miR-126-3p和SLC7A11在肺癌中的研究進(jìn)展
黨裔武 陸會平 陳罡
【關(guān)鍵詞】 miR-126-3p;SLC7A11;肺癌;鐵死亡
中圖分類號:R734.2 ? 文獻(xiàn)標(biāo)志碼:A ? DOI:10.3969/j.issn.1003-1383.2021.05.014
在我國乃至全世界常見癌癥中肺癌是最常見且最致命的,在廣西,其發(fā)病率和病死率也僅次于肝癌[1~3],嚴(yán)重危害人類健康。肺癌又可根據(jù)組織學(xué)分為非小細(xì)胞肺癌(non-small cell lung cancer,NSCLC)和小細(xì)胞肺癌,所有亞型均與吸煙有關(guān)[4],準(zhǔn)確的組織學(xué)和分子分型對肺癌的治療和預(yù)后判斷至關(guān)重要。肺鱗癌(lung squamous cell carcinoma,LUSC)和肺腺癌是最常見的兩種非小細(xì)胞肺癌。隨著精準(zhǔn)醫(yī)療的快速發(fā)展,人們通過分子病理學(xué)技術(shù)準(zhǔn)確判斷表面生長因子受體、程序性死亡配體1等基因的狀態(tài),針對性地指導(dǎo)肺癌患者進(jìn)行酪氨酸激酶抑制劑及免疫檢查點抑制劑治療,從而有效延長患者壽命。然而,分子靶向藥治療的受益者十分有限,且不可避免的藥物不良反應(yīng)和耐藥等問題也是接受靶向藥物治療患者所面臨的巨大挑戰(zhàn)。與此同時,迄今仍未發(fā)現(xiàn)明確的LUSC驅(qū)動基因和藥物治療靶點,針對LUSC的治療方案較為局限。因此,尋找特異性LUSC診療分子靶標(biāo)仍是當(dāng)下肺癌防控的關(guān)鍵任務(wù)。近年眾多文獻(xiàn)報道微小RNA(microRNA,miRNA)與肺癌發(fā)生發(fā)展關(guān)系密切,本文將從miRNA的視角,以miR-126-3p及其潛在作用靶基因溶質(zhì)載體家族成員7成員11(solute carrier family 7 member 11,SLC7A11,又稱xCT)為例,對它們在肺癌中的作用及機(jī)理進(jìn)行綜述。
1 miRNA的概況
miRNA是一系列由18~22個核苷酸組成的內(nèi)源性非編碼RNA,參與人類多種惡性腫瘤的發(fā)生發(fā)展[5]。當(dāng)前的研究認(rèn)為miRNA可能因染色體異常、轉(zhuǎn)錄失調(diào)和表觀修飾等原因在肺癌中表達(dá)失調(diào),通過多分子、多途徑、多步驟綜合調(diào)節(jié)肺癌細(xì)胞增殖、遷移、侵襲和轉(zhuǎn)移,故基于大量臨床前和臨床研究提出可將外周血和組織樣本中檢測到的miRNA作為肺癌早期診斷、疾病進(jìn)展監(jiān)測、治療和預(yù)后預(yù)測的潛在生物標(biāo)志物[6]。
2 miR-126-3p與肺癌的關(guān)系
miR-126-3p參與肺癌的發(fā)生發(fā)展。已有研究證實miR-126-3p在大多數(shù)肺癌組織中表達(dá)顯著下調(diào)[7~12],表達(dá)上調(diào)后可經(jīng)靶向SLC7A5延遲細(xì)胞周期G1期抑制小細(xì)胞肺癌細(xì)胞的增殖[7];低表達(dá)miR-126-3p的肺腺癌患者其病理分期更差、腫瘤直徑更大、淋巴結(jié)轉(zhuǎn)移更為常見[9,11],可通過靶向CCR1抑制NSCLC的生長、遷移和侵襲[13]。然而在外周血血清中,miR-126-3p的表達(dá)趨勢尚存在爭議,如法國和波蘭的NSCLC患者血清中miR-126-3p表達(dá)顯著下調(diào)[14~15],而中國肺癌患者血清及血清外泌體中的miR-126-3p明顯上調(diào)[16~18]。由此可見,miR-126-3p在組織水平的表達(dá)趨勢較為穩(wěn)定,但其血清表達(dá)可能受人群遺傳背景的影響,此現(xiàn)象還需要大樣本檢測進(jìn)一步證實。