免疫沉淀分離外泌體對PC12細胞上清中α-syn含量的影響
楊桐桐 王曉婷 謝俊霞 宋寧
[摘要]目的探討采用免疫沉淀方法分離PC12多巴胺能細胞上清中外泌體對細胞上清中α-突觸核蛋白(α-syn)含量的影響。方法利用多西環素誘導PC12細胞高表達α-syn,培養24 h后收集細胞上清液,采用免疫沉淀方法進行外泌體的分離純化,采用酶聯免疫吸附測定方法測定外泌體分離前后細胞上清中α-syn的含量。結果采用免疫沉淀方法可以分離出細胞上清中的外泌體,分離出的外泌體表達特異性標記物alix和CD63。外泌體分離后,上清中α-syn的含量從(2 999.0±193.0)ng/L下降為(2 276.0±81.8)ng/L,降低了(723.0±111.2)ng/L(t=4.580,P<0.05)。結論免疫沉淀分離外泌體能夠降低PC12細胞上清中α-syn的水平。
[關鍵詞]α突觸核蛋白;外泌體;免疫沉淀法;PC12細胞
[中圖分類號]R338.2[文獻標志碼]A[文章編號]2096-5532(2023)03-0329-04
doi:10.11712/jms.2096-5532.2023.59.087[開放科學(資源服務)標識碼(OSID)]
[網絡出版]https://kns.cnki.net/kcms2/detail/37.1517.R.20230731.1057.003.html;2023-07-3115:48:26
EFFECT OF EXOSOME ISOLATION BY IMMUNOPRECIPITATION ON Α-SYNUCLEIN LEVEL IN THE SUPERNATANT OF PC12 CELLS? YANG Tongtong, WANG Xiaoting, XIE Junxia, SONG Ning (Department of Physiology and Pathophysiology, School of Basic Medicine, Qingdao University Medical College, Qingdao 266071, China)
[ABSTRACT]ObjectiveTo investigate the effect of isolation of exosomes from the supernatant of PC12 dopaminergic cells by immunoprecipitation on the α-synuclein (α-syn) level in cell supernatant. MethodsDoxycycline was used to induce α-syn over-expression in PC12 cells. After 24 h of culture, the supernatant was collected, and the exosomes were isolated and purified by immunoprecipitation. The level of α-syn in the supernatant before and after exosome isolation was determined by enzyme-linked immunosorbent assay. ResultsExosomes in cell supernatant could be isolated by immunoprecipitation, and specific markers alix and CD63 were expressed in the isolated exosomes. After exosome isolation, the level of α-syn in the supernatant decreased from (2 999.0±193.0) ng/L to (2 276.0±81.8) ng/L by (723.0±111.2) ng/L (t=4.580,P<0.05). ConclusionExosome isolation by immunoprecipitation can reduce the level of α-syn in the supernatant of PC12 cells.
