生物質超臨界水制氫研究進展
徐功迅,陳 偉,方 真
生物質超臨界水制氫研究進展
徐功迅,陳 偉,方 真※
(南京農業大學工學院,南京 210031)
生物質超臨界水制氫(supercritical water gasification,SCWG)以超臨界水為介質通過熱化學方式將生物質中的有機物轉化為氫氣等能源氣體。相較于傳統制氫方式,SCWG過程具有反應速度快、氫氣選擇性好、副產物少等優點,是一種高效、經濟、清潔的生物質處理技術。該研究主要圍繞SCWG過程中的影響因素進行系統地分析,介紹了超臨界水特殊的物理化學性質,詳細闡述了生物質主要成分如纖維素、半纖維素和木質素在SCWG過程中的反應機理,以及試驗裝置、原料類型和濃度、反應溫度、停留時間、壓力等工藝因素對SCWG的影響。研究發現纖維素占比較高的作物氣化效果更好,低濃度的進料有利于氣化效率和碳氣化效率的提升,提高裝置升溫速率、適當增加反應溫度和停留時間能夠增加氫氣產率,過大的壓力會形成“溶劑籠”效應降低氫氣產量。對不同類型反應系統研究表明,間歇式反應裝置雖然結構簡單、操作方便但也存在物料與催化劑混合不均勻、不能實現連續化生產而不適用于工業化推廣,連續式反應裝置雖面領著堵塞等問題,但具有性能好、效益高的優點,是工業化推廣的發展方向。對SCWG主要應用的催化劑進行討論發現,均相催化劑雖然具有催化效果但具有較強腐蝕性,非均相催化劑因其具有高催化活性、易回收、穩定性好等優點更適用于大規模SCWG生產過程。同時還研究了金屬催化劑酸度在催化過程中的影響,酸度越高,在SCWG過程中積碳會越明顯,通過添加Cu、Ce、Co、La等合適的第二金屬作為促劑可以改變催化劑性能,增加催化劑使用壽命,提高氫氣選擇性。未來應針對SCWG的試驗裝置、高效催化劑及經濟性分析等核心技術開展研究,加速SCWG的工業化推廣,實現經濟、安全、綠色、高效的氫能供給。該研究期望加深對生物質SCWG理解,為日后研究提供理論指導。
生物質;氫氣;催化劑;超臨界水
0 引 言
當前國內外仍以化石燃料為主要能源[1],造成了嚴重的環境污染問題。自2015年《聯合國氣候變化框架公約》提出以來各國紛紛提出碳減排措施。歐盟出臺《歐洲氣候法》提出在2050年前實現碳中和[2];美國承諾2050年前達到碳凈零排放;中國作為碳排放大國,宣布力爭在2030年前完成碳達峰,爭取2060年實現碳中和[3]。目前世界正處于能源發展的交替時代,急需尋找一種科學技術上可行、環境友好的可替代能源形式[4]。
生物質能是將生物質轉化為有用的能源[5],是自然界中唯一一種可再生的碳源,不僅可以用于燃燒還可以作為原料轉化為各種液體或氣體燃料[6]。氫能作為一種清潔高效的可再生二次能源,可以通過燃燒或者燃料電池的方式利用[7]。氫能在使用中有諸多優點:1)氫在燃燒過程中唯一產物是水,可以做到零碳排放[8];2)氫的熱值達到142.3 MJ/kg,是同等質量化石能源的3~4倍[9]; 3)氫是很多化學產品的重要原料,氫能產業發展將推動工業的發展[10]。通過主要制氫方式對比發現,傳統化石能源制氫雖成本較低但產生污染較大,工業副產制氫和水電解制氫成本相對較高,而生物質制氫具有能耗低、原料廣泛等優點,具有很大發展潛力[7,9,11-13]。
1 生物質超臨界水制氫機理研究
1.1 超臨界水及性質
當溫度超過374 ℃,壓力超過22.1 MPa時,水就會變成超臨界水(supercritical water,SCW)。當水的溫度和壓力超過其沸點,但在臨界點以下仍然保持液態時稱為亞臨界水。表1總結了不同條件下水的性質[14-15]:當水處于超(亞)臨界狀態時物理化學性質會發生巨大變化,主要包括以下幾點:
1)隨著氫鍵斷裂、介電常數降低,SCW更像非極性溶劑[16],主要表現為可以溶解大多數有機溶劑并且可以與氣體互溶,但是無機物溶解度大大降低[17]。SCW能夠為反應物提供均相反應條件[18],是一種很好的反應媒介[19];
2)SCW超低的黏度有更好的分子遷移率,可以加速溶質擴散[20],防止碳沉積和催化劑中毒[21];
3)SCW會抑制離子反應,增強自由基反應,有利于氣體產物生成[22];
4)在臨界點附近,水中H3O+和OH-離子濃度增加,此時非常有利于酸性或者堿性催化反應的進行,SCW本身也可以替代一些酸和堿催化劑促進反應[18,23]。

表1 不同條件下水的性質
分子動力學常用于模型的預測,LIEW等[24]用四位柔性模型對亞臨界和超臨界區域的純水進行了分子動力學模擬,通過空間分布函數研究了水的三維溶解結構和氫鍵,發現除了線性氫鍵的水外,超臨界水還包括分叉氫鍵的水;BOERO等[25]利用Car-Parrinello分子動力學構建出超臨界點附近水的氫鍵網絡結構和介電特性,發現在低密度下水主要分裂成三聚體、二聚體和單分子。SASAKI等[26]利用連續流動裝置發現纖維素在350 ℃和25 MPa的條件下僅4 s就可以完全溶解和轉化為葡萄糖和果糖等小分子物質,并將快速溶解的原因歸結于超臨界流體形成的均相反應條件,因此可以看出超臨界狀態非常有利于生物質的溶解和進一步反應。
1.2 生物質超(亞)臨界氣化反應機理
生物質主要由纖維素、半纖維素和木質素構成,纖維素是一種由葡萄糖單體通過(1,4)糖苷鍵連接而成的多糖,這使得纖維素分子內和分子間形成強氫鍵,使其結晶、耐水溶脹[27-28]。半纖維素由木糖、半乳糖和葡萄糖等多種糖單體組成(圖1),不同作物糖單體的比例也不同。

圖1 水的狀態[29]
半纖維素結構較不穩定,更容易水解[30-31]。木質素是由對香豆醇、松柏醇和芥子醇3種單體組成的一種復雜高分子量化合物,化學性質相對穩定。超臨界水氣化的過程復雜,涉及水解、熱解、焦油重整、水煤氣轉化、甲烷化反應等[32-33],主要反應方程式[34-36]如下:
CHO+(2-)H2O → CO2+ (2-+/2)H2(1)
CHO+(1-)H2O → CO + (1-+/2)H2(2)
纖維素的水解:
(C6H10O5) +H2O → nC6H12O6(3)
葡萄糖重整反應:
C6H12O6→ 6CO + 6H2(4)
木質素水解:
(C10H10O3)+H2O →C10H12O4→ 酚醛樹脂 (5)
蒸汽重整反應:
酚醛樹脂+H2O → CO + CO2+ H2(6)
水煤氣轉化反應:
CO + H2O → CO2+ H2(7)
CO的甲烷化反應:
CO + 3H2→ CH4+ H2O (8)
CO2的甲烷化反應:
CO2+ 4H2→ CH4+ 2H2O (9)
加氫反應:
CO + 4H2→ CH4+ 0.5O2(10)
焦油重整反應:
CHO(tar) + CO2→ 2CO +/2H2(11)
式中和分別表示生物質中H/C和O/C的元素摩爾比,產物中氣體組分的比例很大程度上取決于和的大小。生物質在超臨界水狀態下主要轉化為氫氣、甲烷、二氧化碳、一氧化碳等氣體,在這個過程中超臨界水既作為反應介質又作為反應物參與反應過程。
反應中主要過程及主要產物如圖2所示。在最初的水解過程中,生物質主要分解為糖類、酚類和醇類物質,其中纖維素和半纖維素水解的主要成分是五碳糖和六碳糖,木質素主要水解為酚類,包括愈創木酚、酚醛樹脂等。在超臨界水的狀態下這些中間產物進一步轉化為更簡單的化合物,例如酸(羧酸、琥珀酸等)、醇(豆香醇、芥子醇等)、酚類化合物、醛和芳香烴化合物。其中,酸類化合物主要來自于糖類物質的分解,醇、芳烴、醛等物質主要來自于木質素(苯酚)的變性產物[37-38]。
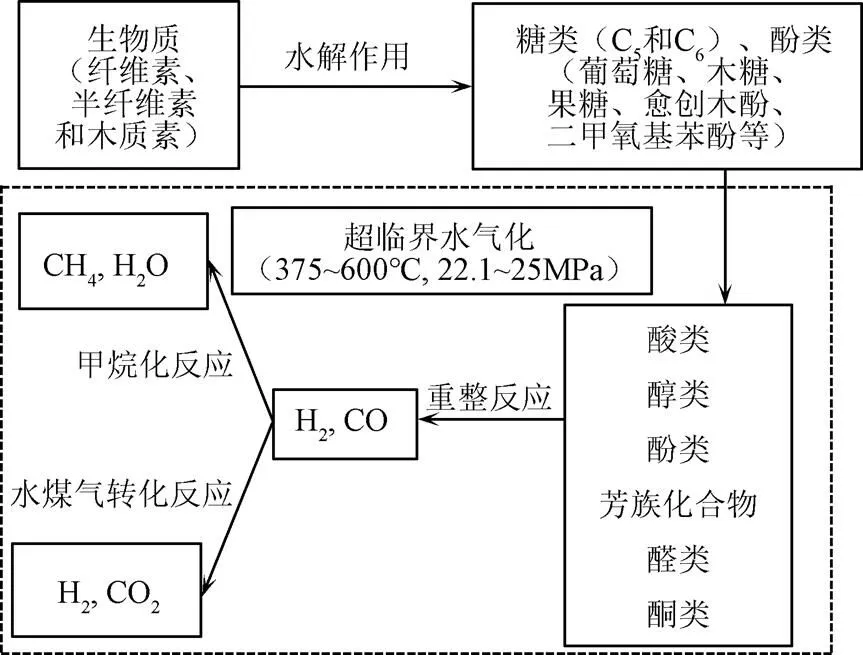
圖2 生物質超臨界水制氫反應路線圖
纖維素有更大的氫含量因此在SCWG過程中比半纖維素對產氫的貢獻更大[39],葡萄糖是纖維素水解過程中的主要產物。KABYEMELA等[40]研究發現在水熱條件下葡萄糖與木糖會發生互相異構化反應,研究證實果糖在SCWG中具有更高活性。CASTELLO等[41]以不同濃度的葡萄糖和苯酚為模型化合物在400 ℃和25 MPa的連續式反應器中試驗提出了反應機理(圖3),并指出在400 ℃離子反應條件下葡萄糖主要分解成赤蘚糖和乙醇醛。赤蘚糖具有呋喃環,進一步反應易形成糠醛,乙醇醛則進一步分解為乙醇;葡萄糖與果糖之間會相互轉化,果糖脫水形成5-羥甲基糠醛(5-HMF),5-HMF部分通過縮合形成重質化合物,部分進一步降解為酸類從而形成氣體產物。