總之,中外學(xué)者一致認(rèn)為血清miR-126-3p表達(dá)水平可作為早期診斷、疾病進(jìn)展和預(yù)后判斷的潛在分子標(biāo)志物[17,19~20]。同時miR-126-3p亦是肺癌治療的潛在靶標(biāo)。研究表明,放療后肺癌患者血清中的miR-126-3p表達(dá)顯著下調(diào)[21];隱丹參酮可顯著上調(diào)非小細(xì)胞肺癌細(xì)胞的miR-126-3p表達(dá)水平,從而抑制細(xì)胞生長和侵襲能力[22]。然而,目前針對miR-126-3p在肺癌藥物治療中的作用及其下游靶標(biāo)及調(diào)控信號途徑的研究尚十分匱乏。為進(jìn)一步探索miR-126-3p作用的分子機(jī)制,本課題組前期通過miRWalk預(yù)測發(fā)現(xiàn)SLC7A11是miR-126-3p的關(guān)鍵靶基因[11]。
3 SLC7A11與肺癌的關(guān)系
3.1 SLC7A11基因的作用機(jī)理
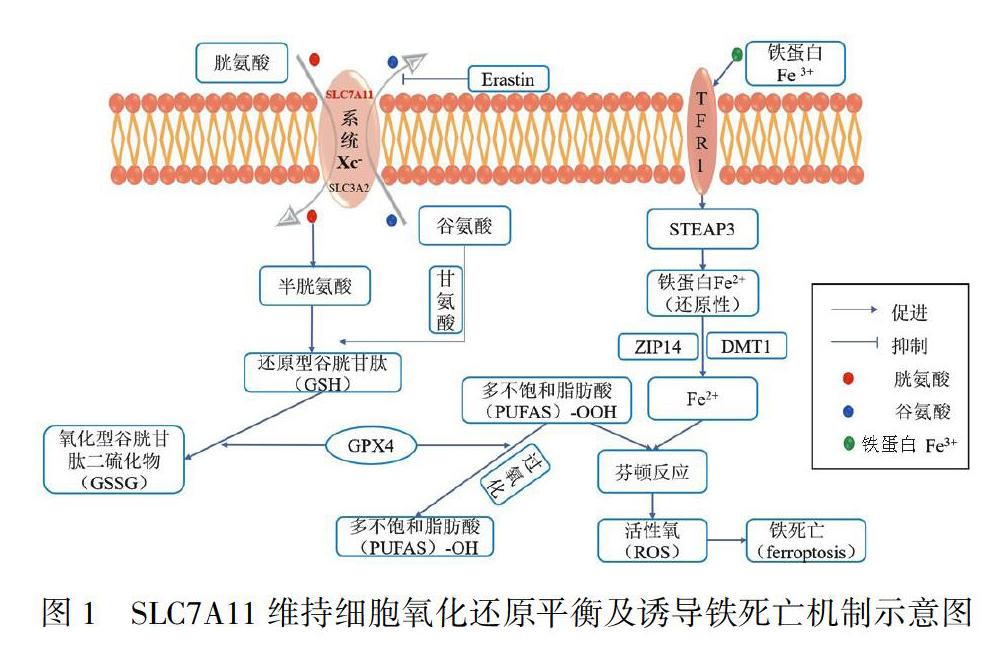
SLC7A11蛋白與SLC3A2組成胱氨酸/谷氨酸反轉(zhuǎn)運(yùn)體(又稱Xc-系統(tǒng),以1∶1的比例為細(xì)胞攝取胱氨酸并交換胞內(nèi)谷氨酸)。SLC7A11對胱氨酸和谷氨酸具有高度特異性,負(fù)責(zé)Xc-系統(tǒng)的基本轉(zhuǎn)運(yùn)活性,并通過調(diào)節(jié)內(nèi)源性抗氧化劑-谷胱甘肽(glutathione,GSH)維持細(xì)胞的氧化還原平衡和調(diào)節(jié)細(xì)胞鐵死亡(圖1),對細(xì)胞的正常生長和發(fā)育至關(guān)重要。已有研究表明,SLC7A11在包括肺癌在內(nèi)的多種人類惡性腫瘤中過表達(dá),高表達(dá)的SLC7A11可通過抑制細(xì)胞鐵死亡而導(dǎo)致患者不良預(yù)后,其機(jī)制研究表明腫瘤細(xì)胞通過上調(diào)SLC7A11表達(dá)維持高水平GSH以抵消自身代謝速率增加所導(dǎo)致的氧化應(yīng)激,從而促進(jìn)腫瘤細(xì)胞增殖侵襲和抑制細(xì)胞鐵死亡[23~24],提示SLC7A11是驅(qū)動腫瘤發(fā)生發(fā)展的關(guān)鍵基因。
3.2 SLC7A11基因在肺癌發(fā)生發(fā)展中的作用
SLC7A11通過調(diào)節(jié)胞內(nèi)胱氨酸/谷氨酸代謝、免疫細(xì)胞浸潤,在肺癌發(fā)生、進(jìn)展和治療中發(fā)揮關(guān)鍵作用[25~30]。國內(nèi)外學(xué)者對非小細(xì)胞肺癌患者的肺癌組織進(jìn)行免疫組化檢測發(fā)現(xiàn)SLC7A11主要定位于細(xì)胞膜[25]或細(xì)胞質(zhì)[27],其mRNA和蛋白水平在肺腺癌和肺鱗癌中均顯著上調(diào),高表達(dá)的SLC7A11與患者總體生存期較差有關(guān)[25,27,31];肺腺癌A549細(xì)胞和LUSC H520、HCC15、HCC95細(xì)胞高表達(dá)SLC7A11,沉默這些細(xì)胞的SLC7A11基因表達(dá)可明顯降低細(xì)胞生長速度、侵襲能力及谷氨酰胺依賴性,過表達(dá)SLC7A11基因不僅顯著降低癌細(xì)胞內(nèi)GSH/GSSG比率以提高細(xì)胞內(nèi)微環(huán)境的氧化性,還可以誘導(dǎo)正常氣道上皮細(xì)胞中的代謝重編程和氧化磷酸化,而這或許是吸煙促使正常細(xì)胞增殖和癌變的機(jī)制[25]。與此同時,一項有趣的研究將LUSC差異表達(dá)基因根據(jù)其表達(dá)水平和臨床特征分為4個亞型:原始型(primitive)、經(jīng)典型(classical)、分泌型(secretory)和基礎(chǔ)型(basal),其中經(jīng)典型基因在吸煙者中顯著過表達(dá),具有獨(dú)特的異源生物代謝功能,與能量代謝(包括氧化磷酸化、檸檬酸循環(huán)、電子傳遞鏈等)、異源生物代謝(包括細(xì)胞色素p450、谷胱甘肽代謝)、細(xì)胞成分(線粒體內(nèi)膜、呼吸鏈)等密切相關(guān)[32],而吸煙及吸煙最嚴(yán)重的患者均高度集中于經(jīng)典型基因。在表達(dá)經(jīng)典型基因的LUSC細(xì)胞系HCC15、NCI-H520和HCC95中SLC7A11的表達(dá)明顯高于其他LUSC細(xì)胞系[25],提示SLC7A11可能通過調(diào)節(jié)細(xì)胞氧化還原平衡驅(qū)動吸煙所致LUSC的發(fā)生發(fā)展。