[KEY WORDS]alpha-synuclein; exosomes; immunoprecipitation; PC12 cells
帕金森病(PD)是一種常見的神經退行性疾病,遺傳、環境以及年齡因素均可能參與PD發病[1-2]。PD的主要病理學表現為黑質中多巴胺能神經元的丟失以及黑質殘存多巴胺能神經元中形成路易小體和路易神經突,路易小體的主要成分是聚集形式的α-突觸核蛋白(α-syn)。近年來研究發現,α-syn具有阮病毒樣特征,可以在細胞間傳播[3]。外泌體是一種直徑30~150 nm的囊泡,可以攜帶包括α-syn在內的細胞內成分在細胞間進行傳遞[4]。由于外泌體特殊的脂膜結構,其內含有的致病蛋白更不容易被細胞間隙的蛋白酶水解且容易被細胞攝取,因此值得進一步研究[5]。然而,對于在細胞間傳播的外泌體形式的α-syn所占比例仍存在較大爭議。有研究表明,利用免疫沉淀方法可以從細胞上清中分離外泌體[6-7]。本實驗旨在探討采用免疫沉淀方法分離PC12多巴胺能細胞上清中外泌體對細胞上清中α-syn含量的影響。
1材料和方法
1.1實驗材料
可誘導高表達α-syn PC12細胞由DAVID C. RUBINSZTEIN教授饋贈;DMEM高糖培養液購自Hyclone公司;胎牛血清和馬血清購自依科賽公司;多西環素購自Sigma公司;CD63抗體購自Novus公司;蛋白A/G瓊脂糖珠購自Santa Cruz公司;flotillin-1抗體購自Abcam公司;alix抗體購自Cell Signaling Technology公司;酶聯免疫吸附測定(ELISA)試劑盒購于Biolegend公司;ECL發光液購于雅酶公司。
1.2高表達α-syn PC12細胞的培養和細胞上清液的收集
將狀態良好的PC12細胞計數后接種于6孔板中,待其密度達到60%左右時,加入2 mg/L 多西環素作用24 h誘導細胞高表達α-syn。更換為無血清培養液,再次培養24 h細胞密度達到90%~95%,收集上清液。上清液在4 ℃下以3 500 r/min離心15 min,去除細胞碎片和死細胞。
1.3免疫沉淀分離細胞上清中的外泌體
將牛血清清蛋白(BSA)溶解于磷酸鹽緩沖液(PBS)中,配制成2 g/L緩沖液。取500 μL PBS/BSA緩沖液稀釋50 μL蛋白A/G瓊脂糖珠并在4 ℃下孵育過夜。以PBS洗滌珠子3次后重懸于100 μL抗flotillin-1抗體(用2 g/L PBS/BSA緩沖液以1∶100比例稀釋)中,4 ℃孵育4 h。以PBS洗滌珠子3次后收集蛋白A/G瓊脂糖珠,加入離心后的細胞上清液200 μL,4 ℃孵育3 h。在4 ℃下以1 000 r/min離心1 min,去除蛋白A/G瓊脂糖珠后收集上清液。
1.4ELISA檢測
采用ELISA法測定外泌體分離前后細胞上清中α-syn的含量,嚴格按照試劑盒要求進行操作。
1.5蛋白質免疫印跡檢測
用10 g/L SDS細胞裂解液洗脫上述的瓊脂糖珠,用于免疫印跡檢測。蛋白質用80 g/L SDS-聚丙烯酰胺凝膠電泳分離,并轉移到硝酸纖維素膜上。膜在4 ℃下孵育過夜,使用外泌體特異標記蛋白一抗alix(1∶1 000)和CD63(1∶1 000)檢驗免疫沉淀的效果,加辣根過氧化物酶標記的山羊抗小鼠二抗(1∶10 000)孵育,用ECL發光液顯影。使用Image J軟件進行條帶灰度值分析。
1.6統計學處理
應用Prism 5軟件進行統計學分析,所得計量資料數據以±s表示,采用配對t檢驗進行兩組均數的比較,以P<0.05為差異有統計學意義。
2結果
2.1免疫沉淀分離外泌體的鑒定
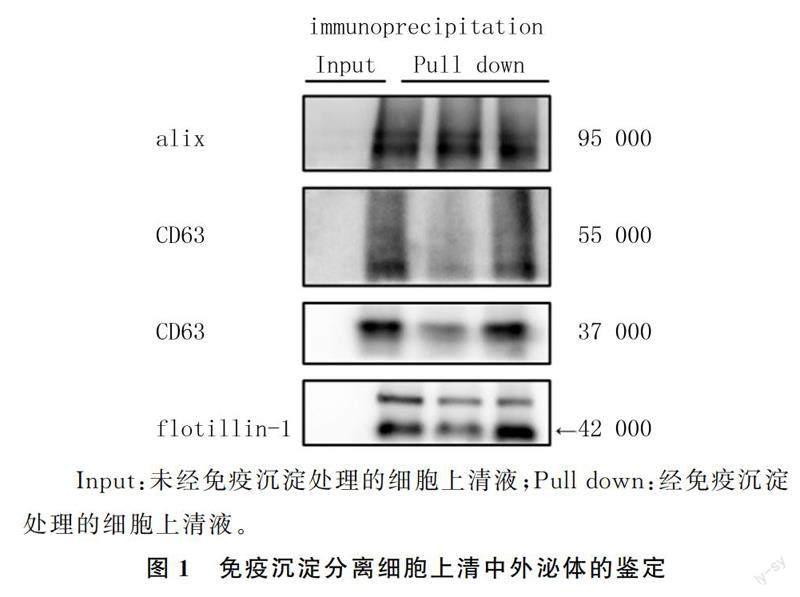
用flotillin-1抗體下拉細胞上清液中的外泌體,使用外泌體標記蛋白alix和CD63進行免疫印跡檢測。結果顯示,未經免疫沉淀處理的細胞上清液無蛋白質印跡,瓊脂糖珠洗脫液有明顯的蛋白質富集,表明采用免疫沉淀方法可以從細胞上清中分離外泌體。見圖1。
2.2分離外泌體對PC12細胞上清中α-syn含量的影響