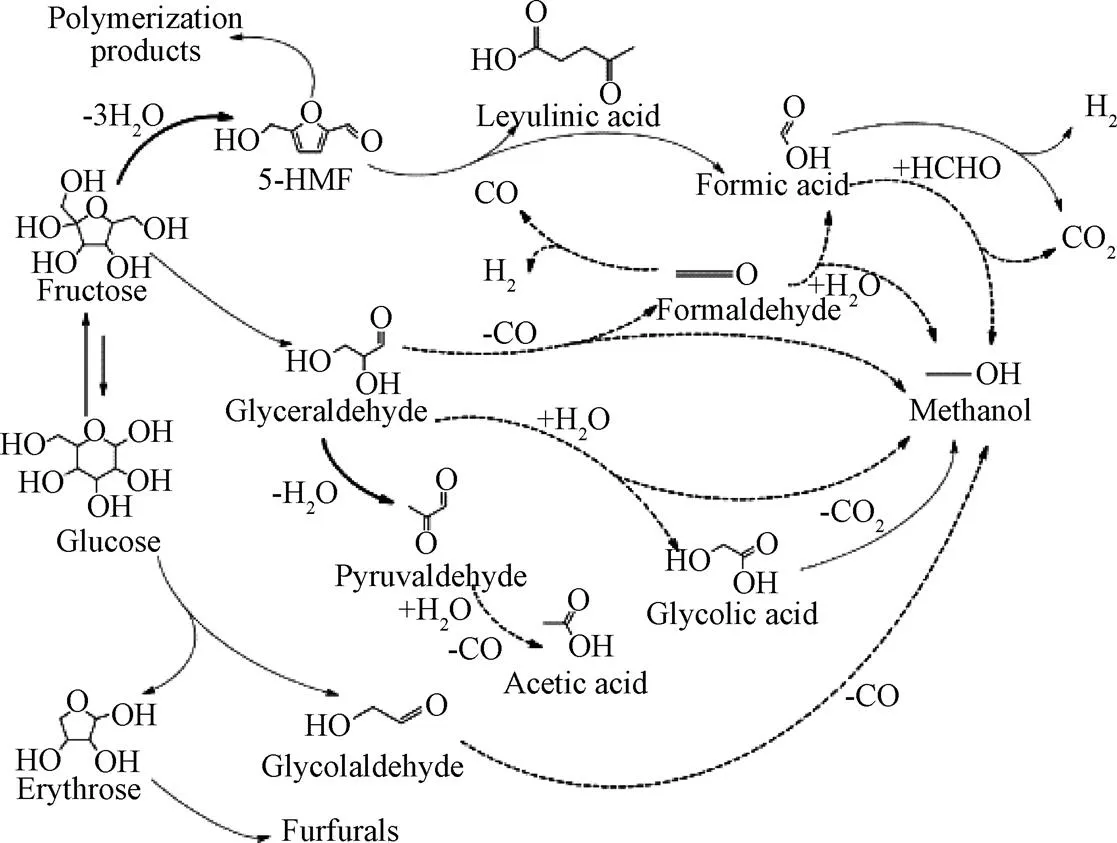
圖3 葡萄糖降解機理[41]
CASTELLO等[41]提出苯酚也是葡萄糖的降解產物之一,因為葡萄糖降解產生的糠醛會產生苯酚[42],大量研究表明在水熱條件下葡萄糖會分解成5-HMF、苯酚等酚類化合物以及酸類化合物等,這些物質進一步分解為呋喃、芳烴、有機酸、醇、酮等物質,其中有機酸、醇、酮進一步分解為氫氣等氣體,呋喃、芳烴等化合物更多的轉化為焦炭[43-44],而烴類的重整反應和水煤氣轉化過程中易在催化劑表面發生結焦造成催化劑失活[45]。酚類物質被認為是生物質氣化過程中的巨大障礙,因為在超臨界狀態下酚類物質可以通過加氫脫氧等反應生成難以氣化的芳烴,產生的中間產物如呋喃、酚類和芳烴等物質易促進聚合反應轉化生成焦炭導致氣化效率降低[46]。
半纖維素是由C5和C6糖組成的一種無定型的生物聚合物,GOODWIN等[47]以木糖為模型化合物探究半纖維素在SCWG過程中降解機理(圖4),認為木糖反應主要有2條途徑:第1種途徑是通過脫水生成糠醛,糠醛分解成水溶性腐殖質(water soluble humus, WSHS)和脂類化合物,再進一步分解成氣體產物;第2種途徑是木糖分解成甲酸甲酯和甘油醛,進一步分解為酸類化合物進而降解為氣體產物,在此過程中生成的氣體產物還會通過水煤氣變換和一氧化碳的甲烷化反應進行轉化。
木質素的結構較為復雜,是生物質中最難氣化的組分。木質素的存在會抑制氣體形成的速率[32],在氣化過程中主要產生4個相:油相(如酚類、PAH等)、水相(如醇類、醛類)、氣相(如氫氣、一氧化碳、二氧化碳、甲烷等)、固相(焦炭等物質)[32,48]。木質素氣體產物主要是二氧化碳和甲烷[49],較高的氫氣產率需要高溫才能實現。在水熱降解過程中木質素的醚鍵斷裂形成甲氧基酚等酚類物質,甲氧基苯酚通過脫甲氧基反應產生烷基苯酚和甲醇。其中甲醇通過2種方式轉化為氣體產品,一種是直接分解成H2和CO,另一種是通過與水反應生成CO2和H2,進一步發生水煤氣轉化反應和加氫反應生成CH4和氫氣等氣體[50](圖5)。烷基苯酚則通過自由基反應轉化為酚和烷烴自由基,烷烴通過自由基反應與氫自由基結合生成甲烷等氣體,酚類物質則通過氫化等反應分解,或者通過縮聚反應生成生物炭。木質素在水解過程中還會形成有苯環的高分子化合物,在超臨界狀態下,苯酚通過脫羥基反應生成苯環,再通過聚合反應生成聯苯和苯基苯酚等物質,這兩種物質是生成焦炭的主要原料之一[51]。

圖4 木糖降解機理[47]

圖5 甲醇反應路徑圖[50]
2 生物質超臨界水制氫影響因素
2.1 試驗裝置
試驗裝置的升溫速率會影響SCWG的產物,在低溫區停留過久會導致焦炭等副產物生成[52],高加熱速率會促進中間產物(甲酸和乙酸等)形成,抑制酚類化合物形成[53],防止焦油焦炭產生[54-55]。目前應用于SCWG的試驗裝置主要有間歇式和連續式2種[34],圖6展示了典型的間歇式與連續式反應裝置。間歇式反應器具有結構簡單、操作方便、原料適應性強、周期性使用過程中易于清理反應裝置內部產生的焦炭等優點,但溫度、壓力等因素不易精準控制,易產生較大誤差,同時也存在物料與催化劑混合不均勻等缺點[46,56]。連續式反應裝置可以較為精準的控制試驗參數,并實現連續化商業生產,在氣化中生成的焦炭易堵塞反應器是目前面臨的主要問題[57]。FANG等[18,37,58]利用可視鉆石反應器(圖7)發現木質纖維素易溶于高壓熱水中,并設計出連續裝置使生物質在均相條件下快速溶解、水解和分解為小分子化合物,為大規模連續氣化快速產氫奠定了試驗基礎。流化床反應器不僅具有克服反應器堵塞的能力還能夠增強傳熱傳質,在過去幾年中被認為是解決SCWG焦炭堵塞的有效方案[59-60],YAKABOYLU等[60]以干淀粉為原料使用流化床反應裝置在600 ℃條件下反應,發現產生的焦油等副產物量較少并且運行2 h均沒有發生堵塞問題。CHEN等[61]對SCWG-Solar系統進行評估提出將太陽能利用于SCWG預熱過程可以減少能耗,是未來大規模高效應用SCWG的可行方案之一。
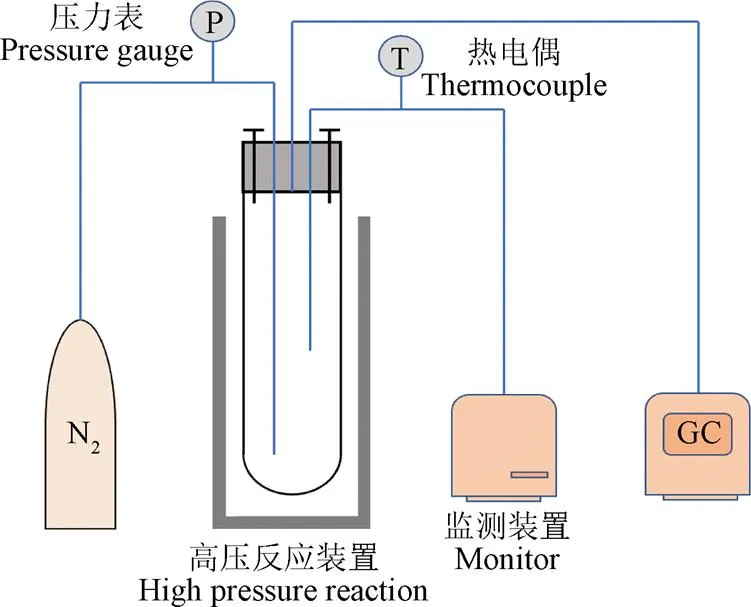
a. 典型間歇式(高壓反應釜)反應器
a. Typical batch (high-pressure reactor) reactor

b. 典型連續反應器

圖7 微型可視鉆石反應器[18]
2.2 原料類型及濃度
農作物秸稈中纖維素約占質量的35%~55%,半纖維素占20%~40%,木質素占10%~25%[62-63]。不同農作物類型的纖維素、半纖維素和木質素含量不同,在SCWG中產物也有很大不同,通常來說纖維素的生物可降解性大于木質素,因此纖維素占比高的作物氣體轉化率大于木質素占比高的作物[64]。生物質中還有蛋白質、礦物質、灰分等其他組分,其中蛋白質的存在會降低氣體產率,因為蛋白質降解形成氨基酸與葡萄糖水解反應會相互干擾。蛋白質分解過程中的丙氨酸會產生自由基清除劑,抑制自由基反應,而SCWG過程中大部分氣體通過自由基反應生成[65],此外生物質中部分礦物質可以充當催化劑的效果[66]。
YANIK等[67]以玉米秸稈、棉花秸稈等8種不同生物質作為反應原料,使用間歇式高壓反應釜在500 ℃進行氣化,氫氣產量在4.05~4.65 mol/kg生物質,發現不同作物的氨基酸、蛋白質、油脂含量等都會影響氫氣產量。WILLIAMS等[68]以纖維素、淀粉、葡萄糖和木薯廢棄物為研究對象,在間歇式高壓反應裝置中試驗發現葡萄糖的氫氣產率最高,木薯廢棄物的氫氣產率最低。淀粉和纖維素雖然都是葡萄糖的聚合物,但在氣化過程中纖維素產出的烴類化合物產出較多,淀粉產出的氫氣、一氧化碳和油類物質較多,試驗表明相同單體的聚合物連接方式不同在SCWG中效果也不同。郭烈錦院士團隊以不同種陸生植物、水生植物以及生物質廢料等為原料氣化,發現陸生禾本植物具有較高的活性,其次為水生植物、有機廢料等[69]。YOSHIDA等[70]使用木質素、纖維素及其混合物在400 ℃、25 MPa條件下利用鎳催化劑進行氣化,發現當原料中有軟木質素時氣化效率較低,纖維素與軟木質素在超臨界狀態下相互作用會生成焦油使催化劑失活,相較于木質素、纖維素等模型化合物,真實生物質在氣化過程中各組分之間也會相互作用影響氣化效果。
進料濃度也會影響氣化效果,物料濃度高會導致物料與水的接觸面積減小,阻礙蒸汽重整過程,過高的進料濃度會使生物質形成較多焦炭、焦油等中間副產物,容易導致反應器堵塞等問題[57]。GUO等[71]使用連續流動管式反應器以甘油為原料進行研究,在567 ℃下甘油濃度由10%增加至25%過程中,氫氣產率和氣化效率均降低;WANG等[72]使用間歇式反應器在600 ℃條件下以堿性木質素為原料進行試驗,發現當進料濃度由1%增加到6%時氣化效率由79.86%下降到42.20%,表明增加木質素原料濃度抑制了氣化反應。提高物料濃度反而會降低氣化效率,低濃度的進料反而有益于氣化率和碳氣化效率的提高[49],然而高濃度的進料有利于對污水、廢棄生物質的批量處理,能有效提高超臨界水處理廢棄物的效率,實現高濃度進料水平下高氫氣產率是未來的研究方向。
2.3 反應溫度
提高溫度可以增加氣化效率和氫氣產率,但在實際應用過程中也就意味著能耗增加[73],對試驗器材也提出了更高要求,不符合綠色生產理念。因此在較低溫度下實現較高氣化效率是未來研究方向。
溫度在SCWG過程中起著至關重要的作用。從熱力學角度分析,生物質分解需要大量熱量,因此高溫對SCWG非常有利[74]。隨著溫度升高生物質會加速分解,較高的溫度能提高碳氣化效率并且增加氫氣產量[57]。升高溫度會增加自由基的濃度,促進離子積反應向自由基轉化,有利于氣體產物生成[43]。OSADA等[75]將SCWG分為3個溫度區域:1)500 ~700 ℃的超臨界水區域,生物質分解迅速,可以使用活性炭催化劑避免結焦或堿性催化劑促進水煤氣轉化;2)374 ~500 ℃的超臨界水區域,可以利用金屬催化劑促進生物質水解;3)當溫度低于374 ℃時,為亞臨界水狀態,此時生物質水解非常緩慢,需要催化劑才能促進氣體形成。PROMDEJ等[76]利用連續式流化床管式反應裝置在300 ~460 ℃、25 MPa條件下,以1.5%葡萄糖為原料進行試驗,發現在亞臨界水狀態中產生的焦炭量明顯比超臨界水狀態下要多,在亞臨界條件下葡萄糖的分解以離子積反應為主,在超臨界水狀態下以自由基反應為主。KARAGOZ等[77]使用間歇式高壓反應釜分別在180、250 和280 ℃條件下氣化鋸末,發現會產生2-呋喃甲醛、2-甲氧基苯酚等中間產物,這些產物不能氣化會導致反應器堵塞,而較快的加熱速度能緩解這種現象的出現。大量研究表明,在較低溫度下氣化主要產物為甲烷,隨著溫度升高氫氣成為主要產物。從反應機理上來解釋,隨著溫度升高,重整反應的吸熱特性有利于氫氣和二氧化碳的生成,水煤氣轉化反應的微弱放熱性質會傾向于產生氫氣和二氧化碳,從而抑制一氧化碳的產生[38,78-80]。
