Slight Co-doping tuned magnetic and electric properties on cubic BaFeO3 single crystal
Shijun Qin(覃湜俊) Bowen Zhou(周博文) Zhehong Liu(劉哲宏) Xubin Ye(葉旭斌)Xueqiang Zhang(張雪強) Zhao Pan(潘昭) and Youwen Long(龍有文)
1Beijing National Laboratory for Condensed Matter Physics,Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
2School of Physical Sciences,University of Chinese Academy of Sciences,Beijing 100049,China
3Songshan Lake Materials Laboratory,Dongguan 523808,China
Keywords: floating-zone single crystal, high-pressure synthesis, chemical doping, magnetic and electrical properties
1. Introduction
Perovskite oxidesAFeO3(A=Ca, Sr, and Ba) with unusually high Fe4+state show very interesting structural and physical properties.[1-7]At room temperature, CaFeO3crystallizes to an orthorhombicPnmastructure. As the temperature decreases to about 290 K, a so-called charge disproportionation (2Fe4+→Fe3++Fe5+) takes place, leading to a metal-insulator transition as well as aPnmatoP21/nstructural phase transition. On further cooling to 116 K, a helical antiferromagnetic (AFM) phase transition is observed,with the spin propagation vector along the pseudocubic[111]direction.[2,3]In comparison with CaFeO3, the crystal structure of SrFeO3changes to a simple cubic perovskite with spacePm-3m,which exhibits a helical AFM ordering atTN1≈134 K. On further cooling, SrFeO3undergoes another two AFM transitions respectively atTN2≈110 K andTN3≈56 K due to the higher-order spiral order adjustment.[4,7-10]Therefore,the helical AFM transitions of SrFeO3seem rather complex, and even topological magnetic structures which have two kinds of multiple-qspin structures has been proposed recently.[11]In spite of complicated spin transitions, SrFeO3always shows metallic electrical transport behavior.[4]Compared with the easily synthesized cubic perovskite phase of SrFeO3, such a simple cubic phase is very difficult to prepare for the analogue BaFeO3. Since the ionic radius of Ba2+is considerably larger than that of Sr2+,[12]conventional annealing always leads to the presence of a hexagonal phase for BaFeO3.[13]Until 2011,the simple cubic perovskite phase BaFeO3was obtained by a topological chemical oxidization method for the precursor BaFeO2.5at lower temperature(473 K)using ozone as an oxidizing agent.However,such a surface oxidization method can only obtain a small amount of powder samples with the thickness about 1 μm.[14]Most recently,large-size single crystals(>3 mm)for the cubic perovskite phase of BaFeO3are successful for growth by using floating zone methods combined with high-pressure treatment techniques.[15]The BaFeO3single crystal also shows three magnetic phase transitions. With decreasing temperature, a spin glass transition is found to occur atTSG≈181 K,followed by two long-range helical AFM transitions at about 117 K and 97 K,respectively. Moreover,a semiconductor-metal-like transition occurs near 117 K.[15]These complicated magnetic behaviors indicate competing ferromagnetic (FM) and AFM interactions in BaFeO3.
Because of the helical magnetic structure,the magnetism ofAFeO3are highly sensitive to external stimuli such as pressure and chemical doping. For example,CaFeO3experiences a structural phase transition and a spin state transition at a critical around 30 GPa.[16]The AFM ground state of SrFeO3can be tuned into an FM one if one uses a pressure up to 7 GPa[17]or doping with Co for Fe by 20%.[18]Compared with SrFeO3,the AFM and FM competition is more remarkable in BaFeO3.[19]Therefore,it is very interesting to study the chemical doping effects on the magnetic and electrical properties of the cubic BaFeO3. In this paper,we found that a tiny Co doping for Fe by 5%in nominal can change the AFM ground state of BaFeO3to an FM state in BaFe0.95Co0.05O3single crystal.Different electrical transport properties are also observed by such a small chemical doping.