ELISA檢測結果顯示,外泌體分離前后的細胞上清液中α-syn的濃度分別為(2 999.0±193.0)、(2 276.0±81.8)ng/L,外泌體分離后較分離前降低了(723.0±111.2)ng/L,差異具有統計學意義(t=4.580,P<0.05)。
3討論
α-syn主要存在于中樞神經系統(CNS),尤其在皮質、海馬、紋狀體、丘腦和小腦等腦區高度表達。據報道,α-syn能調節突觸膜的各種生理過程和性質,例如囊泡的大小,突觸囊泡的運輸、對接和回收以及神經遞質釋放等[8-9]。生理條件下,α-syn呈舒展的可溶性結構,但基因突變、α-syn翻譯后修飾、α-syn濃度升高以及環境因素都可能引起α-syn聚集形成寡聚體或者不溶性的纖維結構[1]。α-syn的聚集是PD最明顯的神經病理學特征,目前研究認為,α-syn的病理改變在PD的發病機制中起著核心作用[10-12]。越來越多來自PD病人、動物模型以及培養細胞的證據表明,α-syn可以在細胞之間傳播,而且細胞外α-syn在PD發生發展的過程中起著重要作用[13-14]。
外泌體是由多種細胞分泌的小囊泡,可以攜帶包括α-syn在內的多種細胞內成分在細胞之間進行傳遞[4,15]。早期內體膜向內出芽形成的管腔內泡(ILVs)逐漸成熟為多泡體(MVBs),MVBs與質膜融合,被釋放到細胞外的ILVs即為外泌體[16-18]。外泌體從各種細胞分泌并釋放到細胞外空間,參與了細胞間的物質傳遞,許多致病蛋白已被證實與外泌體有關,例如阿爾茨海默病中的β淀粉樣蛋白(Aβ)[19-20]和tau[21]、PD中的α-syn[22-23]。此外,狹小的空間可以使大分子處于高濃度狀態,從而產生“大分子擁擠現象”,可以促進致病蛋白的聚集,因此通過外泌體形式進行傳播的致病蛋白致病效率更高、更值得關注[24]。
外泌體α-syn是細胞外α-syn在細胞間傳播的重要形式。有研究顯示,使用來自PD病人腦脊液的外泌體處理H4細胞,在受體細胞內誘導了α-syn的進一步聚集,表明了腦脊液外泌體α-syn在疾病進展中的可能作用[22]。該研究還表明,腦脊液中外泌體形式的α-syn含量只占到2.17%,大部分的α-syn都是以游離形式存在。另外一項研究表明,與健康對照相比,PD病人腦脊液外泌體中總α-syn和聚集形式的α-syn含量均顯著降低,這通常被認為是α-syn在腦內過度沉積的證明[25]。然而,血漿中CNS來源的外泌體形式的α-syn含量增加[26]。這可能是由于PD病人CNS中過量有害的α-syn外排清除導致的。α-syn一般在溶酶體降解,據報道PD病人的溶酶體功能降低[27]。這些需要溶酶體降解的蛋白進入MVBs,MVBs與質膜融合,將外泌體釋放到胞外,這可能是病理條件下,神經元清除α-syn的機制[28]。最近有研究報道,血漿外泌體α-syn還可用于PD、多系統萎縮(MSA)等神經變性疾病的診斷,例如PD病人血漿中神經細胞來源的外泌體α-syn顯著升高[26],MSA病人血漿中少突膠質細胞來源的外泌體α-syn顯著降低[29]。由于血漿來源的外泌體更易獲得,因此血漿外泌體中α-syn或其他致病蛋白的水平變化,可能是神經相關疾病診斷的新靶點[30-32]。
有研究認為,外泌體α-syn只占分泌至細胞外α-syn的一小部分,對疾病的傳播沒有顯著影響[24]。但如上所述,少量腦脊液中的外泌體α-syn足以引起受體細胞內源性α-syn聚集[22]。因此有理由認為,PD病人腦脊液外泌體中α-syn的含量雖然很低,但足以在疾病進展中起到關鍵作用。另一項研究在體外培養過表達α-syn的SH-SY5Y多巴胺能細胞系,利用免疫磁珠從細胞上清液中特異性分離外泌體,發現細胞上清液中大約75%的α-syn來源于外泌體[6]。在本實驗中,通過免疫磁珠分離外泌體后,上清液中α-syn下降了大約30%。這些結果表明,外泌體α-syn在細胞外液中的比例可能與不同細胞類型有關,對比腦脊液,可能體外培養的神經細胞系上清中外泌體形式的α-syn比例更高。但無論細胞外液中外泌體α-syn的比例高低,由于在各種體液成分中容易獲得,外泌體α-syn在包括PD在內神經變性疾病的發病機制、早期診斷和治療策略中的作用都值得進一步關注。
[參考文獻]