2.4 停留時間
反應物在反應器內停留的時間或者持續的時間稱為停留時間,在一定時間范圍內氫氣產率會隨著停留時間的增加而增加[81]。YOUSSEF等[82]以豬糞為原料氣化,發現反應時間為60 min時甲烷和二氧化碳的產量最高,反應時間由30 min變化到60 min時氫氣產量幾乎不變,當反應時間達到90 min時,氫氣產量反而降低。OSADA等[83]以甘蔗渣廢料為原料氣化,發現反應時間為15 min時氣體產物的碳產量為10%,且不隨著時間的增加而增加,反應30 min時甘蔗渣完全氣化氣且氫氣產量幾乎恒定。RESENDE等[80]建立了纖維素和木質素SCWG的動力學模型,發現短時間內氫氣主要通過重整反應生成,較長時間主要通過水煤氣轉化反應產生,而一氧化碳、二氧化碳和甲烷等氣體會由中間產物水熱反應產生。CHEN等[84]利用石英管反應器將食品廢棄物用于SCWG,通過建立模型發現碳氣化效率隨反應溫度的升高而升高,反應活性在氣化初期急劇升高,隨后隨反應時間的延長而降低。
2.5 壓 力
在超臨界水狀態下,氫氣產量和氣化效率與壓力之間的關系非常復雜。為達到超臨界狀態,反應壓力需要大于22 MPa,在反應過程中適合的反應壓力范圍為22~30 MPa[85]。
溶質在溶劑中的擴散速率取決于溶劑黏度,溶劑黏度是壓力和溫度的函數,因此壓力可以影響溶質的溶解程度進而影響反應速率。在SCW狀態下溶劑的“籠效應”得到增強,此時溶劑籠可以通過隔離反應物來降低溶質與溶質之間的反應速率,加強溶質與溶劑之間的反應速率。因此高壓有利于水煤氣轉化,但是阻礙了原料的分解速率。GOKKAYA等[86]在500 ℃條件下對苯酚進行氣化,發現當壓力增大時碳氣化效率反而下降。LU等[87]使用流化床間歇式高壓反應器對生物質模型化合物(纖維素和葡萄糖)和真實生物質(稻稈、麥稈、花生殼等)進行氣化,發現高壓有利于水煤氣轉化進而促進氫氣產量。MADENOGLU等[88]在間歇式高壓反應釜中以葡萄糖為原料氣化,發現相同溫度下增大壓力會促進葡萄糖分解稱為醛和酮類化合物,隨著壓力增加碳氣化效率和氫氣產量均減小。
2.6 催化劑
傳統的SCWG過程需要高溫高壓條件,不僅功耗大而且對于反應裝置要求也非常高。向反應體系中加入催化劑可以降低反應的活化能,使反應在較低溫度下進行,能有效減少運營成本、提高氫氣選擇性[89]、減少SCWG過程焦油和焦炭產生[90]。催化劑主要是通過催化生物質水解、脫水等形成中間體,快速實現C-C鍵斷裂,尤其對于酚類化合物而言,氣化過程必須足夠快速才能避免產生的中間體聚合形成焦炭等物質。同時催化劑會解離H2O,在催化劑表面產生活性自由基,這些自由基將與分解的小分子CHO結合,最終釋放出CO和CO2,從水分解和裂解的CHO中吸附的氫原子將結合形成H2。催化劑主要分為均相催化劑和非均相催化劑2種。
2.6.1 均相催化劑
均相催化劑便于操作,能夠與原料均勻混合。堿類催化劑具有低成本和高活性的特性,是最廣泛應用的均相催化劑。堿性催化劑可以吸收CO2促使水煤氣轉化向生成氫氣方向移動[91-92]。CHEN等[93]使用流化床反應裝置對污水污泥SCWG進行了研究,得到催化劑的活性順序為由大到小依次為:KOH、K2CO3、NaOH、Na2CO3、KOH,通過對比發現堿性催化劑不會顯著改變產物中碳的分布情況,而氫氣產率增加主要是堿性催化劑促進超臨界水狀態下的水煤氣轉化反應。ONWUDILI等[94]用間歇式高壓反應釜在550 ℃、36 MPa下以葡萄糖為原料進行研究,發現高濃度的NaOH對氫氣選擇性更高。FENG等[95]通過分析模擬軌跡并跟蹤目標原子,指出C-H-O自由基是產生氫氣或氫離子的主要反應物,解釋了堿金屬鹽催化劑(NaOH和KOH)可以提高氫氣產量的原因。雖然堿性催化劑降低了纖維素降解的起始溫度,有利于氣體產物生成[96],但在實際操作過程中需要持續添加新鮮催化劑,并且反應過后產生的含堿催化劑廢液難以處理[17,97],因此使用范圍受到限制。
2.6.2 非均相催化劑
非均相催化劑具有易于回收、化學性質穩定等優點而被廣泛研究,主要包括有金屬催化劑、金屬氧化物催化劑和碳基催化劑等。
ELLIOTT等[98]發現在SCWG過程活性最好的金屬是Ru、Rh、Ni,它們可以改善甲烷化反應。Ni基催化劑因催化活性與貴金屬相當,但是價格較為低廉因而得到大量研究[99]。Ni能有效促進C-O鍵斷裂,同時促進生物質分解的中間體(苯酚、酸類、醛類)降解,進而增加氣化效率[100-101]。ADAMU等[102]發現Ni基催化劑具有金屬/載體協同效應,特別是當浸漬在金屬氧化物載體(如Al2O3)上。Ni具有很強的催化C-C鍵斷裂能力,從而提高碳轉化率[101,103],還能有效催化水溶性產物的蒸汽重整反應和甲烷化反應[104]。SINAG等[100]提出在葡萄糖SCWG過程中沉積的碳主要通過2個路徑實現,一是通過中間液體產物分解,二是副產氣;在超臨界狀態下葡萄糖分解為糠醛和有機酸,糠醛容易分解為焦炭沉積,Ni基催化劑能夠促進有機酸分解為氣體產物。ZHU等[105]以不同濃度的硝酸鎳和硝酸鋯為原料,采用超臨界水合成法成功制備了一系列ZrO2負載的Ni納米催化劑(Ni、0.8Ni-0.2ZrO2、0.6Ni-0.4ZrO2、0.4Ni-0.6ZrO2和ZrO2),以甘油為原料試驗表明0.4Ni-0.6ZrO2獲得了最高的氫氣產率,因為它在水煤氣轉化反應中具有將CO轉化為H2的氣體的高活性,表征發現超臨界水合成法制備的Ni/ZrO2催化劑表現出優異的晶體結構和形態穩定性,以及對甘油的SCWG具有良好的抗結焦能力。
盡管Ni基催化劑具有很多優點,但是不可避免的面臨著易燒結、水熱性質不穩定、形成的焦油焦炭等會影響Ni的選擇性和穩定性等問題[17,106-107]。改變載體材料和選擇合適的促進劑可以有效提高Ni基催化劑的穩定性,良好的載體材料能夠為催化劑保證機械強度、提供活化中心并增加活性金屬的分散性[108](圖8)。研究表明-Al2O3作為催化劑載體會在高溫水熱條件下發生相變轉化為-Al2O3,這種相變會導致表面積和孔體積損失以及平均孔徑增加[109],使活性元素溶解在水中從而造成催化劑失活[110]。LI等[111]利用溶膠-凝膠法制備了ZrO2、TiO2和Ta2O5負載的Ni基催化劑(分別表示為Ni-Zr、Ni-Ti和Ni-Ta),以甘油為原料在連續流動反應裝置中進行SCWG試驗發現Ni-Zr和Ni-Ta利用SCW的結晶環境可以實現“催化劑原位活化”效果,是用于長期催化SCWG的良好候選者,LI認為Ni與載體的燒結和催化劑材料的浸出是SCW環境中可能會導致催化劑失活的原因。XIE等[112]采用浸漬法制備了一系列不同沸石的鎳催化劑,使用高壓間歇式反應器以微藻為原料進行SCWG試驗發現沸石的強酸性位點可以增加碳氣化效率,證明沸石也可以作為SCWG的催化劑載體。

圖8 鎳基催化劑SCWG催化纖維素生物質反應路徑圖[109]
酸度是決定催化劑活性、穩定性和抗積碳能力的重要參數,因為糖類化合物會在催化劑酸性位點上發生脫水反應,使用具有氫化活性金屬和強酸性位點的雙功能催化劑會導致連續脫水/氫化,因此會產生高級烷烴。酸性位點還會促進聚合反應導致焦油狀物質的形成[109]。催化劑的酸度越高在SCWG中積碳會越明顯[113],Ni基催化劑的強酸性加速了焦炭形成[114]。雖然焦炭對催化劑活性位點覆蓋會導致的催化劑失活,但可以通過高溫煅燒和還原的方式恢復催化劑活性。而大量試驗表明在催化劑使用過程中失活是不可逆的,因此推斷在SCWG催化劑催化過程中燒結是導致催化劑失活最主要的原因,尤其集中在高負載的金屬催化劑上。催化劑燒結將導致微晶長大、催化劑表面孔隙和孔徑分布發生變化、比表面積和活性位點減少、氫氣選擇性降低[45,115]。選擇合適的促劑將會提高Ni基催化劑氫氣選擇性和抗燒結能力。表2總結了不同金屬元素作為Ni基催化劑促進劑對氣化效率的影響。