2. Experimental details
The polycrystalline BaFe0.95Co0.05O2.5precursors were prepared by a solid-state reaction method.[15,20]Appropriate amounts of highly pure (99.9%) BaCO3, Fe2O3, and Co3O4were stoichiometrically weighed,and fully mixed and ground.The mixed powders were heated at 1373 K for 24 hours under flowing Ar gas. The resulting product was reground in air and pressed into a rod of 4.0 mm in diameter and 8.0 mm in length at 200 MPa for annealing at 1373 K for 12 hours in flowing Ar.The annealing rod was adopted to grow the oxygen deficient BaFe0.95Co0.05O2.5single crystals by using the floating zone method with a growth rate of 2.4 mm/h at Ar atmosphere. To obtain oxygen stoichiometric BaFe0.95Co0.05O3single crystal,the oxygen deficient precursor of BaFe0.95Co0.05O2.5single crystal was treated on a large volume cubic-anvil-type highpressure apparatus at 5 GPa and 1023 K for 60 min with the usage of exceeding KClO4oxidizing agent.[15,18,20]
Powder x-ray diffraction(XRD)and Laue back reflection were used to identify the crystal quality and structure on a Huber diffractometer at room temperature with CuKα1radiation. The diffraction range of 2θangle is from 10°to 100°with a step of 0.005°. Structural refinement for the XRD data was performed based on the Rietveld method using the GSAS program.[21]An Setaram TG-DTA system was used to perform thermogravimetric (TG) analysis with a heating rate of 10 K/min to 1300 K in Ar flow. The temperaturedependent magnetic susceptibility and isothermal magnetization were measured using a superconducting quantum interference device magnetometer(Quantum Design,MPMS3-VSM).Specific heat and resistivity data were obtained on a physical property measurement system (Quantum Design, PPMS-9T)at zero magnetic field.
3. Results and discussion
Figure 1(a) shows the precursor single crystal of BaFe0.95Co0.05O2.5and full oxidization BaFe0.95Co0.05O3single crystal with~3 mm in diameter and height. In order to facilitate the anisotropy study of the physical properties of the single crystal,we used Laue diffraction to determine the crystal planes and cut specific planes for physical property measurements. The bulk XRD pattern was performed on the selected (100) plane of BaFe0.95Co0.05O3crystal with the size of 2 mm×2 mm×1 mm as shown in Fig. 1(b), onlyh00 peaks can be observed, suggesting that the as-made crystals are of single domain without observed twins. The insets of Fig. 1(b) show the sharp Laue diffraction spots of the high symmetrical(001),(110),and(111)planes,which further confirm the high quality of BaFe0.95Co0.05O3single crystal we obtained. Furthermore,this doped single crystal were crushed into powders for XRD measurement. The powder XRD pattern as well as the Rietveld refinement results are presented in Fig. 1(c). One can find that all the diffraction peaks can be well fitted based on a simple cubic perovskite structure with space groupPm-3m. The refined structural parameters are shown in Table 1. Compared with the parent single crystal of BaFeO3witha=3.96833 ?A,[15]the refined lattice constant of BaFe0.95Co0.05O3crystal slightly increases to 3.97131(7) ?A.The lattice expansion results from the covalent effect of B-O in cubic BaFeO3weakened by Co doping,and the B-O bond length increased.
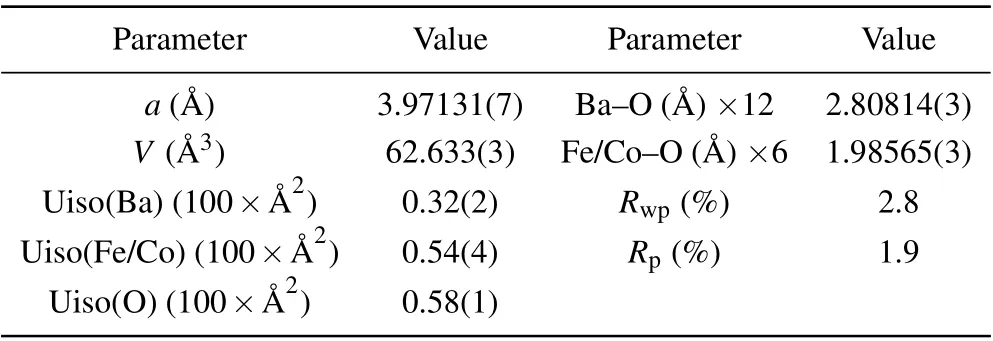
Table 1. Refined structure parameters for BaFe0.95Co0.05O3 single crystal at room temperaturea.

