抗寒無核葡萄雜種胚挽救及分子標記輔助選擇
朱佩佩,羅燚佳,向雯,張明磊,張劍俠
抗寒無核葡萄雜種胚挽救及分子標記輔助選擇
朱佩佩,羅燚佳,向雯,張明磊,張劍俠
西北農林科技大學園藝學院/旱區作物逆境生物學國家重點實驗室/農業部西北地區園藝作物生物與種質創制重點實驗室,陜西楊凌 712100
【】利用胚挽救技術創制抗寒無核葡萄新種質,為選育抗寒無核葡萄新品種奠定基礎。分別以抗寒歐美雜交無核葡萄品種‘Jupiter’和歐山雜種有核優系‘00-1-5’(‘玫瑰香’ב山葡萄黑龍江實生’)為父本,5個歐亞種無核品種‘Ruby Seedless’‘秦紅1號’‘秦紅2號’‘Crimson Seedless’和‘秦秀’為母本雜交,在各母本最佳胚珠取樣時期取樣,以固-液雙相培養基MM3和ER分別作為胚發育培養基進行胚珠培養,比較不同母本和2種培養基對胚發育率、萌發率、成苗率及畸形苗率的影響;以WPM+0.2 mg?L-16-BA+0.1 mg?L-1IAA固體培養基作為胚萌發培養基,獲得胚挽救后代,經煉苗后移栽至田間;分別在2MS+0.2 mg?L-16-BA+0.1 mg?L-1IAA培養基中添加0、1.0和1.6 mg?L-1ZnSO4進行畸形苗轉化正常苗培養,以篩選最適宜轉化培養基;利用無核基因分子標記SCF27-2000對胚挽救后代進行無核性狀分子檢測。5個雜交組合的胚珠接種至MM3培養基的數量為2 158個,獲得發育胚175個和胚挽救苗118株;接種至ER培養基的胚珠數為894個,獲得發育胚74個和胚挽救苗58株。以‘00-1-5’為父本的2個雜交組合中,‘秦紅2號’比‘秦秀’更適合作為母本,其雜種胚的發育率和成苗率最高,分別為17.04%和7.41%;以‘Jupiter’為父本的3個雜交組合中,‘Ruby Seedless’בJupiter’效果最好,胚發育率和成苗率分別為13.71%和10.67%,其次是‘秦紅1號’בJupiter’,胚發育率和成苗率分別為14.39%和4.71%,而‘Crimson Seedless’בJupiter’胚發育率和成苗率最低,分別為9.55%和1.76%。接種至MM3培養基的胚珠,獲得的胚發育率和成苗率均高于ER培養基。畸形苗率最高的是‘秦紅1號’בJupiter’,而‘Ruby Seedless’בJupiter’未出現畸形苗,ER培養基比MM3培養基形成的畸形苗率高。‘秦紅2號’ב00-1-5’組合畸形苗接種至2MS+1.6 mg?L-1ZnSO4+0.2 mg?L-16-BA+0.1 mg?L-1IAA轉化培養基效果最好,轉化率為40.00%。利用無核標記SCF27-2000對5個雜交組合176株雜種后代檢測表明,159個株系擁有無核基因分子標記。‘Ruby Seedless’‘秦紅1號’和‘秦紅2號’適宜作為胚挽救育種的母本,而‘Crimson Seedless’和‘秦秀’不適合作為胚挽救育種的母本,培養基MM3比ER更適宜作為胚發育培養基,擁有無核基因分子標記的159株胚挽救苗為培育抗寒無核葡萄新品種提供了種質基礎。
無核葡萄;抗寒性;胚挽救;新種質;分子標記輔助選擇
0 引言
【研究意義】無核葡萄是育種的一個重要目標。目前世界上栽培的無核葡萄品種大多屬于歐亞種葡萄(L.),品質優良,但抗寒性差,生產上缺乏抗寒無核葡萄品種,限制了其在寒冷地區的發展。原產中國的野生山葡萄(Rupr.)可耐-40℃的低溫,是葡萄屬中極為重要的抗寒種質資源[1-2],此外,原產北美洲的美洲葡萄(L.)也是重要的抗寒種質資源[2]。利用抗寒的歐山雜種(×)或歐美雜種(×)作父本,與種子敗育型歐亞種無核葡萄品種作母本雜交,借助胚挽救技術,在雜種胚敗育前提供種胚正常發育所需的營養物質使其萌發成苗,是創制抗寒無核葡萄新種質的有效途徑,將為進一步選育抗寒無核葡萄新品種奠定基礎。【前人研究進展】1982年,美國葡萄育種家RAMMING和EMERSHAD[3]首次以無核葡萄品種的胚珠進行離體培養,獲得了2株雜種,標志著無核葡萄胚挽救技術的創立。由于傳統的無核葡萄育種只能以有核品種作母本與無核品種作父本雜交,雜交后代中無核雜種的幾率極低,育種效率低,周期長;而胚挽救技術以無核葡萄作母本與無核或有核品種作父本雜交,擴大了母本選擇的范圍,提高了育種的效率,從而使該技術在許多國家得到廣泛的研究和應用[4-7]。迄今,國內外已通過該技術選育出‘Melissa’‘Autumn Crisp’‘Early Sugar’‘秦紅無核’和‘秦紅1號’等47個無核葡萄新品種[8]。作為一項新的無核葡萄育種技術,影響其成功的因素很多,如父母本基因型、花前噴施激素、取樣時期、基本培養基及其形態、添加有機物種類與濃度、培養條件等[9-13]。親本基因型是影響胚挽救效果的一個關鍵因素,特別是母本基因型,前人研究認為‘Flame Seedless’[4]、‘Ruby Seedless’[4,9]、‘Perlette’[9]、‘秦紅2號’[9]和‘Askari’[13]等適宜作胚挽救母本。徐海英等[6]研究認為胚過早發生敗育的品種不適宜作胚挽救的母本,如‘Thompson Seedless’和‘Himrod’等,但Pommer等[12]認為早熟和中熟品種比晚熟品種作母本的胚挽救效果更好。