3.3 SLC7A11基因在肺癌靶向治療中的前景
SLC7A11亦可作為肺癌靶向治療的潛在靶標(biāo)。在肺癌分子靶向治療和免疫治療領(lǐng)域的研究表明,has-mir-373和has-mir-372通過競爭結(jié)合上調(diào)了SLC7A11的表達(dá),從而調(diào)節(jié)肺腺癌的免疫浸潤[26];但抑制SLC7A11會選擇性殺死KRAS突變的肺腺癌細(xì)胞和抑制體內(nèi)腫瘤生長[27];并且SLC7A11可作為PD-L1低表達(dá)且EGFR野生型NSCLC的潛在藥物靶標(biāo)[28];而人皮膚成纖維細(xì)胞SLC7A11的表達(dá)則有助于發(fā)現(xiàn)存在厄洛替尼治療后皮疹風(fēng)險的肺癌患者[33]。與此同時,SLC7A11也可作為中醫(yī)藥治療肺癌的有效靶點,如蘿卜硫素(sulforaphane)通過抑制SLC7A11表達(dá)和誘導(dǎo)鐵死亡促進(jìn)小細(xì)胞肺癌細(xì)胞死亡[30];雙氫青蒿素通過對PRIM2/SLC7A11調(diào)控軸的抑制來阻止肺癌細(xì)胞增殖和克隆形成,并促進(jìn)細(xì)胞發(fā)生鐵死亡[29]。提示SLC7A11是LUSC極富潛力的治療、療效和預(yù)后預(yù)測靶點。
4 小結(jié)
在肺癌中表達(dá)下調(diào)的miR-126-3p和高表達(dá)的SC7A11往往提示患者預(yù)后不良,二者均可作為肺癌治療的潛在靶標(biāo),并且SLC7A11是miR-126-3p的潛在靶基因,可能miR-126-3p通過靶向上調(diào)SLC7A11的表達(dá)而促進(jìn)LUSC發(fā)生發(fā)展,但這一假設(shè)需要設(shè)計嚴(yán)謹(jǐn)?shù)姆肿由飳W(xué)實驗進(jìn)行驗證。與此同時,鑒于SLC7A11對細(xì)胞鐵死亡的調(diào)節(jié)作用,miR-126-3p/SLC7A11調(diào)控軸調(diào)控細(xì)胞鐵死亡的機(jī)制尚未見相關(guān)的研究。總之,本綜述全面闡述和分析了目前miR-126-3p和SLC7A11在肺癌中的研究進(jìn)展,為肺癌尤其LUSC的診療提供新思路,有助于推動LUSC發(fā)生發(fā)展機(jī)制的研究。
參 考 文 獻(xiàn)
[1]國家癌癥中心.2018中國腫瘤登記年報[M].北京:人民衛(wèi)生出版社,2019:129.
[2]李秋林,曹驥,容敏華,等.2016年廣西腫瘤登記地區(qū)惡性腫瘤發(fā)病和死亡分析[J].中國癌癥防治雜志,2020,12(1):44-51.
[3]BRAY F,F(xiàn)ERLAY J,SOERJOMATARAM I,et al.Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J].CA Cancer J Clin,2018,68(6):394-424.
[4]WU D,PANG Y,WILKERSON M D,et al.Gene-expression data integration to squamous cell lung cancer subtypes reveals drug sensitivity[J].Br J Cancer,2013,109(6):1599-1608.
