alkaline condition with an enhancement factor of 7.4 compared to that of commercial Pt/C. The ADTs have been further conducted to confirm that the strategies are not only beneficial for enhancing ORR activity but also for long-term durability. We attributed the enhanced electrochemical performance to the electronic and stabilizing effect caused by the Rh-doping, as well as the optimized stronger atomic interaction due to the ordered intermetallic structure. In addition, we also found this catalyst presented the highly methanol tolerance behavior compared to commercial Pt/C due to its special elemental composition. This work highlights the indispensable role of Rh atoms in achieving high-performance ORR catalysts as well as the potential of ordered intermetallics as candidate electrocatalysts in the field of DMFCs.
Acknowledgment:The authors acknowledge the support from the Tencent Foundation through the XPLORER PRIZE.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.