此外,父本對胚挽救效果也有一定的影響[9-10]。培養基及其形態是決定胚挽救成敗的另一關鍵因素。Razi等[13]認為NN培養基培養效果好,也有使用ER培養基作為胚發育培養基[10],Pommer等[12]使用Cain培養基作為胚發育培養基,但筆者課題組研究獲得的MM3培養基(改良ER培養基)被認為更適合以中國野生葡萄作為父本的胚挽救育種[9,14]。關于培養基的形態,Spiegel-Roy等[11]研究認為固相培養基最好,但RAMMING等[15]認為液體培養基效果好。TIAN等[16]認為固-液雙相培養基最適合作為胚發育培養基。由此可見,胚發育培養基及其形態有必要做進一步的研究。對于通過胚挽救技術獲得的雜種,一般是在樹體結果后通過田間鑒定是否為無核性狀,來確定取舍,育種速度較慢,而利用分子標記技術可以實現對目標性狀在幼苗期的鑒定,極大縮短品種選育時間[17]。目前已獲得的葡萄無核基因分子標記主要有SCAR標記SCC8-1018[18]、SCF27-2000[19],RAPD標記GSLP1-569[20],SSR標記p3-VvAGL11-216[21]、VMC7F2-198[22]和VvSD10[23]。其中以SCF27-2000對無核葡萄品種檢測具有較廣泛的適用性[14,24]。【本研究切入點】針對目前無核葡萄胚挽救中胚發育率、萌發率和成苗率仍較低,以及生產上缺乏抗寒無核葡萄品種的問題,本研究對胚挽救的主要影響因素(母本和胚發育培養基)進行研究,配置無核品種×抗寒歐山雜種、無核品種×抗寒歐美雜種雜交組合,以篩選出適宜作胚挽救的母本,比較2種胚發育培養基MM3和ER的胚挽救效果,并篩選出適宜畸形苗轉化為正常苗的培養基,同時對胚挽救后代進行早期無核性狀的分子標記輔助選擇。【擬解決的關鍵問題】通過比較母本基因型、篩選胚發育培養基、篩選畸形苗轉化正常苗培養基及分子標記輔助選擇,為提高無核葡萄胚挽救效率提供參考依據,同時創制抗寒無核葡萄新種質,為進一步選育抗寒無核葡萄新品種奠定材料基礎。
1 材料與方法
2018年4月至2019年5月在西北農林科技大學葡萄種質資源圃、旱區作物逆境生物學國家重點實驗室與園藝學院葡萄育種與分子生物學實驗室進行。
1.1 親本材料
共設計5個雜交組合,分別是‘秦紅2號’ב00-1-5’、‘秦秀’ב00-1-5’、‘Ruby Seedless’בJupiter’、‘秦紅1號’בJupiter’和‘Crimson Seedless’בJupiter’。其中父本‘Jupiter’為抗寒歐美雜交無核品種,引自美國;另一父本‘00-1-5’(‘玫瑰香’ב山葡萄黑龍江實生’)為抗寒歐山雜種有核優系,由筆者課題組選育。父、母本均為二倍體品種(系)。
1.2 方法
1.2.1 葡萄田間雜交 在父本植株上一個花序中有5%小花開放時取花穗,制備新鮮花粉,然后干燥、低溫保存。母本在開花前3 d,選擇健壯植株上發育一致的花穗進行人工去雄(圖1-A)。2—3 d后,于上午7:00—9:00進行授粉,連續授粉3 d以保證授粉效率。
1.2.2 胚珠培養 5個母本品種‘秦秀’‘Crimson Seedless’‘秦紅2號’‘秦紅10號’和‘Ruby Seedless’的取樣時期分別為花后42 d[9]、51 d[9]、51 d[24]、51 d[24]和61 d[9]。對采集的幼果先用自來水進行表面沖洗,然后在超凈工作臺中依次用濃度為75%乙醇和1%次氯酸鈉進行表面消毒,再用無菌水沖洗3次,最后用手術刀剝取胚珠,接種于胚發育培養基中。
將5個組合‘秦秀’ב00-1-5’、‘秦紅2號’ב00-1-5’、‘Ruby Seedless’בJupiter’、‘Crimson Seedless’בJupiter’和‘秦紅1號’בJupiter’的胚珠接種在MM3固-液雙相培養基中,研究不同母本對胚挽救效果的影響。
將材料較多的2個組合‘秦紅2號’ב00-1-5’和‘Ruby Seedless’בJupiter’的胚珠,分別接種在固-液雙相培養基ER和MM3中,比較不同培養基對胚挽救效果的影響。
兩種培養基添加物均為0.5 g?L-1水解酪蛋白+1 mmol?L-1絲氨酸+0.5 mg?L-1GA3+1.0 mg?L-1IAA+ 0.5 mg?L-16-BA+60 g?L-1蔗糖+3 g?L-1活性炭+7 g?L-1瓊脂(pH 6),其中液相部分不加瓊脂。采用容量為200 mL的培養瓶,每瓶接種20個胚珠。培養瓶上覆蓋黑布進行遮光培養,溫度為(25±1)℃,培養9—10周。
1.2.3 胚萌發培養 胚珠培養9—10周后,在超凈工作臺中借助解剖鏡剝取裸胚,接種到胚萌發培養基中(圖1-B),置于(25±1)℃、每日光照16 h的培養室培養。40 d后,胚萌發成苗(圖1-C—E)。萌發培養基為WPM固體培養基,培養基成分為:WPM+0.2 mg?L-16-BA+0.1 mg?L-1IAA+20 g?L-1蔗糖+1.5 g?L-1活性炭+7 g?L-1瓊脂(pH 6)。
幼苗在煉苗移栽前需要進行繼代培養,培養基為1/2 MS+0.2 mg?L-1IBA+30 g?L-1蔗糖+7 g?L-1瓊脂(pH 6)。