表2 不同金屬促進劑對Ni基催化劑影響
活性炭(activated carbon,AC)催化劑可以使用作物秸稈、貝殼等天然生物質在惰性氣體條件下制備。AC可以作為催化劑載體或者單獨作為催化劑使用,在SCWG過程中AC可以促進碳氣化效率。MATSUMURA等[121]使用填充床式反應器以葡萄糖為原料,加入椰殼AC在600 ℃條件下幾乎可以實現100%的碳氣化效率。LEE等[122]以葡萄糖為原料在575 ~725 ℃、28 MPa條件下通過對使用前后的催化劑進行表征發現AC的結構幾乎沒有改變,證明AC是一種不受超臨界水狀態影響的穩定材料。QUAN等[123]發現將Ce引入催化劑顯著提高了炭基催化劑的穩定性,表征發現在使用過的催化劑上形成了無定形碳和絲狀碳兩種碳,無定形碳比絲狀碳更容易氧化,Ce的加入可以減少反應過程中非晶碳的生成,增強碳基催化劑穩定性。催化劑的負載量和載體材料不同都會影響氣體的組分比率和產率[124]。YAO等[125]以麥秸、稻殼和棉稈為原料在500 ℃管式爐中快速熱解得到3種生物炭用作催化劑載體,采用工業品AC進行對照試驗,當氣化溫度為800 ℃,Ni負載量為15%時,Ni/AC催化劑的性能最佳,棉花生物炭為載體的Ni氣化產氫活性最高,為64.02%,而水稻生物炭為載體的Ni氣化產氫活性最低。
碳納米管(carbon nanotubes,CNT)具有比表面積大、熱穩定性好、機械強度高等優點而被廣泛應用于催化劑載體[126]。RASHIDI等[127]利用間歇式高壓反應裝置以甘蔗渣為原料,采用浸漬法制備具有不同鎳負載量的Ni/CNT納米催化劑,發現由于CNT巨大的內表面,在SCWG過程中使用Ni/CNTs納米催化劑可顯著提高氫氣產量。LI等[126]采用沉淀法制備了Ni負載在Mg促進的-Al2O3、α-Al2O3和CNT催化劑,使用連續式流動裝置以甘油為原料進行SCWG試驗,發現在SCW的高壓下,CNT表現出優異的晶體穩定性,減少了活性金屬流失,能在長期SCWG過程中保證機械穩定。
金屬氧化物催化劑具有耐高溫、價格低廉、易于氧化再生、易于運輸儲存等優點。CAO等[128]在600 ℃條件下以葡萄糖為模型化合物,對12種金屬氧化物(V2O5、Cr2O3、MnO2、Fe2O3、Co2O3、CuO、ZnO、ZrO2、MoO3、SnO2、WO3和CeO2)進行SCWG,發現大多數氧化物對氫氣形成的影響很小,但對CO形成的影響很大,其中Cr2O3,CuO、WO3和V2O5是最有效的制氫催化劑。ZHANG等[129]研究了不同價態的鐵基催化劑在催化木質素方面的表現,發現鐵基催化劑在SCWG中有得天獨厚的優勢,尤其零價態的鐵H2產量明顯高于不同價態的氧化鐵催化體系,低價態的鐵基催化劑(Fe和FeO)具有低配位數活性鐵原子,有利于CO產生,高價態的鐵(Fe2O3和Fe3O4)有足夠的晶格氧,容易將CO氧化成CO2。CAO等[130]以黑液為原料測試了14種金屬氧化物在SCWG中效果,發現金屬氧化物不僅可以提供催化活性位點并且氧含量也是影響氣體組成的重要因素。
3 總結與展望
本文通過對生物質SCWG(supercritical water gasification)機理總結和影響因素系統的梳理得到以下結論:1)在SCWG過程中有機酸、醛類、酮類等小分子中間體是形成氣體的主要來源,而酚類和呋喃則主要是通過聚合反應生成固體碳顆粒;2)間歇式反應器適用于研究相行為和反應機制,而連續式反應裝置適合參數研究,提高升溫速率可以抑制焦油、焦炭等副產物產生;3)適當提高進料濃度有利于提高氫氣產量,但進料濃度過高易形成較多的焦炭、焦油等中間產物導致氫氣產量降低,還易導致反應器堵塞等問題;4)低溫高壓水熱環境有利于離子反應,隨著溫度升高氫鍵數目減少,自由基反應增強,氣化效率會提高;5)在高壓下溶劑籠會導致分解反應速率降低,有利于碳的形成,不利于氫氣產生,選擇合適的壓力對于SCWG過程十分重要;6)催化劑的酸性是影響SCWG過程的重要因素,酸性強會加速焦炭的形成造成催化劑失活,Ce、La、Ru等金屬元素作為合適的促劑可以增強催化劑活性。
為了能夠進一步加深對生物質SCWG的理解并投入于日常生產生活,未來還需要在以下幾個方向加強研究:1)現階段對于反應路徑的研究主要集中在對于生物質模型化合物,對于實際生物質的反應路徑和反應機理還需要進一步研究;2)高溫高壓的反應環境對反應裝置提出了更高要求,目前需要解決反應裝置腐蝕、堵塞、鹽沉積等問題;3)需要進一步優化催化劑的添加量,探索催化機理,同時進一步探究催化劑失活原因;4)為進一步降低成本,要對催化劑的穩定性和回收利用性進行研究,開發高選擇性可重復使用的催化劑;5)目前沒有實際應用的超臨界水制氫系統,因此對于實際生產中的能耗建模、經濟性分析等相關工作較少。盡管以上的諸多問題還需要不斷的研究和探索,但是生物質SCWG能夠清潔高效的將生物質廢棄物轉化為清潔的氫能,既實現了廢棄物的無害化處理,又能生產能源,是一種非常有前景的技術。
[1] 王偉興,康慶華,董帆,等. 國內外能源利用現狀分析[J]. 云南化工,2019,46(6):48-49.
WANG Weixing, KANG Qinghua, DONG Fan, et al. Analysis of energy utilization situation at home and abroad[J]. Yunnan Chemical Industry, 2019, 46(6): 48-49. (in Chinese with English abstract)
[2] 蘭瑩,秦天寶. 《歐洲氣候法》:以“氣候中和”引領全球行動[J].環境保護,2020,48(9):7.
LAN Ying, QIN Tianbao. European Climate Law: Leading global action with “climate neutrality”[J]. Environmental Protection, 2020, 48(9): 7. (in Chinese with English abstract)
[3] 劉宇,羊凌玉,李欣蓓,等. 碳中和目標實現下中國轉型發展路徑研究[J]. 北京理工大學學報(社會科學版),2022,24(4):27-36.
LIU Yu, YANG Lingyu, LI Xinbei, et al. Research on China’s transformation development path under the carbon neutrality goal[J]. Transactions of Beijing Institute of Technology (Social Sciences Edition), 2022, 24(4): 27-36. (in Chinese with English abstract)
[4] DRESSELHAUS M S, THOMAS I L. Alternative energy technologies[J]. Nature, 2001, 414(6861): 332-337.
[5] ELLABBAN O, ABU-RUB H, BLAABJERG F. Renewable energy resources: Current status, future prospects and their enabling technology[J].Renewable and Sustainable Energy Reviews, 2014, 39: 748-764.
[6] HALL D O, SCRASE J I. Will biomass be the environmentally friendly fuel of the future?[J]. Biomass Bioenerg, 1998, 15(4/5): 357-367.
[7] 岳國君,林海龍,彭元亭,等. 以生物質為原料的未來綠色氫能[J]. 化工進展,2021,40(8):7.
YUE Guojun, LIN Hailong, PENG Yuanting, et al. Future green hydrogen energy using biomass as raw material[J]. Chemical Industry Progress, 2021, 40(8): 7. (in Chinese with English abstract)
