草地早熟禾轉錄因子PpMYB44基因克隆及非生物脅迫響應分析



收稿日期:2024-02-16;修回日期:2024-05-30
基金項目:國家自然科學基金(31701958);黑龍江省自然科學基金項目(QC2017026);黑龍江省普通高等學校青年創新人才培養計劃項目(UNPYSCT-2018102);黑龍江省基本科研業務費項目(145109518);齊齊哈爾大學學位與研究生教育教學改革研究項目(QUZLTSJG2023014)資助
作者簡介:陳陽(1986-),女,漢族,黑龍江齊齊哈爾人,副教授,博士研究生,主要從事草坪草逆境研究,E-mail:Chenyang8368215@126.com;*通信作者Author for correspondence,E-mail:Jinyifeng8368215@ 163.com
摘要:MYB轉錄因子在植物次生代謝調節、激素信號轉導和抗逆等生理生化過程中發揮重要作用。為揭示MYB轉錄因子對草地早熟禾(Poa pratensis L.)逆境脅迫條件下的響應,本研究克隆了PpMYB44基因,并進行了生物信息學分析,應用qRT-PCR技術對該基因在組織中的表達特異性及非生物脅迫處理下的表達進行了檢測。結果顯示,PpMYB44包含典型結構域PLN03091超家族,與其在黑麥草(Lolium perenne)中的同源基因相似度較高。草地早熟禾PpMYB44基因存在組織特異性,其相對表達量由高到低依次為根>莖>葉;PpMYB44顯著響應干旱、鹽、磷和氮脅迫,干旱、氮脅迫抑制PpMYB44基因表達;鹽和磷顯著促進其表達;經植物激素SA、GA處理后,PpMYB44相對表達量下調,而IAA及GABA促進該基因表達。本研究為豐富草地早熟禾轉錄因子MYB家族在非生物脅迫下的調節機制提供了理論基礎。
關鍵詞:草地早熟禾;轉錄因子;PpMYB44;非生物脅迫;表達分析
中圖分類號:S688.4 文獻標識碼:A 文章編號:1007-0435(2024)10-3062-09
Cloning and Analysis of the Abiotic Stress Response of PpMYB44 Gene in Poa pratensis L.
CHEN Yang1,2, HUANG Xiao-qin1, YOU Xue1, YU Hao-ran1, LIU Dian-hui1, ZHAO Qing-feng1, JIN Yi-feng1,2*
(1.College of Life Science and Agro-Forestry, Qiqihar University, Qiqihar, Heilongjiang Province 161006, China;2. Heilongjiang Province Key Laboratory of Resistance Gene Engineering and Preservation of Biodiversity in Cold Areas, Qiqihar, Heilongjiang Province 161006, China)
Abstract:MYB transcription factors play a crucial role in various physiological and biochemical processes, including the regulation of plant secondary metabolism,hormone signal transduction,and stress resistance. To investigate the response of MYB transcription factors in kentucky bluegrass (Poa pratensis L.) to stress conditions,we cloned and bioinformatically analyzed the PpMYB44 gene. We also examined the gene’s expression specificity in different tissues and under abiotic stress treatments using qRT-PCR technology. Our results showed that PpMYB44 contains the typical domain PLN03091 superfamily and has a higher degree of similarity to its homologous gene in ryegrass. The expression of the PpMYB44 gene is specific to different tissues,with the highest relative expression in roots,followed by stems and leaves. PpMYB44 significantly responds to drought,salt,phosphorus,and nitrogen stress,with drought and nitrogen stress inhibiting its expression,while salt and phosphorus significantly promoting its expression. Moreover,treatments with the plant hormones SA and GA down-regulated the relative expression of PpMYB44,whereas IAA and GABA promoted its expression. This study provides a theoretical basis for enhancing our understanding of the regulatory mechanism of MYB transcription factors in bluegrass under abiotic stress.