1.2.4 畸形苗觀察及轉化培養基篩選 胚萌發培養5—6周后,觀察不能正常萌發的畸形苗,包括子葉扭曲無根苗、白化苗、玻璃化苗、矮小苗等,比較不同母本組合及同一組合分別在MM3和ER固-液雙相培養基上的畸形苗率。
將‘秦紅2號’ב00-1-5’組合產生的畸形苗,分別接種至添加0、1.0和1.6 mg?L-1ZnSO4的2MS+0.2 mg?L-16-BA+0.1 mg?L-1IAA培養基中,培養30 d后統計畸形苗轉化為正常苗的百分率。
1.2.5 無核基因分子標記輔助選擇 采用CTAB法提取雜交后代株系基因組DNA,利用葡萄無核基因SCAR標記SCF27-2000的引物(F:5′-CAGGTGGGA GTAGTGGAATG-3′;R:5′-CAGGTGGGAGTAAGA TTTGT-3′)對雜交組合親本及后代進行PCR檢測,凡能擴增出2 000 bp大小的條帶即初步確定為具有無核性狀。

A:去雄后的花穗;B:胚;C—E:幼胚萌發成苗;F:胚挽救苗移栽至田間
PCR反應程序為:94℃預變性4 min;95℃變性30 s,62℃復性90 s,72℃延伸1 min,共35個循環;72℃延伸1 min,12℃終止。
1.2.6 數據統計與分析 記錄各組合胚的發育數、萌發數、成苗數和畸形苗數,統計胚的發育率、萌發率、成苗率、畸形苗率及畸形苗轉化率,計算方法如下:
胚的發育率(%)=從胚珠中剝取胚的個數/胚珠數×100
胚的萌發率(%)=萌發胚的個數/胚珠數×100
胚的成苗率(%)=成苗數/胚珠數×100
畸形苗率(%)= 畸形苗數/胚珠數×100
畸形苗轉化率(%)=轉化成的正常苗數/畸形苗數×100
使用SPSS 22.0軟件,對同一雜交組合3次生物
學重復中胚的發育率、萌發率、成苗率、畸形苗率和畸形苗轉化率進行差異顯著性分析(<0.05)。
2 結果
2.1 不同母本對胚挽救效果的影響
5個雜交組合中不同母本的胚發育率、萌發率和成苗率的結果(表1)顯示,以MM3為胚發育培養基,當以‘00-1-5’為父本,‘秦紅2號’為母本的雜交組合的胚發育率和成苗率顯著高于‘秦秀’ב00-1-5’,分別為17.04%和7.41%。以‘Jupiter’為父本,‘Ruby Seedless’為母本胚挽救效果最好,發育率和成苗率為13.71%和10.67%;其次是‘秦紅1號’為母本的雜交組合,而‘Crimson Seedless’作母本胚的發育率和成苗率最低,與其他兩個組合均具有顯著性差異(<0.05)。

表1 不同母本對胚挽救效果的影響
不同小寫字母代表在<0.05水平上差異顯著。下同
Different lowercase letters represent significant differences at<0.05 level. The same as below
2.2 不同發育培養基對胚挽救效果的影響
將‘Ruby Seedless’בJupiter’和‘秦紅2號’ב00-1-5’的胚珠分別接種至MM3和ER基本培養基。對于‘Ruby Seedless’בJupiter’組合,接種至MM3培養基所獲得的胚發育率和成苗率顯著高于ER培養基(<0.05)(表2);組合‘秦紅2號’ב00-1-5’在MM3培養基中,胚的發育率和成苗率比ER培養基高,兩種培養基中胚的發育率和成苗率之間差異顯著(<0.05)。
2.3 不同母本及發育培養基對畸形苗率的影響
對5個雜交組合幼胚在MM3培養基中的畸形苗率的調查結果(表3)表明,畸形苗率最高的是‘秦紅1號’בJupiter’,為5.96%,而組合‘Ruby Seedless’בJupiter’未出現畸形苗,其他3個組合介于1.17%—5.03%。
不同發育培養基對不同雜交組合畸形苗率的影響結果見表4。對于‘秦紅2號’ב00-1-5’組合,在ER培養基上獲得的畸形苗率更高,顯著高于MM3培養基,為4.15%。而‘Ruby Seedless’בJupiter’在MM3和ER培養基上均未出現畸形苗。
2.4 畸形苗轉化培養基的篩選
‘秦紅2號’ב00-1-5’組合的45株畸形苗轉化結果(表5)表明,畸形苗轉接至2MS+1.6 mg?L-1ZnSO4+0.2 mg?L-16-BA+ 0.1 mg?L-1IAA轉化培養基上效果較好,轉化率為40.00%,顯著高于對照組。

表2 胚發育培養基MM3與ER對胚挽救成苗的影響
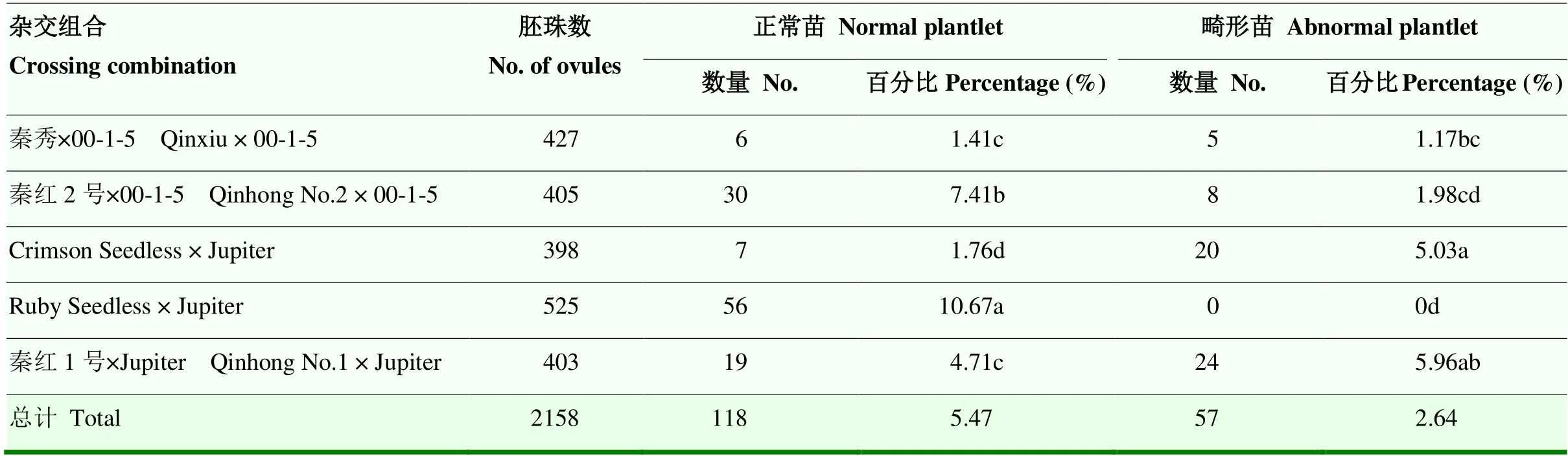
表3 母本對畸形苗形成的影響

表4 MM3和ER培養基對畸形苗率的影響
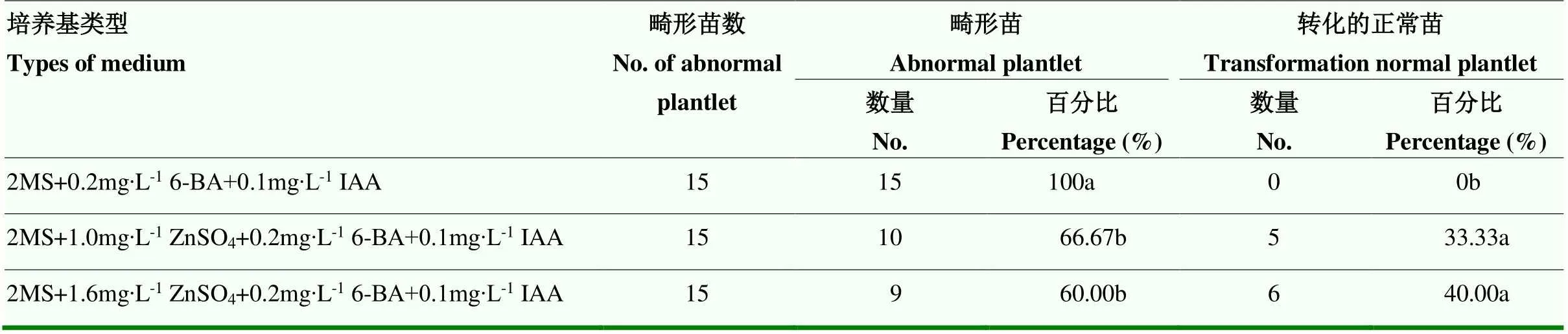
表5 不同ZnSO4濃度對畸形苗轉化率的影響
2.5 利用無核基因分子標記對雜交后代的無核性狀鑒定
利用無核基因分子標記SCF27-2000對‘秦紅2號’ב00-1-5’組合的53個雜種株系進行檢測,表明51個株系擴增出特異條帶(圖2),該組合無核率為96.23%。

M:Marker (2k Plus DNA marker) 1:秦紅2號;2:00-1-5;3—55:‘秦紅2號’ב00-1-5’組合雜交后代。“+”表示具有2000 bp特異帶;“-”表示無2000 bp特異帶。下同