[8] BALAT M. Potential importance of hydrogen as a future solution to environmental and transportation problems[J]. International Journal of Hydrogen Energy, 2008, 33(15): 4013-4029.
[9] PARTHASARATHY P, NARAYANAN K S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield-A review[J]. Renewable Energy, 2014, 66: 570-579.
[10] 鄒才能,李建明,張茜,等. 氫能工業現狀,技術進展,挑戰及前景[J]. 天然氣工業,2022,42(4):20.
ZOU Caineng, LI Jianming, ZHANG Qian, et al. Current situation, technological progress, challenges and prospects of hydrogen energy industry[J]. Natural Gas Industry, 2022, 42(4): 20. (in Chinese with English abstract)
[11] 曹軍文,張文強,李一楓,等. 中國制氫技術的發展現狀[J]. 化學進展,2021,33(12):30.
CAO Junwen, ZHANG Wenqiang, LI Yifeng, et al. Development status of hydrogen production technology in China[J]. Advances in Chemistry, 2021, 33(12): 30. (in Chinese with English abstract)
[12] 劉翠偉,裴業斌,韓輝,等. 氫能產業鏈及儲運技術研究現狀與發展趨勢[J]. 油氣儲運,2022,41(5):17.
LIU Cuiwei, PEI Yebin, HAN Hui, et al. Research status and development trend of hydrogen energy industry chain and the storage and transportation technologies [J]. Oil and Gas Storage and Transportation, 2022, 41(5): 17. (in Chinese with English abstract)
[13] YAO J G, KRAUSSLER M, BENEDIKT F, et al. Techno-economic assessment of hydrogen production based on dual fluidized bed biomass steam gasification, biogas steam reforming, and alkaline water electrolysis processes[J]. Energy Conversion & Management, 2017, 145: 278-292.
[14] KRUSE A, DINJUS E. Hot compressed water as reaction medium and reactant-properties and synthesis reactions[J]. The Journal of Supercritical Fluids, 2007, 39(3): 362-380.
[15] KRAMMER P, VOGEL H. Hydrolysis of esters in subcritical and supercritical water[J].The Journal of Supercritical Fluids, 2000, 16(3): 189-206.
[16] CHEN J, WANG Q, XU Z, et al. Process in supercritical water gasification of coal: A review of fundamentals, mechanisms, catalysts and element transformation [J]. Energy Conversion & Management, 2021, 237: 114122.
[17] OKOLIE J A, RANA R, NANDA S, et al. Supercritical water gasification of biomass: A state-of-the-art review of process parameters, reaction mechanisms and catalysis[J]. Sustainable Energy & Fuels, 2019, 3(3): 578-598.
[18] FANG Z, MINOWA T, SMITH R L, et al. Liquefaction and gasification of cellulose with Na2CO3and Ni in subcritical water at 350 degrees C[J]. Industrial & Engineering Chemistry Research, 2004, 43(10): 2454-2463.
[19] ABDPOUR S, SANTOS R M. Recent advances in heterogeneous catalysis for supercritical water oxidation/ gasification processes: Insight into catalyst development[J]. Process Safety and Environmental Protection, 2021, 149: 169-184.
[20] KRITZER P, DINJUS E. An assessment of supercritical water oxidation (SCWO) : Existing problems, possible solutions and new reactor concepts[J].Chemical Engineering Journal, 2001, 83(3): 207-214.
[21] HU Y, GONG M, XING X, et al. Supercritical water gasification of biomass model compounds: A review [J]. Renewable and Sustainable Energy Reviews, 2020, 118: 109529.
[22] 張麗莉,陳麗,趙雪峰,等. 超臨界水的特性及應用[J]. 化學工業與工程,2003(1):33-38,54.
ZHANG Lili, CHEN Li, ZHAO Xuefeng, et al. Characteristics and application of supercritical water[J]. Chemical Industry and Engineering, 2003(1): 33-38, 54. (in Chinese with English abstract)
[23] MARINO F , BOVERI M , BARONETTI G , et al. Hydrogen production via catalytic gasification of ethanol. A mechanism proposal over copper-nickel catalysts[J]. International Journal of Hydrogen Energy, 2004, 29(1): 67-71.