Key words:Kentucky Bluegrass;Transcription factor;PpMYB44;Abiotic stress;Expression analysis
草地早熟禾(Poa pratensis L.)是根莖疏叢型多年生冷季型草坪草,其擁有強壯的地下根系,有助于快速形成致密緊湊的草皮,該草種質地細膩柔軟,具備耐寒性和耐旱性[1]。轉錄因子是一類具有轉錄調節功能的蛋白質,通過選擇性地結合靶基因啟動子內的特定順式調控元件,實現對靶基因的精確調控,從而在植物生長發育及逆境響應等多方面發揮作用[2-3]。MYB家族是植物中重要轉錄因子家族之一,主要參與調控植物發育、次級代謝、響應非生物脅迫以及激素信號傳導等[4-5]。MYB轉錄因子在N末端存在高度保守的MYB結構域,該結構域是DNA結合的關鍵模塊,而C末端包含轉錄激活區,根據MYB結構域的數量,可劃分為4個亞家族:1R-MYB,R2R3-MYB,3R-MYB,4R-MYB[6]。1R-MYB可調節植物根部發育及影響細胞形態的發生[7]。R2R3-MYB在植物群體中數量最多,參與激素應答、環境脅迫和調控花青素的合成[8]。3R-MYB在細胞分裂周期發揮調控作用并控制次生代謝[9]。MYB家族中數量最少的是4R-MYB轉錄因子,在亞麻(Linum usitatissimum L.)全基因組中鑒定得到1個4R-MYB轉錄因子LuMYB4R1,其可調控亞麻木質素和纖維發育[10]。
目前針對R2R3-MYB轉錄因子的研究較為深入,根據R2R3結構域和C端基序的保守性,擬南芥(Arabidopsis thaliana)R2R3-MYB轉錄因子可分為25個亞家族,其中R2R3-MYB亞家族S22由AtMYB44,AtMYB70,AtMYB73,AtMYB77四個成員組成。擬南芥AtMYB44可調節花青素的生物合成,并對葉綠素分解代謝具有抑制作用,其過表達可延緩擬南芥葉片衰老[11]。甘薯(Ipomoea batatas)IbMYB44與IbMYB340相互作用,從而降低塊根中紫色色素沉著[12]。MYB44可積極響應低溫及高溫脅迫,野生山葡萄(Vitis amurensis Rupr.)VaMYB44作為耐寒性的負調控因子,VaMYC2可與VaMYB44啟動子結合促進轉錄,參與冷響應[13]。高溫脅迫下,黃瓜(Cucumis sativus L.)CsABI5與CsMYB44相互作用,誘導葉綠素降解[14]。隨著轉錄因子MYB44研究不斷深入,發現其對干旱、鹽、氮及磷酸鹽饑餓脅迫均有響應。擬南芥AtMYB44通過調控脫落酸(Abscisic acid,ABA)信號傳導,氣孔運動和根系生長積極響應干旱脅迫[15]。在文冠果(Xanthoceras sorbifolium Bunge)和不結穗大白菜(Brassica campestris ssp.)