利用無核標記SCF27-2000對‘秦紅1號’בJupiter’組合的19個雜種株系進行檢測,所有株系均擴增出特異條帶(圖3),該組合無核率為100%。
利用無核基因分子標記SCF27-2000分別對‘秦秀’ב00-1-5’‘Ruby Seedless’בJupiter’和‘Crimson Seedless’בJupiter’組合的雜種株系進行檢測,無核率分別為83.33%、90.59%和100%。
3 討論
SAHIJRAM等[7]認為,利用傳統育種方法對種子敗育型無核葡萄作親本進行雜交,很難獲得無核雜種后代。而胚挽救技術能夠解決這一問題,但影響胚挽救成功的因素很多,如親本類型、激素處理、取樣時期、培養基、培養條件等[9,24-26]。NOTSUKA等[5]研究認為,由于無核葡萄胚挽救中選用的母本不同,雜交親和性不同,形成合子胚的比率不同,合子胚敗育時間也不相同,用不同品種作母本進行雜交,胚的發育率不同。本試驗結果表明,‘Ruby Seedless’‘秦紅1號?’和‘秦紅2號?’較適合作母本,這與LIU等[14]和VALDEZ[25]及筆者課題組[9]前期的結果相一致。而‘秦秀’和‘Crimson Seedless’作母本的胚挽救效果較差,但LIU等[14]和SIMIN等[26]認為‘Crimson Seedless’較適合作胚挽救母本,可能是由于每年物候期不同、取樣時期不同和選用的培養基不同影響胚挽救的效果。不同母本由于基因型不同,胚珠內營養狀況不同,受精卵發育程度不同,幼胚離體培養后進一步發育的程度也不同,造成部分幼胚不能萌發成正常植株。當‘Crimson Seedless’‘秦紅1號’和‘秦秀’作母本時,畸形苗率較高,而‘Ruby Seedless’‘秦紅2號’作母本時,畸形苗率較低。以上說明‘Ruby Seedless’‘秦紅1號’和‘秦紅2號’較適合作胚挽救母本。
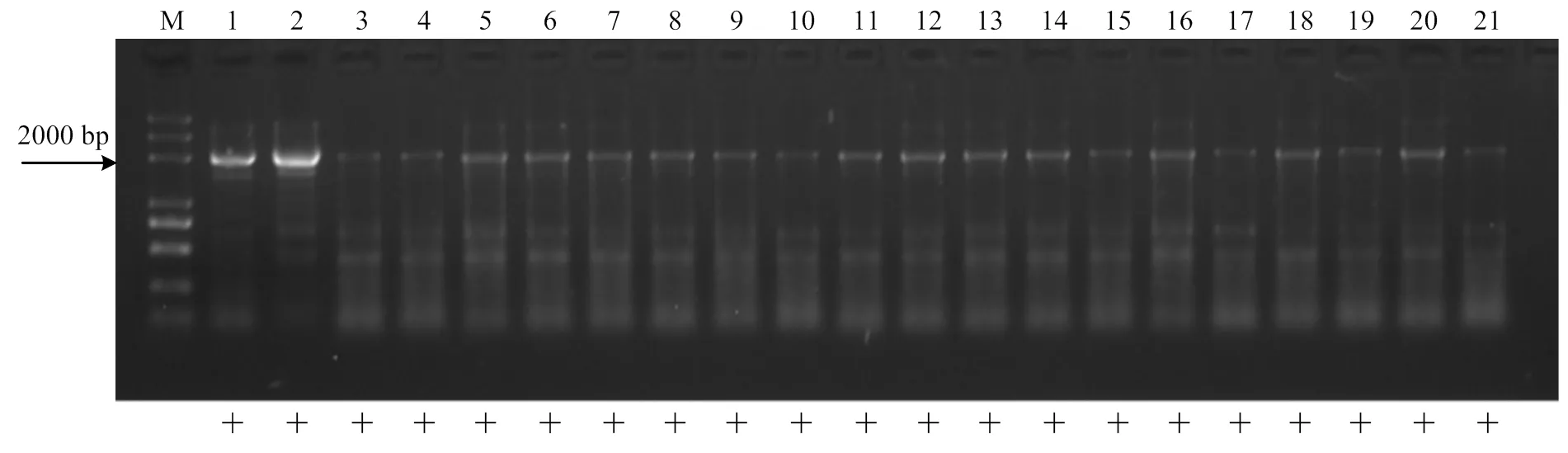
M:Marker (2k Plus DNA marker);1:秦紅1號;2:Jupiter;3—21:‘秦紅1號’בJupiter’組合雜交后代
胚發育培養基為離體胚珠提供了幼胚體外發育所需的生長環境和營養物質,是影響胚挽救效果的關鍵因素之一。前人對胚發育培養基和萌發培養基進行了大量研究[5-7,11-13],目前應用較多的胚發育培養基主要有Bouquet and Davis(BD)培養基(1989)[27]、Nitsch and Nitsch(NN)培養基(1969)[28]、Cain培養基(1983)[12]、Emershad and Ramming(ER)培養基(1984)和MM3培養基等[29-32]。EBADI等[33]以‘Flame Seedless’自然授粉胚珠為研究對象,認為NN培養基比ER培養基效果更好。BURGER等[29]認為采用BD培養基并添加活性炭效果最好。PONCE等[34]認為MS培養基效果最好。KHOSHANDAM等[32]認為NN培養基適合作為胚發育培養基。趙雅楠等[35]認為MM3培養基效果比ER培養基效果好。本研究發現‘Ruby Seedless’בJupiter’接種在MM3培養基中,胚挽救效果最好,胚的成苗率最高,這與LIU等[14]、LI[24]等的結果相一致。MM3比ER培養基中多添加了半胱氨酸鹽酸鹽,半胱氨酸鹽酸鹽具有抗氧化和防止非酶褐變的作用,推測其可以減輕剝取胚珠時造成的損傷,促進胚的進一步發育,減少畸形苗的形成。
發育培養基選定后,在培養基中添加適量激素配比也會顯著促進胚的發育。KUMAR等[30]認為植物激素在植物發育各個方面的調節中具有重要作用,因此被廣泛研究。LI等[4]認為在WPM培養基中添加0.1 μmol?L-16-BA作胚發育培養基效果最好。RAZI等[13]認為IAA的濃度對胚萌發具有顯著影響,‘Askari’בRuby Seedless’和‘Askari’בBidane Sefid’組合在NN培養基上添加1 mg?L-1IAA效果最好。KHOSHANDAM等[32]認為在NN培養基中添加0.35 mg?L-1GA3和1 mg?L-1IAA效果最好。GRAY等[36]發現培養基中添加BA能顯著提高胚萌發率。本研究表明,當以MM3為胚發育培養基時,除添加IAA濃度與RAZI等[13]和KHOSHANDAM等[32]相同外,還應添加0.5 mg?L-1GA3和0.5 mg?L-16-BA,適宜作為抗寒無核葡萄胚挽救的胚發育培養基。KHOSHANDAM等[32]認為NN培養基也可以作為胚萌發培養基。POMMER等[12]用WPM液體培養基在濾紙橋上培養幼胚。EBADI等[33]也認為WPM培養基作為胚萌發培養基效果較好。
有研究表明,在胚挽救過程中,部分萌發的胚畸形生長,不能形成正常幼苗[25]。LESHEM等[37]認為培養基中添加激素后能促進和誘導畸形苗的產生,可能是由于生長素和細胞分裂素的比例不協調所造成。LIU等[38]認為胚挽救過程中畸形苗的出現可能與胚發育水平相關。Ji等[39]認為2MS+0.4 mg?L-16-BA+0.1 mg?L-1IBA+10 μmol?L-1ZnSO4作轉化正常植株的培養基,轉化率最高,為27.9%,本研究與此結果類似。