Fig. 1. (a) Morphology of BaFe0.95Co0.05O2.5 and BaFe0.95Co0.05O3 single crystals. (b) XRD pattern for the high symmetrical (001) plane of the BaFe0.95Co0.05O3 single crystal. The inset shows the Laue diffraction spots of the (100), (110), and (111) planes. (c) Powder XRD pattern measured at room temperature and Rietveld refinement results of pulverized BaFe0.95Co0.05O3 single crystal. The observed (black circles), calculated(pink line), and difference (orange line) patterns are shown. The green ticks indicate the allowed Bragg reflections with space group Pm-3m. (d)Temperature-dependent mass and oxygen content of BaFe0.95Co0.05O3 single crystal.
Considering that the oxygen content has significant influence on physical properties of materials, the TG measurement was performed to examine the oxygen content of BaFe0.95Co0.05O3single crystal. Figure 1(d) shows the sample mass as a function of temperature. Obviously, the oxygen starts to release at about 370 K. At temperatures above 1000 K,the decomposed product becomes stable. According to the mass loss and the final product, which is the precursor BaFe0.95Co0.05O2.5as confirmed by XRD,the calculated oxygen content of BaFe0.95Co0.05O3single crystal is determined to be 3.01(2), indicating the stoichiometric composition for the single crystal we grown. This result in turn suggests the presence of Fe4+/Co4+valence states in BaFe0.95Co0.05O3as reported in SrFeO3[4]and SrCoO3[20]single crystals.
Figure 2(a) shows the temperature dependence of magnetic susceptibility (χ) of BaFe0.95Co0.05O3single crystal with an applying magnetic feild (H=0.1 T) parallel to the(100),(110),and(111)crystal planes in a feild-cooling mode.As the temperature decreases to a critical temperatureTC≈120 K, the magnetic susceptibility experiences a sharp increase, indicting an FM phase transition. One can fnid that there is no signifciant difference in the susceptibility curves with feild along different crystal planes,suggesting negligible magnetocrystalline anisotropy in the cubic perovskite phase of BaFe0.95Co0.05O3. The inset of Fig. 2(a) shows the inverse susceptibility as function of temperature. Above 240 K,the data can be ftited based on the Curie-Weiss law with the functionχ-1=(T-θ)/C. The ftited Weiss temperatureθ=175.7 K is in agreement with the FM ordering. According to the ftited Curie constantC=4.199 emu·K·mol-1, the effective magnetic moment is calculated to be 5.79μB/f.u. If one considers the spin-only contribution for a high-spin Fe3+(Fe4+)state,the effective moment per ion in theory should be 5.89 (4.90)μB. Because of the strong p-d negative charge transfer energy, the high Fe4+(3d4) state can be regard as an Fe3+(3d5) combined with an oxygen holeL, (i.e., a d5Lstate).[15,22]The Curie-Weiss ftiting seems to suggest the formation of such a d5Lstate in BaFe0.95Co0.05O3.
Figure 2(b) depicts the isothermal magnetization curves measured at selected temperatures with the magnetic field parallel to the (100) plane. AboveTC, the linear magnetization behavior is consistent with the paramagnetism. BelowTC(e.g.at 100,15,and 2 K),however,canonical magnetic hysteresis is found to occur.Moreover,the magnetization sharply increases with field and becomes saturated at 1.0 T.The coercive field is quite small(13.6 Oe at 2 K,1 Oe=79.5775 A·m-1),indicating the soft FM feature. The saturated magnetic moment observed at 2 K and 6 T is 3.64μB/f.u.,which is somewhat larger than that of the parent BaFeO3single crystal(3.2μB/f.u.).[15]Figure 2(c)shows the comparison of magnetization with field along the three typical planes of(100), (110),and(111). Basically,there is no essential difference occurring in these three directions. At this stage,it is difficult to distinguish the easiest magnetization axis from [110] and [111] directions. Sharply different from BaFeO3where the magnetization undergoes a clear metamagnetic transition at~0.5 T from the initial AFM ground state to a higher-field FM state, the ground spin state of the Co-5%doped system has already been FM without any metamagnetic variation taking place.

Fig. 2. (a) The temperature-dependent magnetic susceptibility of BaFe0.95Co0.05O3 single crystal measured at 0.1 T with magnetic field parallel to the (100), (110), and (111) planes. The inset shows the Curie-Weiss fitting for the inverse susceptibility in 240 K-300 K. (b)Isothermal magnetization curves measured at selected temperatures with the field parallel to the (100) plane. (c) Comparison of fielddependent magnetization curves with field parallel to the(100), (110),and(111)planes.