[5]LEWIS B P,BURGE C B,BARTEL D P.Conserved seed pairing,often flanked by adenosines,indicates that thousands of human genes are microRNA targets[J].Cell,2005,120(1):15-20.
[6]TANG S,LI S,LIU T,et al.MicroRNAs:emerging oncogenic and tumor-suppressive regulators,biomarkers and therapeutic targets in lung cancer[J].Cancer Lett,2021,502:71-83.
[7]MIKO E,MARGITAI Z,CZIMMERER Z,et al.miR-126 inhibits proliferation of small cell lung cancer cells by targeting SLC7A5[J].FEBS Lett,2011,585(8):1191-1196.
[8]VSA U,VOODER T,KOLDE R,et al.Meta-analysis of microRNA expression in lung cancer[J].Int J Cancer,2013,132(12):2884-2893.
[9]CHEN Q Y,HU H Z,JIAO D M,et al.miR-126-3p and miR-451a correlate with clinicopathological features of lung adenocarcinoma:the underlying molecular mechanisms[J].Oncol Rep,2016,36(2):909-917.
[10]WANG K,CHEN M W,WU W.Analysis of microRNA (miRNA) expression profiles reveals 11 key biomarkers associated with non-small cell lung cancer[J].World J Surg Oncol,2017,15(1):175.
[11]CHEN P,GU Y Y,MA F C,et al.Expression levels and co-targets of miRNA-126-3p and miRNA-126-5p in lung adenocarcinoma tissues:an exploration with RT-qPCR,microarray and bioinformatic analyses[J].Oncol Rep,2019,41(2):939-953.
[12]CHEN S W,LU H P,CHEN G,et al.Downregulation of miRNA-126-3p is associated with progression of and poor prognosis for lung squamous cell carcinoma[J].FEBS Open Bio,2020,10(8):1624-1641.
[13]LIU R,ZHANG Y S,ZHANG S,et al.MiR-126-3p suppresses the growth,migration and invasion of NSCLC via targeting CCR1[J].Eur Rev Med Pharmacol Sci,2019,23(2):679-689.
[14]′SWITLIK W,KARBOWNIK M S,SUWALSKI M,et al.miR-30a-5p together with miR-210-3p as a promising biomarker for non-small cell lung cancer:a preliminary study[J].Cancer Biomark,2018,21(2):479-488.