干旱研究中,過表達XsMYB44和BcMYB44均可通過提高植物滲透調節能力和活性氧(Reactive oxygen species,ROS)穩態來抵抗干旱脅迫[16-17]。研究發現,MYB44可提升植物的耐鹽能力,過表達MYB44可通過抑制ABA信號轉導,增強植株鹽脅迫的耐受性[18]。在氮脅迫下,GhMYB44基因的上調能導致ABA信號通路增強,減緩陸地棉(Gossypium hirsutum L.)根系生長[19]。馬鈴薯(Solanum tuberosum L.)StMYB44通過抑制與磷轉移相關的StPHO1基因的表達來負向調節馬鈴薯中磷的轉運[20]。可見,MYB44轉錄因子在響應多種非生物脅迫方面具有重要意義。
目前,對于草坪草MYB轉錄因子響應非生物脅迫的研究較少,本研究采用RT-PCR克隆得到草地早熟禾PpMYB44基因并對其進行生物信息學分析,采用實時熒光定量PCR分析PpMYB44基因表達的調控機制,為后續闡明草地早熟禾PpMYB44的功能鑒定奠定基礎。
1 材料與方法
1.1 試驗材料及處理方法
選用草地早熟禾‘午夜Ⅱ號’(Poa pratensis ‘Midnight Ⅱ’)為試驗材料,選取顆粒飽滿種子均勻等量撒播于培養土中,將播種完成的育苗缽置于光照培養箱中,培養條件參數參考金一鋒等[21]進行設置。待植株生長至90 d時,選取生長健壯、長勢優良植株,將其根部泥土用清水洗凈后置于1/2 Hoagland營養液中水培,將經過14 d培養后的植株進行脅迫處理,每個處理進行三次生物學重復。參照金一鋒等[21]試驗處理方法:(1)干旱脅迫:采用10% 聚乙二醇6000(Polyethylene glycol 6000,PEG6000)的1/2 Hoagland營養液澆灌植株根部模擬干旱脅迫,處理0,2,16,24 h后取樣,取樣部位為葉部。(2)鹽脅迫:將不同濃度氯化鈉(Sodium chloride,NaCl)(0,30,100,200 mmol·L-1)加入1/2 Hoagland營養液。(3)氮脅迫:以硝酸鈉(Sodium nitrate,NaNO)(0,1.50,15.00 mmol·L-1)為主要氮源,配置1/2 Hoagland營養液。(4)磷脅迫:以磷酸二氫鉀(Potassium dihydrogen phosphate,KHPO)(0,0.10,1.00 mmol·L-1)作為主要磷源,配置1/2 Hoagland營養液。鹽、氮及磷脅迫第21 d取樣,取樣部位為葉部。(5)植物激素處理:水楊酸(Salicylic acid,SA)濃度為1.50 mmol·L-1,吲哚乙酸(Indole acetic acid,IAA)濃度為400 mmol·L-1,赤霉素(Gibberellin,GA)濃度為0.50 mmol·L-1,γ-氨基丁酸(γ-aminobutyric acid,GABA)為2.00 mmol·L-1,每天噴施植物激素,葉片表面直至有液滴滴下,分別在0 d,5 d,10 d,15 d取葉片樣品,存儲于-80℃。