[1]HAYES M T. Parkinsons disease and Parkinsonism[J]. The American Journal of Medicine, 2019,132(7):802-807.
[2]JANKOVIC J. Parkinsons disease: clinical features and diagnosis[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 2008,79(4):368-376.
[3]EMMANOUILIDOU E, MELACHROINOU K, ROUME-LIOTIS T, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival[J]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 2010,30(20):6838-6851.
[4]YUAN L, LI J Y. Exosomes in Parkinsons disease: current perspectives and future challenges[J]. ACS Chemical Neuroscience, 2019,10(2):964-972.
[5]GUPTA A, PULLIAM L. Exosomes as mediators of neuroinflammation[J]. Journal of Neuroinflammation, 2014,11:68.
[6]ALVAREZ-ERVITI L, SEOW Y, SCHAPIRA A H, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission[J]. Neurobiology of Disease, 2011,42(3):360-367.
[7]BENJAMINS J A, NEDELKOSKA L, TOUIL H, et al. Exosome-enriched fractions from MS B cells induce oligodendrocyte death[J]. Neurology (R) Neuroimmunology & Neuroinflammation, 2019,6(3):e550.
[8]IWAI A, MASLIAH E, YOSHIMOTO M, et al. The precursor protein of non-a beta component of Alzheimers disease amyloid is a presynaptic protein of the central nervous system[J]. Neuron, 1995,14(2):467-475.
[9]GALVIN J E, SCHUCK T M, LEE V M, et al. Differential expression and distribution of alpha-, beta-, and gamma-synuclein in the developing human substantia nigra[J]. Experimental Neurology, 2001,168(2):347-355.
[10]SPILLANTINI M G, SCHMIDT M L, LEE V M, et al. Alpha-synuclein in Lewy bodies[J]. Nature, 1997,388(6645):839-840.
[11]MEHRA S, SAHAY S, MAJI S K. α-synuclein misfolding and aggregation: implications in Parkinsons disease pathoge-nesis[J]. Biochimica et Biophysica Acta Proteins and Proteomics, 2019,1867(10):890-908.