[24] LIEW C C, INOMATA H, ARAI K, et al. Three-dimensional structure and hydrogen bonding of water in sub- and supercritical regions: A molecular simulation study[J]. Journal of Supercritical Fluids, 1998, 13(1/2/3): 83-91.
[25] BOERO M, TERAKURA K, IKESHOJI T, et al. Hydrogen bonding and dipole moment of water at supercritical conditions: A first-principles molecular dynamics study [J]. Physical Review Letters, 2000, 85(15): 3245.
[26] SASAKI M, KABYEMELA B, MALALUAN R, et al. Cellulose hydrolysis in subcritical and supercritical water [J]. The Journal of Supercritical Fluids 1998, 13(1/2/3): 261-268.
[27] KARIMI K, TAHERZADEH M J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity[J]. Bioresource Technology, 2016, 200: 1008-1018.
[28] PETERSON A A, VOGEL F, LACHANCE R P, et al. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies[J]. Energy & Environmental Science, 2008, 1(1): 32-65.
[29] 鄭豪,魚濤,屈撐囤,等. 超臨界水的基本特性及應用進展 [J]. 化工技術與開發,2020,49(Z1):62-66.
ZHENG Hao, YU Tao, QU Chengdun, et al. Basic characteristics and application progress of supercritical water[J]. Chemical Technology and Development. 2020, 49(Z1): 62-66. (in Chinese with English abstract)
[30] GARROTE G, DOMINGUEZ H, PARAJO J C. Hydrothermal processing of lignocellulosic materials[J]. Holz Als Roh-Und Werkstoff, 1999, 57(3): 191-202.
[31] BOBLETER O. Hydrothermal degradation of polymers derived from plants [J]. Progress in Polymer Science, 1994, 19(5): 797-841.
[32] OSADA M, SATO T, WATANABE M, et al. Low-temperature catalytic gasification of lignin and cellulose with a ruthenium catalyst in supercritical water[J]. Energy Fuels, 2004, 18(2): 327-333.
[33] CHEN J, LIANG J, XU Z, et al. Assessment of supercritical water gasification process for combustible gas production from thermodynamic, environmental and techno-economic perspectives: A review[J]. Energy Conversion and Management, 2020, 226: 113497.
[34] REDDY S N, NANDA S, DALAI A K, et al. Supercritical water gasification of biomass for hydrogen production[J]. International Journal of Hydrogen Energy, 2014, 39(13): 6912-6926.
[35] FERREIRA-PINTO L, PARIZI M P S, DE ARAUJO P C C, et al. Experimental basic factors in the production of H2via supercritical water gasification[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25365-25383.
[36] 趙小燕,湯文,曹景沛,等. 炭載金屬催化劑在生物質焦油重整中的研究進展[J]. 燃料化學學報,2022,50(12):1547-1563.
ZHAO Xiaoyan, TANG Wen, CAO Jingpei, et al. Research progress of carbon supported metal Catalyst in reforming biomass tar[J]. Journal of Fuel Chemistry and Technology 2022, 50(12): 1547-1563. (in Chinese with English abstract)
[37] FANG Z, SATO T, SMITH R L, et al. Reaction chemistry and phase behavior of lignin in high-temperature and supercritical water[J]. Bioresource Technology, 2008, 99(9): 3424-3430.
[38] KRUSE A. Supercritical water gasification [J]. Biofuels Bioproducts & Biorefining, 2010, 2(5):415-437.
[39] YOSHIDA T, MATSUMURA Y. Gasification of cellulose, xylan, and lignin mixtures in supercritical water[J].Engineering Chemistry Research, 2001, 40(23): 5469-5474.
[40] KABYEMELA B M, ADSCHIRI T, MALALUAN R M, et al. Kinetics of glucose epimerization and decomposition in subcritical and supercritical water[J]. Industrial & Engineering Chemistry Research, 1997, 36(5): 1552-1558.
[41] CASTELLO D, KRUSE A, FIORI L. Low temperature supercritical water gasification of biomass constituents: Glucose/phenol mixtures[J]. Biomass and Bioenergy, 2015, 73: 84-94.
[42] KRUSE A, HENNINGSEN T, SMAG A, et al. Biomass gasification in supercritical water: influence of the dry matter content and the formation of phenols[J]. Industrial & Engineering Chemistry Research ,2003, 42(16): 345-360.
[43] KRUSE A, DINJUS E. Hot compressed water as reaction medium and reactant-2. Degradation reactions[J]. The Journal of Supercritical Fluids, 2007, 41(3): 361-379.
[44] KLINGLER D, VOGEL H. Influence of process parameters on the hydrothermal decomposition and oxidation of glucose in sub- and supercritical water[J]. The Journal of Supercritical Fluids, 2010, 55(1): 259-270.
[45] LU Y, JIN H, ZHANG R. Evaluation of stability and catalytic activity of Ni catalysts for hydrogen production by biomass gasification in supercritical water[J]. Carbon Resources Conversion, 2019, 2(1): 95-101.
[46] 陸柒安,張軍,趙亮,等. 生物質超臨界水氣化制氫研究進展[J]. 現代化工,2018,38(12):29-33.
LU Qi’an, Zhang Jun, Zhao Liang, et al. Research progress on hydrogen production through biomass gasification in supercritical water [J]. Modern Chemical Industry, 2018, 38(12): 29-33. (in Chinese with English abstract)
[47] GOODWIN A K, RORRER G L. Reaction rates for supercritical water gasification of xylose in a micro-tubular reactor[J]. Chemical Engineering Journal, 2010, 163(1): 10-21.
[48] WATANABE M, INOMATA H, OSADA M, et al. Catalytic effects of NaOH and ZrO2for partial oxidative gasification of n-hexadecane and lignin in supercritical water[J]. Fuel, 2003, 82(5): 545-552.
[49] RESENDE F L P, FRALEY S A, BERGER M J, et al. Noncatalytic gasification of lignin in supercritical water[J]. Energy Fuels, 2008, 22(2): 1328-1334.
[50] van BENNEKOM J G, VENDERBOSCH R H, ASSINK D, et al. Reforming of methanol and glycerol in supercritical water[J]. The Journal of Supercritical Fluids, 2011, 58(1): 99-113.
[51] HUELSMAN C M, SAVAGE P E. Reaction pathways and kinetic modeling for phenol gasification in supercritical water[J]. The Journal of Supercritical Fluids, 2013, 81: 200-209.
[52] CHEN Y, YI L, WEI W, et al. Hydrogen production by sewage sludge gasification in supercritical water with high heating rate batch reactor [J]. Energy, 2022, 238: 121740.
[53] LI S, GUO L. Stability and activity of a co-precipitated Mg promoted Ni/Al2O3catalyst for supercritical water gasification of biomass [J]. International Journal of Hydrogen Energy, 2019, 44(30): 15842-15852.
[54] Z?HRER H, VOGEL F. Hydrothermal catalytic gasification of fermentation residues from a biogas plant [J]. Biomass and Bioenergy, 2013, 53: 138-148.
[55] KARAYILDIRIM T, SINAG A, KRUSE A. Char and coke formation as unwanted side reaction of the hydrothermal biomass gasification[J]. Chemical Engineering & Technology, 2008, 31(11): 1561-1568.
[56] VENKITASAMY C, HENDRY D, WILKINSON N, et al. Investigation of thermochemical conversion of biomass in supercritical water using a batch reactor[J]. Fuel, 2011, 90(8): 2662-2670.
[57] GUO L J, LU Y J, ZHANG X M, et al. Hydrogen production by biomass gasification in supercritical water: A systematic experimental and analytical study[J]. Catal Today, 2007, 129(3/4): 275-286.
[58] FANG Z, FANG C. Complete dissolution and hydrolysis of wood in hot water[J]. Aiche Journal, 2008, 54(10): 2751-2758.
[59] LU Y J, JIN H, GUO L J, et al. Hydrogen production by biomass gasification in supercritical water with a fluidized bed reactor[J]. International Journal of Hydrogen Energy, 2008, 33(21): 6066-6075.
[60] YAKABOYLU O, ALBRECHT I, HARINCK J, et al. Supercritical water gasification of biomass in fluidized bed: First results and experiences obtained from TU Delft/Gensos semi-pilot scale setup[J]. Biomass and Bioenergy, 2018, 111: 330-342.
[61] CHEN J, XU W, ZUO H, et al. System development and environmental performance analysis of a solar-driven supercritical water gasification pilot plant for hydrogen production using life cycle assessment approach[J].Energy Conversion & Management, 2019, 184: 60-73.
[62] MCKENDRY P. Energy production from biomass (part 1): Overview of biomass[J]. Bioresour Technol, 2002, 83(1): 37-46.
[63] NANDA S, MOHANTY P, PANT K K, et al. Characterization of north american lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels[J]. Bioenergy Reserach, 2013, 6(2): 663-677.
[64] MANKAR A R, PANDEY A, MODAK A, et al. Pretreatment of lignocellulosic biomass: A review on recent advances[J]. Bioresour Technol, 2021, 334: 12.
[65] KRUSE A, MANIAM P, SPIELER F. Influence of proteins on the hydrothermal gasification and liquefaction of biomass. 2. Model compounds[J]. Industrial & Engineering Chemistry Research, 2007, 46(1): 87-96.
[66] 張清葉,李好學. 生物質氣化制氫產氫率影響因素研究[J]. 湖北農業科學,2012(23):5442-5444.