胚挽救苗獲得后,需要對其進行無核性狀檢測,保留具有目的性狀的后代。田間鑒定方法準確有效,但等待結果的時間較長。分子標記輔助選擇實現了對基因型的直接選擇,是早期輔助選擇的有效手段[17]。目前應用較多的葡萄無核基因分子標記有SCAR標記SCC8-1018、SCF27-2000和RAPD標記GSLP1- 569[9,14,35]。前期研究表明,SCF27-2000標記可以有效區分無核與有核親本,被許多研究者使用[9,14,24],利用該分子標記鑒定的無核率與田間測定的實際無核率相關性達81%[19]。而SCC8-1018和GSLP1-569只能區分部分無核與有核品種,特別是GSLP1-569僅對‘無核白’及其親緣關系較近的無核品種具有良好的適用性[9,14]。因此,本研究采用SCF27-2000對親本及其雜交后代進行無核性狀鑒定。由于分子標記與無核基因的連鎖程度不同,連鎖程度越高,檢測無核性狀的準確率越高,因此,篩選連鎖緊密的無核基因分子標記仍然是本領域研究的重要內容。對于已獲得的胚挽救后代,除了采用無核基因分子標記SCF27-2000進行早期鑒定外,仍需要與傳統的田間鑒定相結合,以篩選出真正的無核雜種單株。
胚挽救雜種后代無核性狀的遺傳較為復雜,目前已有許多無核性狀遺傳的假說,包括單顯性基因假說[40]、單隱性基因假說[41]、數量性狀假說[42]、2隱性基因假說[43]、3互補顯性基因假說[44]、1顯性3隱性假說[45]與1主效基因3微效基因假說[46]等。由于葡萄無核機理尚未研究清楚,現有的假說都不夠全面[8,47]。RAMMING等[15]認為無核品種×無核品種無核后代比率理論上可達100%,本研究中無核品種×無核品種雜交后代無核率在90.59%—100%,與RAMMING等[15]的假說基本一致。但是,無核品種×有核品種雜交后代無核率達到83.33%—96.23%,與筆者前期研究結果一致[9],但高于NOTSUKA等[5]、LIU等[14]和趙雅楠等[35]的結果,推測不同組合對無核性狀的遺傳力不同,還可能與細胞質遺傳有關[48]。因此,選擇無核性狀傳遞力強的無核葡萄品種作為胚挽救的母本,有利于獲得無核雜種。
4 結論
母本基因型對胚挽救效果有顯著影響。以‘00-1-5’作父本時,‘秦紅2號’作母本的胚挽救效果較好;以‘Jupiter’作為父本時,‘Ruby Seedless’作母本的胚挽救效果較好。以‘Ruby Seedless’和‘秦紅2號’作母本時,雜種胚的成苗率較高且畸形苗率較低,說明‘Ruby Seedless’和‘秦紅2號’適宜作胚挽救母本。‘秦紅1號’作母本雜種胚的成苗率較高,但是畸形苗率也比較高;而‘Crimson Seedless’和‘秦秀’作母本時,雜種胚的成苗率低且畸形苗率較高,說明‘Crimson Seedless’和‘秦秀’不適合作母本。對于‘Ruby Seedless’בJupiter’和‘秦紅2號’ב00-1-5’2個組合,在MM3固-液雙相培養基上的成苗率均比ER固-液雙相培養基高,畸形苗率比ER固-液雙相培養基低,表明MM3比ER培養基更適合作為胚發育培養基。利用無核基因分子標記SCF27-2000對胚挽救后代進行無核性狀早期鑒定,初步判定獲得的159個胚挽救苗是潛在的無核株系。
[1] LI G R, CHENG S S, JI W, HOU X J, HU H L.embryo rescue of F-1 progenies from crosses between seedless grapes and Chinese wild types. Bangladesh Journal of Botany, 2019, 48(3): 755-761.
[2] ZHANG J, WU X, NIU R K, LIU Y C, LIU N, XU W H, WANG Y C. Cold-resistance evaluation in 25 wild grape species. Vitis, 2012, 51(4): 153-160.
[3] RAMMING D W, EMERSHAD R L.embryo culture of seeded and seedless. HortScince, 1982, 17: 487.
[4] LI S S, LI Z Y, ZHAO Y N, ZHAO J, LUO Q W, WANG Y J. New disease-resistant, seedless grapes are developed using embryo rescue and molecular markers. 3 Biotech, 2020, 10(1): 1-12.
[5] NOTSUKA K, TSURU T, SHIRAISHI M. Seedless-seedless grape hybridization via in-ovule embryo culture. Journal of the Japanese Society for Horticultural Science, 2001, 70(1): 7-15.
[6] 徐海英,閻愛玲, 張國軍.葡萄二倍體與四倍體品種間雜交胚珠的離體培養.果樹學報, 2001, 18(6): 317-320.