In SrFe1-xCoxO3solid solution, the Co-doping induced FM phase does not appear until the substitution content of Co increase to 20%.[18]Therefore,the cubic BaFeO3is more sensitive to be tailored than SrFeO3.The propagation vector expression of the spiral spin ordering of the cubic Sr/BaFeO3isQ=φ×(2π/a),[11,14,23]whereais the lattice constant. The spin arrangement thus depends largely on the value of the spiral angleφ. For example, ifφ=0, the spins will all align in parallel and an FM state occurs. The value ofφfor BaFeO3and SrFeO3is 0.06 and 0.112,[23]respectively. Therefore, a slight Co-doping only by 5%can induce the ground spin state transition from helical AFM to collinear FM for BaFeO3. In addition, Mostovoy[24]in theory proposed a phase diagram from helical AFM order to FM order by considering the charge transfer energyΔ,p-dtransition integral (pdσ),p-ptransition amplitudetp-p, and the superexchange coupling integralJbetweent2gspins. In such a phase diagram, the FM order appears in the region with highΔ/(pdσ) value. When the lattice expands due to chemical doping, the (pdσ) decreases rapidly. Meanwhile, the charge transfer energy does not change much. As a result, the FM order is more likely to occur with increasing lattice constant. Since the Co doping favors lattice increase,the FM ground rather than the helical AFM one emerges in the current BaFe0.95Co0.05O3single crystal.
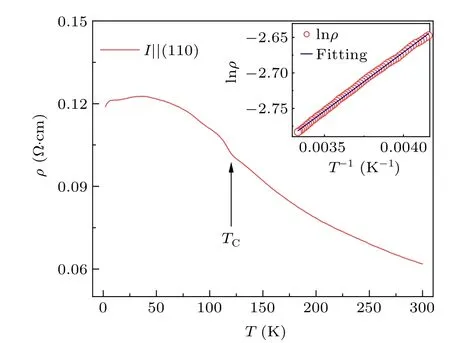
Fig. 3. Temperature dependence of resistivity for BaFe0.95Co0.05O3single crystal measured with electrical current parallel to the(110)plane. The inset shows the ftiting result using the thermal excitation model in 240 K-300 K.
Figure 3 shows the temperature dependence of resistivity for BaFe0.95Co0.05O3single crystal with the current parallel to the (110) plane. The value of resistivity at 300 K is about 6.2×10-2Ω·cm. As the temperature decreases, the resistivity slightly increases, suggesting a semiconducting electrical transport behavior. Once the temperature decreases toTC,the resistivity displays a small increase. On further cooling to less than~50 K,the resistivity is nearly unchanged any more,implying a bad metal-like behavior at lower temperatures. In 240 K-300 K, the temperature dependence of resistivity can be fitted the thermal excitation model(see the inset of Fig.3),yielding the activation energy to be 14.3 meV.Such a small activation energy as well as the bad metal-like electrical transport suggest the itinerant electronic behavior of BaFe0.95Co0.05O3single crystal belowTC.
To get a deeper insight into the transport properties for BaFe0.95Co0.05O3, the temperature dependence of specific heat (Cp) was measured. As showed in Fig. 4, aλ-type anomaly is found to occur in the specific heat curve nearTC, indicating the second-order nature for the long-range FM phase transition. At the temperatures below 12 K, the specific heat data can be well fitted using the functionCp=γT+βT3/2+αT3(see the inset of Fig. 4). The fitted parameters areγ=1.52(8)×10-2J·mol·K-2,β=2.44(3)×10-3J·mol·K-5/2, andα= 1.67(4)×10-4J·mol·K-4. In comparison, the value ofγcoefficient is larger than that ofβandα, suggesting the solid contribution of itinerant electrons in BaFe0.95Co0.05O3. In the parent BaFeO3, the fitted value ofγ=2.78×10-2J·mol·K-2,[15]which is larger than that of the current Co-doped one.Actually,one can find an apparent metallization transition at the long-range spin ordering temperature,but the resistivity experiences a small increase in BaFe0.95Co0.05O3. The Co introduction thus looks unfavorable for the presence of itinerant electronic behavior,probably due to the reduced p-d hybridization caused by lattice expansion.