[12]J??KO H, LENKIEWICZ A M, WILKANIEC A, et al. The interplay between parkin and alpha-synuclein; possible impli-cations for the pathogenesis of Parkinsons disease[J]. ActaNeurobiologiae Experimentalis, 2019,79(3):276-289.
[13]GEORGE S, REY N L, TYSON T, et al. Microglia affect α-synuclein cell-to-cell transfer in a mouse model of Parkinsons disease[J]. Molecular Neurodegeneration, 2019,14(1):34.
[14]ATIK A, STEWART T, ZHANG J. Alpha-synuclein as a biomarker for Parkinsons disease[J]. Brain Pathology, 2016,26(3):410-418.
[15]PEGTEL D M, GOULD S J. Exosomes[J]. Annual Review of Biochemistry, 2019,88:487-514.
[16]KALLURI R, LEBLEU V S. The biology,? function,? and biomedical applications of exosomes[J]. Science, 2020,367(6478):eaau6977.
[17]HEIJNEN H F, SCHIEL A E, FIJNHEER R, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules[J]. Blood, 1999,94(11):3791-3799.
[18]HOWITT J, HILL A F. Exosomes in the pathology of neurodegenerative diseases[J]. The Journal of Biological Chemistry, 2016,291(52):26589-26597.
[19]WANG Y P, BALAJI V, KANIYAPPAN S, et al. The release and trans-synaptic transmission of Tau via exosomes[J]. Molecular Neurodegeneration, 2017,12(1):5.
[20]RAJENDRAN L, HONSHO M, ZAHN T R, et al. Alzheimers disease beta-amyloid peptides are released in association with exosomes[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006,103(30):11172-11177.
[21]SHI M, KOVAC A, KORFF A, et al. CNS tau efflux via exosomes is likely increased in Parkinsons disease but not in Alzheimers disease[J]. Alzheimers & Dementia: the Journal of the Alzheimers Association, 2016,12(11):1125-1131.
[22]STUENDL A, KUNADT M, KRUSE N, et al. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinsons disease and dementia with Lewy bodies[J]. Brain: a Journal of Neurology, 2016,139(Pt 2):481-494.
[23]DANZER K M, KRANICH L R, RUF W P, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers[J]. Molecular Neurodegeneration, 2012,7:42.
[24]EMMANOUILIDOU E, VEKRELLIS K. Exocytosis and spreading of normal and aberrant α-synuclein[J]. Brain Patho-logy, 2016,26(3):398-403.
[25]HONG Z, TIAN C, STEWART T, et al. Development of a sensitive diagnostic assay for parkinson disease quantifying α-synuclein-containing extracellular vesicles[J]. Neurology, 2021,96(18):e2332-e2345.
[26]SHI M, LIU C Q, COOK T J, et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinsons disease[J]. Acta Neuropathologica, 2014,128(5):639-650.
[27]CHU Y P, DODIYA H, AEBISCHER P, et al. Alterations in lysosomal and proteasomal markers in Parkinsons disease: relationship to alpha-synuclein inclusions[J]. Neurobiology of Disease, 2009,35(3):385-398.
[28]LEE T H, DASTI E, MAGNUS N, et al. Microvesicles as mediators of intercellular communication in cancer: the emerging science of cellular ‘debris[J]. Seminars in Immunopathology, 2011,33(5):455-467.
[29]YU Z W, SHI M, STEWART T, et al. Reduced oligodendrocyte exosome secretion in multiple system atrophy involves SNARE dysfunction[J]. Brain: a Journal of Neurology, 2020,143(6):1780-1797.
[30]JIANG C, HOPFNER F, KATSIKOUDI A, et al. Serum neuronal exosomes predict and differentiate Parkinsons di-sease from atypical Parkinsonism[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 2020,91(7):720-729.
[31]MUSTAPIC M, EITAN E, WERNER J K Jr, et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes[J]. Frontiers in Neuroscience, 2017,11:278.
[32]DUTTA S, HORNUNG S, KRUAYATIDEE A, et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinsons disease from multiple system atrophy[J]. Acta Neuropathologica, 2021,142(3):495-511.
(本文編輯馬偉平)