XU H Y, YAN A L, ZHANG G J. Study on theembryo culture of the crossed progeny between diploid and tetraploid grape cultivars.Journal of Fruit Science, 2001, 18(6): 317-320. (in Chinese)
[7] SAHIJRAM L, MURTHY B N S, SHIKHAMANY S D. Introgression of downy mildew resistance into grape cv. Thompson Seedless through hybrid embryo rescue. Indian Journal of Horticulture, 2005, 62(2): 107-111.
[8] 李莎莎, 王躍進. 葡萄無核基因及無核育種研究進展. 園藝學報, 2019, 46(9): 1711-1726.
LI S S, WANG Y J. Advances in seedless gene researches and seedless breeding in grapevine., 2019, 46(9): 1711-1726. (in Chinese)
[9] ZHU P P, GU B, LI P Y, SHU X, ZHANG X, ZHANG J X. New cold-resistant, seedless grapes developed using embryo rescue and marker-assisted selection. Plant Cell Tissue and Organ Culture (PCTOC), 2019, 140(3): 551-562.
[10] LI J, WANG X H, WANG X P, WANG Y J. Embryo rescue technique and its applications for seedless breeding in grape. Plant Cell Tissue Organ Cult (PCTOC), 2015, 120(3): 861-880.
[11] SPIEGEL-ROY P, SAHAR N, BARON J, LAVI U.culture and plant formation from grape cultivars with abortive ovules and seeds.Journal of the American Society for Horticultural Science, 1985, 110: 109-112.
[12] POMMER C V, RAMMING D W, EMERSHAD R L. Influence of grape genotype, ripening season, seed trace size, and culture date on in ovule embryo development and plant formation. Bragantia, 1995, 54(2): 237-249.
[13] RAZI M, MARANDI R J, BANEH H D, DOULATI BANEH H, HOSSEINI B, DARVISHZADEH R. Effect of paternal genotypes sprays with BA and IAA concentration on embryo rescue of F1progenies from ‘Askari’ (L.) cultivar. Journal of Agricultural Science & Technology, 2013, 15(5): 1023-1032.
[14] LIU Q, ZHANG J, WANG Y, YU D, XIA H. Breeding for cold-resistant, seedless grapes from Chinese wildusing embryo rescue. New Zealand Journal of Experimental Agriculture, 2015, 44(2): 136-151.
[15] RAMMING D W, EMERSHAD R L, SPIEGEL ROY P, SAHAR N, BARON I. Embryo culture of early ripening seeded grape () genotypes.HortScience, 1990, 25(3): 339-342.
[16] TIAN L L, WANG Y J. Seedless grape breeding for disease resistance by using embryo rescue. Vitis Geilweilerhof, 2015, 47(1): 15-19.
[17] 方宣鈞, 吳為人, 唐紀良. 作物DNA標記輔助育種. 北京:科學出版社, 2001: 2-6.