[15]′SWITLIK W Z,KARBOWNIK M S,SUWALSKI M,et al.Serum miR-210-3p as a potential noninvasive biomarker of lung adenocarcinoma:a preliminary study[J].Genet Test Mol Biomarkers,2019,23(5):353-358.
[16]ZHU Y,LI T,CHEN G,et al.Identification of a serum microRNA expression signature for detection of lung cancer,involving miR-23b,miR-221,miR-148b and miR-423-3p[J].Lung Cancer,2017,114:6-11.
[17]FENG M,ZHAO J Y,WANG L,et al.Upregulated expression of serum exosomal microRNAs as diagnostic biomarkers of lung adenocarcinoma[J].Ann Clin Lab Sci,2018,48(6):712-718.
[18]WU Q W,Y U LL,LIN X Q,et al.Combination of serum miRNAs with serum exosomal miRNAs in early diagnosis for non-small-cell lung cancer[J].Cancer Manag Res,2020,12:485-495.
[19]SANFIORENZO C,ILIE MI,BELAID A,et al.Two panels of plasma microRNAs as non-invasive biomarkers for prediction of recurrence in resectable NSCLC[J].PLoS One,2013,8(1):e54596.
[20]ULIVI P,PETRACCI E,MARISI G,et al.Prognostic role of circulating miRNAs in early-stage non-small cell lung cancer[J].J Clin Med,2019,8(2):E131.
[21]TANG Y,CUI Y,LI Z,et al.Erratum to:radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells[J].J Exp Clin Cancer Res,2016,35:20.
[22]WANG H J,ZHANG Y S,ZHANG Y G,et al.Cryptotanshinone inhibits lung cancer invasion via microRNA-133a/matrix metalloproteinase 14 regulation[J].Oncol Lett,2019,18(3):2554-2559.
[23]趙雨霏,陶圓,顏曉菁.SLC7A11基因在惡性腫瘤中的研究進(jìn)展[J].中國腫瘤臨床,2019,46(15):795-799.
[24]CHEN X,Y U CH,KANG R,et al.Cellular degradation systems in ferroptosis[J].Cell Death Differ,2021:1-14.
[25]JI X M, QIAN J, RAHMAN S M J, et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression[J]. Oncogene, 2018, 37(36):5007-5019.
[26]WEI B Y,KONG W,MOU X Y,et al.Comprehensive analysis of tumor immune infiltration associated with endogenous competitive RNA networks in lung adenocarcinoma[J].Pathol Res Pract,2019,215(1):159-170.
[27]HU K W,LI K,LV J,et al.Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma[J].J Clin Invest,2020,130(4):1752-1766.
[28]HU W L,WANG G S,YARMUS L B,et al.Combined methylome and transcriptome analyses reveals potential therapeutic targets for EGFR wild type lung cancers with low PD-L1 expression[J].Cancers (Basel),2020,12(9):E2496.
[29]YUAN B,LIAO F,SHI Z Z,et al.Dihydroartemisinin inhibits the proliferation,colony formation and induces ferroptosis of lung cancer cells by inhibiting PRIM2/SLC7A11 axis[J].Onco Targets Ther,2020,13:10829-10840.
[30]IIDA Y,OKAMOTO-KATSUYAMA M,MARUOKA S,et al.Effective ferroptotic small-cell lung cancer cell death from SLC7A11 inhibition by sulforaphane[J].Oncol Lett,2021,21(1):71.
[31]UDDIN M N,AKTER R,LI M Y,et al.Expression of SARS-COV-2 cell receptor gene ACE2 is associated with immunosuppression and metabolic reprogramming in lung adenocarcinoma based on bioinformatics analyses of gene expression profiles[J].Chem Biol Interact,2021,335:109370.
[32]WILKERSON M D,YIN X Y,HOADLEY K A,et al.Lung squamous cell carcinoma mRNA expression subtypes are reproducible,clinically important,and correspond to normal cell types[J].Clin Cancer Res,2010,16(19):4864-4875.
[33]WICKERSHAM K E,HODGES T K,EDELMAN M J,et al.Differential gene expression in erlotinib-treated fibroblasts[J].Nurs Res,2019,68(2):110-126.
(收稿日期:2021-02-18 修回日期:2021-03-12)
(編輯:王琳葵 梁明佩)