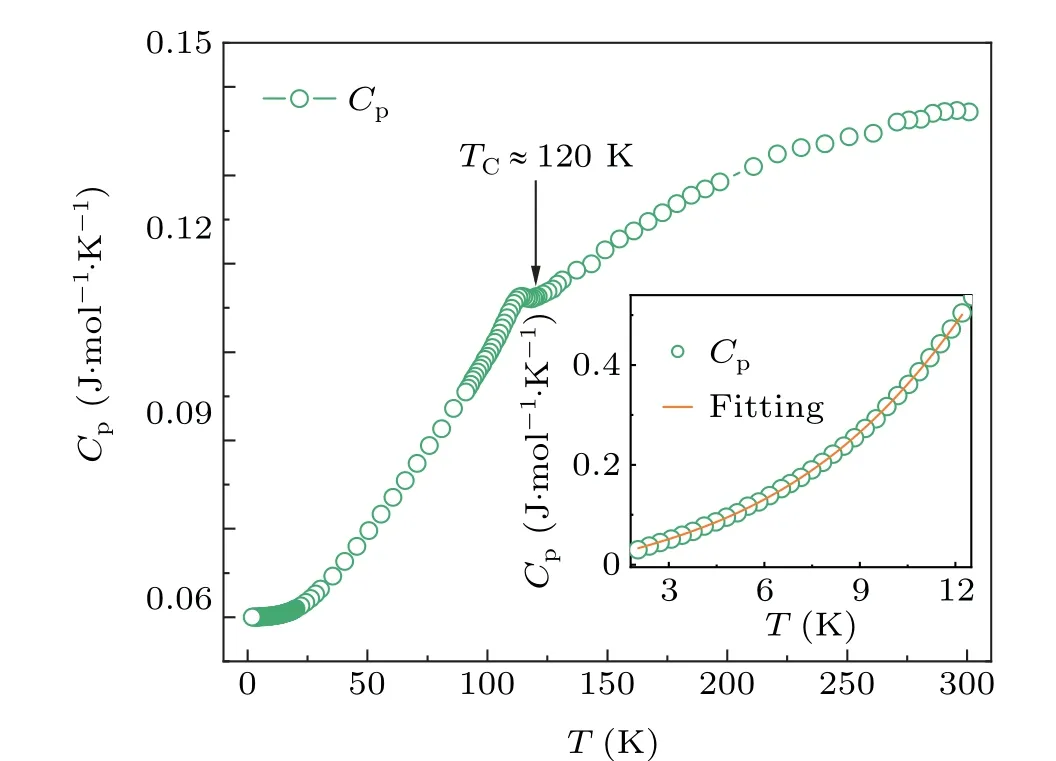
Fig.4. Temperature dependence of specific heat for BaFe0.95Co0.05O3 single crystal. The inset shows the fitting result for specific heat date below 12 K using the function Cp=γT+βT3/2+αT3.
4. Conclusion
In summary, the single crystal of BaFe0.95Co0.05O3was prepared by the combination of floating zone methods with high-pressure techniques for the first time. The crystal crystallizes to a simple cubic perovskite structure withPm-3msymmetry. The TG analysis confirms the stoichiometric oxygen content for the as-made single crystal, suggesting the presence of Fe4+/Co4+state. Compared with parent BaFeO3,the slight introduction of Co in BaFe0.95Co0.05O3only induces a small lattice expansion,but essentially changes the magnetism from the initial helical AFM ground state to an FM one with the Curie temperatureTC≈120 K.The resistivity and specific heat measurements indicate that the FM ordering favors a bad metal-like electrical transport behavior in BaFe0.95Co0.05O3.The present work shows that Co-doping is an effective method to modify the magnetic and electrical properties for the cubic perovskite of BaFeO3.
Acknowledgments
Project supported by the National Natural Science Foundation of China (Grant Nos. 11934017 and 11921004),the National Key Research and Development Program of China(Grant Nos.2021YFA1400300,2018YFE0103200,and 2018YFA0305700), the Beijing Natural Science Foundation (Grant No. Z200007), and the Fund from the Chinese Academy of Sciences(Grant No.XDB33000000).
- Chinese Physics B的其它文章
- Erratum to“Accurate determination of film thickness by low-angle x-ray reflection”
- Anionic redox reaction mechanism in Na-ion batteries
- X-ray phase-sensitive microscope imaging with a grating interferometer: Theory and simulation
- Regulation of the intermittent release of giant unilamellar vesicles under osmotic pressure
- Bioinspired tactile perception platform with information encryption function
- Quantum oscillations in a hexagonal boron nitride-supported single crystalline InSb nanosheet