FANG X J, WU W R, TANG J L. DNA Marker-Assisted Crop Breeding. Beijing: Science Press, 2001: 2-6. (in Chinese)
[18] LAHOGUE F, THIS P, BOUQUET A. Identification of a codominant scar marker linked to the seedlessness character in grapevine. Theoretical and Applied Genetics, 1998, 97(5/6): 950-959.
[19] MEJíAN, HINRICHSEN P. A new, highly assertive SCAR marker potentially useful to assist selection for seedlessness in table grape breeding. Acta Horticulturae, 2003, 603: 559-564.
[20] 王躍進, LAMIKANRA O. 檢測葡萄無核基因DNA探針的合成與應用. 西北農林科技大學學報(自然科學版), 2002, 30(3): 42-46.
WANG Y J, LAMIKANRA O. Application and synthesis on the DNA probe for detecting seedless genes in grapevine. Journal of Northwest A & F University (Natural Science Edition), 2002, 30(3): 42-46. (in Chinese)
[21] KARAAGAC E, VARGAS A M, ANDRéS M T D, CARRE?O I, IBá?EZ J, CARRE?O J, MARTíNEZ-ZAPATER J, CABEZAS J. Marker assisted selection for seedlessness in table grape breeding. Tree Genetics & Genomes, 2012, 8(5): 1003-1015.
[22] CABEZAS J A, CERVERA M T, RUIZ-GARCIA L, CARRENO J, MARTINEZ-ZAPATER J M. A genetic analysis of seed and berry weight in grapevine. Genome, 2006, 49(12): 1572-1585.
[23] 馬亞茹, 馮建燦, 劉崇懷, 樊秀彩, 孫海生, 姜建福, 張穎. 葡萄無核性狀的SSR新分子標記開發及應用. 中國農業科學, 2018, 51(13): 2622-2630.
MA Y R, FENG J C, LIU C H, FAN X C, SUN H S, JIANG J F, ZHANG Y. Development and application of SSR new molecular marker for seedless traits in grape. Scientia Agricultura Sinica, 2018, 51(13): 2622-2630. (in Chinese)
[24] LI Z Q, Li T M, WANG Y J, XU Y. Breeding new seedless grapes using in ovulo embryo rescue and marker-assisted selection. In Vitro Cell Developmental Biology-Plant, 2015, 51(3): 241-248.
[25] VALDEZ J G. Immature embryo rescue of grapevine (L.) after an extended period of seed trace culture. Vitis, 2005, 44(1): 17-23.
[26] SIMIN U, KESGIN M, DILLI Y. The success ofembryo rescue technique in hybridization of seedless grape varieties.World Congress of Vine and Wine, 2015, 38(5): 01008.
[27] KIM S H, KWON J H, PARK Y S, HEO J Y.embryo rescue for the production of hypotetraploids after cross between hypotetraploid and tetraploid grape cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 2020, 48(1): 503-508.
[28] EMERSHAD R L, RAMMING D W.embryo culture ofL. cv. ‘Thompson Seedless’. American Journal of Botany, 1984, 71(6): 873-877.
[29] BURGER P, GOUSSARD P G.culture of ovules and embryos from seedless grapes (L.). South African Journal for Enology & Viticulture, 1996, 17(2): 31-37.
[30] KUMAR R, KHURANA A, SHARMA AK. Role of plant hormones and their interplay in development and ripening of fleshy fruits.Journal of Experimental Botany, 2014, 65(16): 4561-4575.
[31] BHARATHY P V, KARIBASAPPA G S, PATIL S G, AGRAWAL D C. In ovulo rescue of hybrid embryos in Flame seedless grapes-influence of pre-bloom sprays of benzyladenine. Scientia Horticulturae, 2005, 106(3): 353-359.
[32] KHOSHANDAM L, BANEH H D, MARANDI R J, Darwishzadeh R. Effect of BA and ovule developmental stages on embryo rescue in Perlette grape (L.) cultivar. Journal of Natural and Social Sciences, 2017, 6(1): 1-9.
[33] EBADI A, AALIFAR M, FARAJPOUR M, MOGHADDAM M R F. Investigating the most effective factors in the embryo rescue technique for use with ‘Flame Seedless’ grapevine (). Journal of Horticultural Science & Biotechnology, 2016, 91(5): 441-447.
[34] PONCE M T, GUI?AZú M E, TIZIO R. Brief note improved in vitro embryo development of stenospermic grape by putrescine. Biocell, 2002, 26(2): 263-266.
[35] 趙雅楠, 駱強偉, 王躍進. 利用胚挽救技術創制無核抗寒葡萄新種質. 中國農業科學, 2018, 51(21): 4119-4130.
ZHAO Y N, LUO Q W, WANG Y J. Breeding for grape germplasm involved in seedlessness with cold-resistant using embryo rescue. Scientia Agricultura Sinica, 2018, 51(21): 4119-4130. (in Chinese)
[36] GRAY D J, MORTENSEN J A, BENTON C M, DURHAM R E, MOORE G A. Ovule culture to obtain progeny from hybrid seedless bunch grapes. Journal of the American Society for Horticultural Science, 1990, 115(6): 1019-1024.
[37] LESHEM B and SACHS T. ‘Vitrified’ Dianthus-teratomata in vitro due to growth factor imbalance. Annals of Botany, 1985, 56(5): 613-617.
[38] LIU S M, SYKES S R, CLINGELEFFER P R. Improved in ovulo embryo culture for stenospermocarpic grapes (L.). Australian Journal of Agriculture Research, 2003, 54(9): 869-876.
[39] JI W, LI Z Q, YAO W K, GONG P J, WANG Y J. Abnormal seedlings emerged during embryo rescue and its remedy for seedless grape breeding. Korean Journal of Horticultural Science & Technology, 2013, 31(4): 483-489.
[40] STOUT A B. Progress in breeding for seedless grapes. Proceeding American Society for Horticultural Science, 1939, 37: 627-629.
[41] WEINBERGER J H, HARMON F N. Seedlessness ingrapes. Proceedings of the American Society for Horticultural Science, 1964, 85: 270-274.
[42] SANDHU A S, JAWANDA J S, UPPAL D K. Inheritance of seed characters in hybrid populations of intercultivar crosses of grapes (L.). Journal of Research-Punjab Agricultural University (India), 1984, 21: 39-44.