ZHANG Qingye, LI Haoxue. Research on influencing factors of hydrogen production rate from biomass gasification[J]. Hubei Agricultural Sciences, 2012(23): 5442-5444. (in Chinese with English abstract)
[67] YANIK J, EBALE S, KRUSE A, et al. Biomass gasification in supercritical water: Part 1. Effect of the nature of biomass[J]. Fuel, 2007, 86(15): 2410-2415.
[68] WILLIAMS P T, ONWUDILI J. Subcritical and supercritical water gasification of cellulose, starch, glucose, and biomass waste[J]. Energy Fuels, 2006, 20(3): 1259-1265.
[69] 郝小紅,郭烈錦. 超臨界水生物質催化氣化制氫實驗系統與方法研究[J]. 工程熱物理學報,2002, 23(2):143-146.
HAO Xiaohong, GUO Liejin. Experimental system and method for hydrogen production by catalytic gasification of supercritical aquatic materials[J]. Journal of Engineering Thermophysics, 2002, 23(2):143-146. (in Chinese with English abstract)
[70] YOSHIDA T, OSHIMA Y, MATSUMURA Y. Gasification of biomass model compounds and real biomass in supercritical water [J]. Biomass and Bioenerg, 2004, 26(1): 71-78.
[71] GUO S M, GUO L J, CAO C Q, et al. Hydrogen production from glycerol by supercritical water gasification in a continuous flow tubular reactor[J]. International Journal of Hydrogen Energy, 2012, 37(7): 5559-5568.
[72] WANG H M, MIAO R R, YANG Y, et al. Study on the catalytic gasification of alkali lignin over Ru/C nanotubes in supercritical water[J]. Journal of Fuel Chemistry and Technology, 2015, 43(10): 1195-1201.
[73] YANG C, WANG S Z, YANG J Q, et al. Hydrothermal liquefaction and gasification of biomass and model compounds: A review[J]. Green Chemistry, 2020, 22(23): 8210-8232.
[74] SU H, KANCHANATIP E, WANG D, et al. Production of H2-rich syngas from gasification of unsorted food waste in supercritical water[J]. Waste Management, 2020, 102: 520-527.
[75] OSADA M, SATO T, WATANABE M, et al. Catalytic gasification of wood biomass in subcritical and supercritical water[J]. Combustion Science and Technology, 2006, 178(1/2/3): 537-552.
[76] PROMDEJ C, MATSUMURA Y. Temperature effect on hydrothermal decomposition of glucose in sub- and supercritical water[J]. Industrial & Engineering Chemistry Research, 2011, 50(14): 8492-8497.
[77] KARAGOZ S, BHASKAR T, MUTO A, et al. Low-temperature hydrothermal treatment of biomass: Effect of reaction parameters on products and boiling point distributions[J]. Energy Fuels, 2004, 18(1): 234-241.
[78] SUSANTI R F, DIANNINGRUM L W, YUM T, et al. High-yield hydrogen production from glucose by supercritical water gasification without added catalyst[J]. International Journal of Hydrogen Energy, 2012, 37(16): 11677-11690.
[79] KERSTEN S R A, PRINS W, VAN DER DRIFT B, et al. Principles of a novel multistage circulating fluidized bed reactor for biomass gasification[J]. Chemical Engineering Science, 2003, 58(3/4/5/6): 725-731.
[80] RESENDE F L P, SAVAGE P E. Kinetic model for noncatalytic supercritical water gasification of cellulose and lignin[J]. Aiche Journal, 2010, 56(9): 2412-2420.
[81] SUSANTI R F, VERIANSYAH B, KIM J D, et al. Continuous supercritical water gasification of isooctane: A promising reactor design[J]. International Journal of Hydrogen Energy, 2010, 35(5): 1957-1970.
[82] YOUSSEF E A, ELBESHBISHY E, HAFEZ H, et al. Sequential supercritical water gasification and partial oxidation of hog manure[J]. International Journal of Hydrogen Energy, 2010, 35(21): 11756-11767.
[83] OSADA M, YAMAGUCHI A, HIYOSHI N, et al. Gasification of sugarcane bagasse over supported ruthenium catalysts in supercritical water[J]. Energy Fuels, 2012, 26(6): 3179-3186.
[84] CHEN J, FAN Y, E J, et al. Effects analysis on the gasification kinetic characteristics of food waste in supercritical water[J]. Fuel, 2019, 241: 94-104.
[85] 羅威,廖傳華,陳海軍,等. 生物質超臨界水氣化制氫技術的研究進展[J]. 天然氣化工:C1化學與化工,2016,41(01):84-90.
LUO Wei, LIAO Chuanhua, CHEN Haijun, et al. Research progress in hydrogen production from biomass via supercritical water gasification [J]. Natural Gas Chemical Industry: C1 Chemistry and Chemical Industry, 2016,41(01):84-90. (in Chinese with English abstract)
[86] GOKKAYA D S, SAGLAM M, YILKSEL M, et al. Supercritical water gasification of phenol as a model for plant biomass[J]. International Journal of Hydrogen Energy, 2015, 40(34): 11133-11139.
[87] LU Y J, GUO L J, JI C M, et al. Hydrogen production by biomass gasification in supercritical water: A parametric study[J]. International Journal of Hydrogen Energy, 2006, 31(7): 822-831.
[88] MADENOGLU T G, SAGLAM M, YUKSEL M, et al. Simultaneous effect of temperature and pressure on catalytic hydrothermal gasification of glucose[J]. The Journal of Supercritical Fluids, 2013, 73: 151-160.
[89] LENG L, ZHANG W, LENG S, et al. Bioenergy recovery from wastewater produced by hydrothermal processing biomass: Progress, challenges, and opportunities[J]. Science of the Total Environment, 2020, 748: 142383.
[90] ELLIOTT D C . Catalytic hydrothermal gasification of biomass[J]. Biofuels Bioproducts & Biorefining, 2010, 2(3):254-265
[91] ELIF D, NEZIHE A. Hydrogen production by supercritical water gasification of fruit pulp in the presence of Ru/C [J]. International Journal of Hydrogen Energy, 2016, 41(19): 8073-8083.
[92] SU W, ZHAO M, XING Y, et al. Supercritical water gasification of hyperaccumulators for hydrogen production and heavy metal immobilization with alkali metal catalysts[J]. Environmental Research, 2022, 214: 114093.
[93] CHEN Y A, GUO L J, CAO W, et al. Hydrogen production by sewage sludge gasification in supercritical water with a fluidized bed reactor [J]. International Journal of Hydrogen Energy, 2013, 38(29): 12991-12999.
[94] ONWUDILI J A, WILLIAMS P T. Hydrogen and methane selectivity during alkaline supercritical water gasification of biomass with ruthenium-alumina catalyst[J]. Applied Catalysis B, 2013, 132: 70-79.
[95] FENG H, SUN J, WU Z, et al. Investigation of recycled phenol effects on supercritical water gasification of coal using ReaxFF MD simulation[J]. Fuel, 2021, 303: 121177.
[96] DING N, AZARGOHAR R, DALAI A K, et al. Catalytic gasification of cellulose and pinewood to H2in supercritical water[J]. Fuel, 2014, 118: 416-425.
[97] TAUFIQ-YAP Y H, SIVASANGAR S, SURAHIM M. Catalytic supercritical water gasification of empty palm fruit bunches using ZnO-doped Ni-CaO catalyst for hydrogen production[J]. Bioenergy Research, 2019, 12(4): 1066-1076.
[98] ELLIOTT D C, SEALOCK L J, BAKER E G. Chemical- processing in high-pressure aqueous environments.2. development of catalysts for gasification[J]. Industrial & Engineering Chemistry Research, 1993, 32(8): 1542-1548.
[99] NGUYEN H T, YODA E, KOMIYAMA M. Catalytic supercritical water gasification of proteinaceous biomass: Catalyst performances in gasification of ethanol fermentation stillage with batch and flow reactors[J]. Chemical Engineering Science, 2014, 109: 197-203.
[100] SINAG A, KRUSE A, RATHERT J. Influence of the heating rate and the type of catalyst on the formation of key intermediates and on the generation of gases during hydropyrolysis of glucose in supercritical water in a batch reactor[J]. Industrial & Engineering Chemistry Research, 2004, 43(2): 502-508.
[101] SHEIKHDAVOODI M J, ALMASSI M, EBRAHIMI-NIK M, et al. Gasification of sugarcane bagasse in supercritical water; evaluation of alkali catalysts for maximum hydrogen production[J]. Journal of the Energy Institute, 2015, 88(4): 450-458.
[102] ADAMU S, BINOUS H, RAZZAK S A, et al. Enhancement of glucose gasification by Ni/La2O3-Al2O3towards the thermodynamic extremum at supercritical water conditions[J]. Renewable Energy, 2017, 111: 399-409.
[103] AZADI P, FARNOOD R. Review of heterogeneous catalysts for sub- and supercritical water gasification of biomass and wastes[J]. International Journal of Hydrogen Energy, 2011, 36(16): 9529-9541.
[104] MINOWA T, ZHEN F, OGI T. Cellulose decomposition in hot-compressed water with alkali or nickel catalyst[J]. Journal of Supercritical Fluids, 1998, 13(1): 253-259.
[105] ZHU B, LI S, WANG W, et al. Supercritical water synthesized Ni/ZrO2catalyst for hydrogen production from supercritical water gasification of glycerol[J]. International Journal of Hydrogen Energy, 2019, 44(59): 30917-30926.