[43] SPIEGEL-ROY P, BARON Y, SAHAR N. Inheritance of seedlessness in seeded × seedless progeny ofL. Vitis, 1990, 29: 79-83.
[44] LEDBETTER C A, BURGOS L. Inheritance of stenospermocarpic seedlessness inL. Journal of Heredity, 1994, 85(2): 157-160.
[45] BOUQUET A, DANGLOT Y. Inheritance of seedlessness in grapevine (L.). Vitis, 1996, 35(1): 35-42.
[46] DOLIGEZ A, BOUQUET A, DANGLOT Y, LAHOGUE F, RIAZ S, MEREDITH C, EDWARDS K, THIS P. Genetic mapping of grapevine (L.) applied to the detection of QTLs for seedlessness and berry weight.Theoretical & Applied Genetics, 2002, 105(5): 780-795.
[47] ROYTCHEV V. Inheritance of grape seedlessness in seeded and seedless hybrid combinations of grape cultivars with complex genealogy. American Journal of Enology and Viticulture, 1998, 49(3): 302-305.
[48] COSMIDES L M, TOOBY J. Cytoplasmic inheritance and intragenomic conflict. Journal of Theoretical Biology, 1981, 89(1): 83-129.
Rescue and Molecular Marker Assisted-Selection of the Cold-Resistant Seedless Grape Hybrid Embryo
ZHU PeiPei, LUO YiJia, XIANG Wen, ZHANG MingLei, ZHANG JianXia
College of Horticulture, Northwest Agriculture and Forestry University/State Key Laboratory of Crop Stress Biology in Arid Areas/Key Laboratory of Horticultural Plant Germplasm Resource Utilization in Northwest China, Ministry of Agriculture, Yangling 712100, Shaanxi
【】The traditional methods of breeding seedless grape can only use the seeded variety as the female parent and the seedless variety as the male parent to cross, and the rate of the seedless hybrid is extremely low. As a new breeding technology, the embryo rescue can use seedless grapes as female parents, which expands the selection range of female parents, and greatly improves the efficiency of seedless grape breeding. However, the embryo development rate, germination rate and seedling formation rate in seedless grape embryo rescue are still low, which restricts the application of this technique. 【】The main objective of this paper was to screen suitable female parents and embryo development medium for embryo rescue, and to create new cold-resistant seedless grape germplasm by embryo rescue and molecular marker assisted-selection, so as to provide a basis for improving the effect of seedless grape embryo rescue and for further breeding new cold-resistant seedless grape varieties.【】Using the cold-resistant seedless grape cultivar Jupiter (Euro-American hybrid) and the seeded hybrid 00-1-5 (×) as the male parents and five seedless grape cvs. (Ruby Seedless, Qinhong No.1, Qinhong No.2, Crimson Seedless and Qinxiu) as the female parents, respectively, the hybridization was carried out between seedless grape and cold-resistant grape. The immature fruits of each female parent at the suitable sampling time were collected for ovule culture. The solid-liquid biphasic MM3 and ER medium were used as the embryo development medium to compare the effects of different medium and female parents on the rate of embryo development, germination, normal seedlings and abnormal seedlings. WPM + 0.2 mg?L-16-BA + 0.1 mg?L-1IAA solid medium was used as embryo germination and seedling formation medium. The embryo-rescue seedlings obtained after domestication in the greenhouse were transplanted to the field in spring. Inaddition, 2MS + 0.2 mg?L-16-BA + 0.1 mg?L-1IAA medium was used for transforming deformed seedlings into normal seedlings, which was added with 0, 1.0 and 1.6 mg?L-1ZnSO4, respectively. Finally, the molecular marker SCF27-2000 linked to the seedless gene was employed to detect the seedless hybrids for embryo-rescue seedlings. 【】For five cross combinations, 175 embryos from 2 158 ovules cultured in MM3 were obtained, which were further germinated and formed 118 hybrid plants. 74 embryos from 894 ovules cultured in ER medium were obtained and further germinated and formed 58 hybrid plants. Using 00-1-5 as male parent, seedless cv. Qinhong No.2 was much more suitable to be the female parent than Qinxiu, its embryo development rate and seedling rate were the highest, reaching 17.04 % and 7.41%, respectively. Using Jupiter as the male parent, Ruby Seedless × Jupiter had the best effect, with embryo development and seedling rate of 13.71% and 10.67%; the next was Qinhong No.1 × Jupiter, the embryo development rate and seedling rate were 14.39% and 4.71%, respectively. However, Crimson Seedless × Jupiter had the lowest embryo development rate and seedling rate, reached to 9.55% and 1.76%, respectively. The rates of embryo development and seedling formation of ovules inoculated into the MM3 medium were higher than that of the ER medium. The highest rate of deformed seedlings was Qinhong No.1 × Jupiter, while Ruby Seedless × Jupiter had no deformed seedlings. The rate of deformed seedlings in the ER medium was higher than that in the MM3 medium. The deformed seedlings of combination Qinhong No.2 × 00-1-5 were transformed to 2MS+1.6 mg?L-1ZnSO4+0.2 mg?L-16-BA+0.1 mg?L-1IAA, producing the best results with a conversion rate of 40.00%. Using the molecular marker SCF27-2000 linked to seedless character, 176 hybrid strains from 5 cross combinations were detected and there were 159 strains with the seedless molecular markers. 【】 Ruby Seedless, Qinhong No.1 and Qinhong No.2 were suitable as the seedless female parents for embryo rescue in vitro, but Crimson Seedless and Qinxiu were not relevant. MM3 medium was more appropriate for embryo rescue in vitro than the ER medium. The 159 plants from embryo rescue with the molecular marker linked to the seedless gene were potential seedless hybrids.
seedless grape; cold-resistance; embryo rescue; new germplasm; molecular marker- assisted selection

10.3864/j.issn.0578-1752.2021.06.012
2020-06-30;
2020-08-28
陜西省重點項目-農業(2017ZDXM-NY-026)、楊凌示范區科技計劃項目(2018NY-29)
朱佩佩,E-mail:825965951@qq.com。通信作者張劍俠,E-mail:zhangjx666@126.com
(責任編輯 趙伶俐)