[106] PINKARD B R, GORMAN D J, TIWARI K, et al. Supercritical water gasification: Practical design strategies and operational challenges for lab-scale, continuous flow reactors[J]. Heliyon, 2019, 5(2): e01269.
[107] GUO Y, WANG S Z, XU D H, et al. Review of catalytic supercritical water gasification for hydrogen production from biomass[J]. Renewable and Sustainable Energy Reviews, 2010, 14(1): 334-343.
[108] SU H, YAN M, WANG S. Recent advances in supercritical water gasification of biowaste catalyzed by transition metal-based catalysts for hydrogen production[J]. Renewable and Sustainable Energy Reviews, 2022, 154: 111831.
[109] AZADI P, AFIF E, AZADI F, et al. Screening of nickel catalysts for selective hydrogen production using supercritical water gasification of glucose[J]. Green Chemistry, 2012, 14(6): 1766-1777.
[110] ELLIOTT D C. Catalytic hydrothermal gasification of biomass[J]. Biofuels Bioproducts & Biorefining-Biofpr, 2008, 2(3): 254-265.
[111] LI S, SAVAGE P E, GUO L. Stability and activity maintenance of sol-gel Ni-MxOy (M=Ti, Zr, Ta) catalysts during continuous gasification of glycerol in supercritical water[J]. Journal of Supercritical Fluids, 2019, 148: 137-147.
[112] XIE L F, DUAN P G, JIAO J L, et al. Hydrothermal gasification of microalgae over nickel catalysts for production of hydrogen-rich fuel gas: Effect of zeolite supports[J]. International Journal of Hydrogen Energy, 2019, 44(11): 5114-5124.
[113] HOSSAIN M Z, CHOWDHURY M B I, CHARPENTIER P A. Effect of surface acidity of Al2O3supported metal catalysts on catalytic activity and carbon deposition during SCWG of glucose[J]. Biomass and Bioenergy, 2019, 124: 142-150.
[114] SU H, KANCHANATIP E, WANG D, et al. Catalytic gasification of food waste in supercritical water over La promoted Ni/Al2O3catalysts for enhancing H2production[J]. International Journal of Hydrogen Energy, 2020, 45(1): 553-564.
[115] LI S, LU Y, GUO L, et al. Hydrogen production by biomass gasification in supercritical water with bimetallic Ni-M/γAl2O3catalysts (M=Cu, Co and Sn)[J]. International Journal of Hydrogen Energy, 2011, 36(22): 14391-14400.
[116] LU Y J, LI S, GUO L J, et al. Hydrogen production by biomass gasification in supercritical water over Ni/gamma Al2O3and Ni/CeO2-gamma Al2O3catalysts[J]. International Journal of Hydrogen Energy, 2010, 35(13): 7161-7168.
[117] LEE I G. Effect of metal addition to Ni/activated charcoal catalyst on gasification of glucose in supercritical water[J]. International Journal of Hydrogen Energy, 2011, 36(15): 8869-8877.
[118] ZHANG L H, XU C B, CHAMPAGNE P. Activity and stability of a novel Ru modified Ni catalyst for hydrogen generation by supercritical water gasification of glucose[J]. Fuel Guildford, 2012. 96(1):541-545
[119] LI S, LU Y J, GUO L J, et al. Hydrogen production by biomass gasification in supercritical water with bimetallic Ni-M/gamma Al2O3catalysts (M=Cu, Co and Sn) [J]. International Journal of Hydrogen Energy, 2011, 36(22): 14391-14400.
[120] YAN B, WU J Z, XIE C, et al. Supercritical water gasification with Ni/ZrO2catalyst for hydrogen production from model wastewater of polyethylene glycol[J]. Journal Supercrit Fluids, 2009, 50(2): 155-161.
[121] MATSUMURA Y, XU X, ANTAL M J. Gasification characteristics of an activated carbon in supercritical water[J]. Carbon, 1997, 35(6): 819-824.
[122] LEE I G, IHM S K. Catalytic gasification of glucose over Ni/Activated charcoal in supercritical water[J]. Industrial & Engineering Chemistry Research, 2009, 48(3): 1435-1442.
[123] QUAN C, WANG H H, GAO N B. Development of activated biochar supported Ni catalyst for enhancing toluene steam reforming [J].International Journal of Energy Research, 2020, 44(7): 5749-5764.
[124] SHAN Y Q, YIN L X, DJANDJA O S, et al. Supercritical water gasification of waste water produced from hydrothermal liquefaction of microalgae over Ru catalyst for production of H2rich gas fuel[J]. Fuel, 2021,292(1):120288.
[125] YAO D D, HU Q, WANG D Q, et al. Hydrogen production from biomass gasification using biochar as a catalyst/ support[J]. Bioresour Technol, 2016, 216: 159-164.
[126] LI S, SAVAGE P E, GUO L. Stability and activity maintenance of Al2O3- and carbon nanotube-supported Ni catalysts during continuous gasification of glycerol in supercritical water[J]. The Journal of Supercritical Fluids, 2018, 135: 188-197.
[127] RASHIDI M, TAVASOLI A. Hydrogen rich gas production via supercritical water gasification of sugarcane bagasse using unpromoted and copper promoted Ni/CNT nanocatalysts[J]. The Journal of Supercritical Fluids, 2015, 98: 111-118.
[128] CAO C Q, ZHANG Y, CAO W, et al. Transition metal oxides as catalysts for hydrogen production from supercritical water gasification of glucose[J]. Catalysis Letters, 2017, 147(4): 828-836.
[129] ZHANG H, CHEN F, ZHANG J, et al. Supercritical water gasification of fuel gas production from waste lignin: The effect mechanism of different oxidized iron-based catalysts[J]. International Journal of Hydrogen Energy, 2021, 46(59): 30288-30299.
[130] CAO C, XIE Y, MAO L, et al. Hydrogen production from supercritical water gasification of soda black liquor with various metal oxides[J]. Renewable Energy, 2020, 157: 24-32.
Research progress of supercritical water hydrogen production from biomass
XU Gongxun, CHEN Wei, FANG Zhen※
(,,210031,)
Green, safe, and reliable clean energy is ever increasing under the "double carbon" goal. Among them, the waste biomass can be converted into green fuel, particularly with the application of many thermochemical and biochemical technologies. Supercritical Water Gasification (SCWG) can be a promising potential to convert the organic matter in the biomass into hydrogen using supercritical water as a medium. The feedstock can be used as a resource for biomass and the waste. SCWG process shares the fast reaction speed, excellent hydrogen selectivity, and fewer by-products, compared with traditional hydrogen production. In addition, water as the reactant in the SCWG process can avoid high energy consumption during drying, and thus reduce the cost. Previous systematic analysis has been made on the SCWG influence factors. In this review, the special physical and chemical properties of supercritical water were introduced to expound the main components (such as cellulose, hemicellulose, and lignin biomass) in the SCWG process reaction, and the experimental device, reaction temperature, residence time, and pressure in the influence factors. It was found that the batch reactor was suitable for the phase behavior and reaction mechanism, due to the simple structure and strong applicability of raw materials. By contrast, the continuous reaction device was used to more accurately control the experimental parameters, and then to realize the continuous commercial production, thus suitable for the parameter research. The hydrogen yield was improved to increase the heating rate of the device during operation, the reaction temperature, and residence time, but to reduce the feed concentration within a certain range. However, there was the complicated influence of pressure on the hydrogen yield. The solvent cage was often used under high pressure, leading to the reduction of the decomposition reaction rate unsuitable for the production of hydrogen. It was necessary to select the appropriate pressure, according to the actual situation. The homogeneous and heterogeneous catalysts were utilized in the SCWG. Specifically, the homogeneous catalyst performed a better catalytic effect on the water gas conversion was reaction, but the strong corrosion was caused the equipment to clog. The heterogeneous catalyst presented high catalytic activity, easy recovery, and excellent stability, more suitable for large-scale SCWG production. At the same time, there were some influences of the acidity of the metal catalyst in the catalytic process. The strong acidity of the catalyst accelerated the formation of carbon deposition, resulting in the catalyst deactivation. Appropriate secondary metals were added, such as Ce, La, and Ru. The performance of the catalyst was accelerated to increase the service life of catalyst for the better hydrogen selectivity. Future research can be focused on the equipment with corrosion resistance and salt deposition resistance, or constantly optimizing operating parameters, while the deactivation mechanism of catalysts, even to optimize the number of catalysts, and the catalysts with high activity and reusable. The existing technical barriers and development prospects of SCWG were analyzed to combine with the current technical development of SCWG. The finding can also provide theoretical guidance for the biomass SCWG in the future.
biomass; hydrogen; catalysts; supercritical water
2023-01-11
2023-02-15
國家自然科學基金面上項目(21878161);南京農業大學高層次人才引進項目(68Q-0603)
徐功迅,研究方向為生物質能源轉化。Email:wsywxns@126.com
方真,博士,教授,博士生導師,研究方向為生物燃料及在內燃機中的利用。Email:zhenfang@njau.edu.cn
10.11975/j.issn.1002-6819.202301053
S216
A
1002-6819(2023)-07-0024-12
徐功迅,陳偉,方真. 生物質超臨界水制氫研究進展[J]. 農業工程學報,2023,39(7):24-35. doi:10.11975/j.issn.1002-6819.202301053 http://www.tcsae.org
XU Gongxun, CHEN Wei, FANG Zhen. Research progress of supercritical water hydrogen production from biomass[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2023, 39(7): 24-35. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.202301053 http://www.tcsae.org