1.2 草地早熟禾PpMYB44基因克隆
提取草地早熟禾葉片總RNA,通過2%瓊脂糖凝膠電泳分析,并對總RNA進行濃度與純度鑒定,以總RNA為模板合成cDNA,置于-80℃保存。利用本課題組轉錄組數據(NCBI登錄號:PRJNA517968)為基礎,結合GenBank已公布的小麥(Triticum aestivum L.)MYB44(XM_048673639.1)、黑麥草(Lolium perenne)MYB44(XM_ 047193738.1)設計特異性引物,見表1。根據所得cDNA模板及引物對PpMYB44基因進行RT-PCR擴增,PCR反應總量為25 μL:2×EsTaq MasterMix 12.50 μL,cDNA 1.00 μL,PpMYB44-F/R各1.00 μL,ddHO 9.50 μL補齊體系。PCR擴增程序設定為預變性94℃ 2 min,然后共30個循環的變性94℃ 30 s,退火56℃ 30 s,延伸72℃ 30 s,最后徹底延伸72℃ 2 min,最終獲得草地早熟禾PpMYB44基因序列。
1.3 草地早熟禾PpMYB44基因生物信息學分析
利用NCBI中BLAST對PpMYB44的核酸序列與編碼氨基酸進行比對分析,通過在線分析工具:ExPASy,SOPMA,SMART,SwissModel,PSORT等對PpMYB44氨基酸組成及理化性質、蛋白結構、亞細胞定位等進行預測分析。
1.4 草地早熟禾PpMYB44基因組織特異性及非生物脅迫下表達模式分析
通過提取草地早熟禾‘午夜Ⅱ號’不同組織部位的RNA及非生物脅迫處理組的葉部RNA,以其為模板合成cDNA第一鏈。qRT-PCR總體積為25 μL,反應體系如下:cDNA 2.00 μL,Q-PpMYB44-F/R各1.00 μL,TB Green Premix Ex Taq II 12.50 μL,ddHO 9.50 μL。擴增程序由40個循環組成:95℃預變性30 s;95℃ 5 s,60℃ 30 s。UBQ為內參基因,進行生物學、試驗重復各3次,采用2-ΔΔCt法處理數據。
2 結果與分析
2.1 草地早熟禾PpMYB44基因編碼區克隆及生物信息學分析
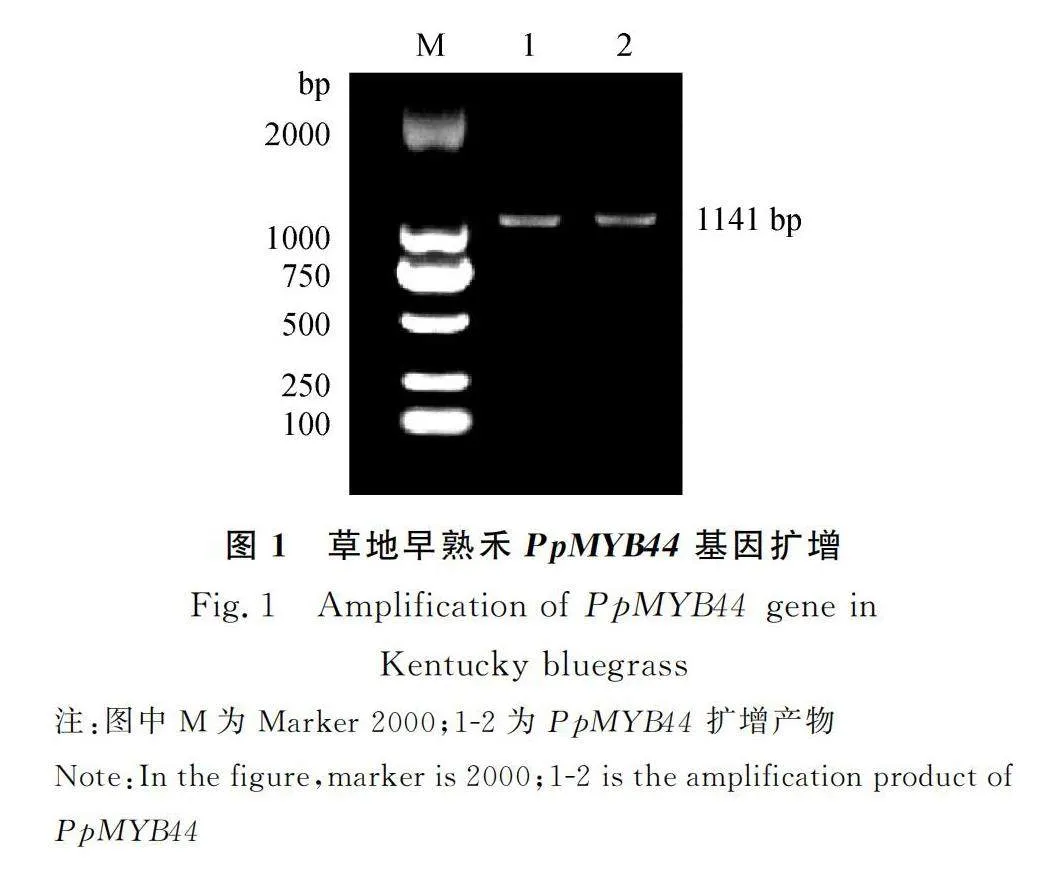
對草地早熟禾葉片總RNA進行反轉錄,以合成的cDNA為模板,RT-PCR擴增得到PpMYB44基因目的片段1141 bp,見圖1,其中開放閱讀框為948 bp,共編碼315個氨基酸,且包含有典型結構域PLN03091 super family(圖2)。
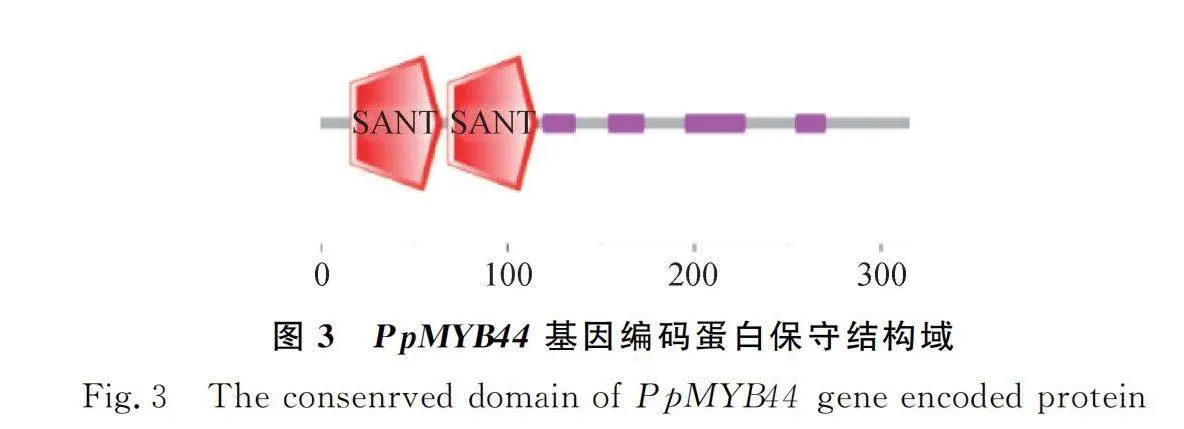
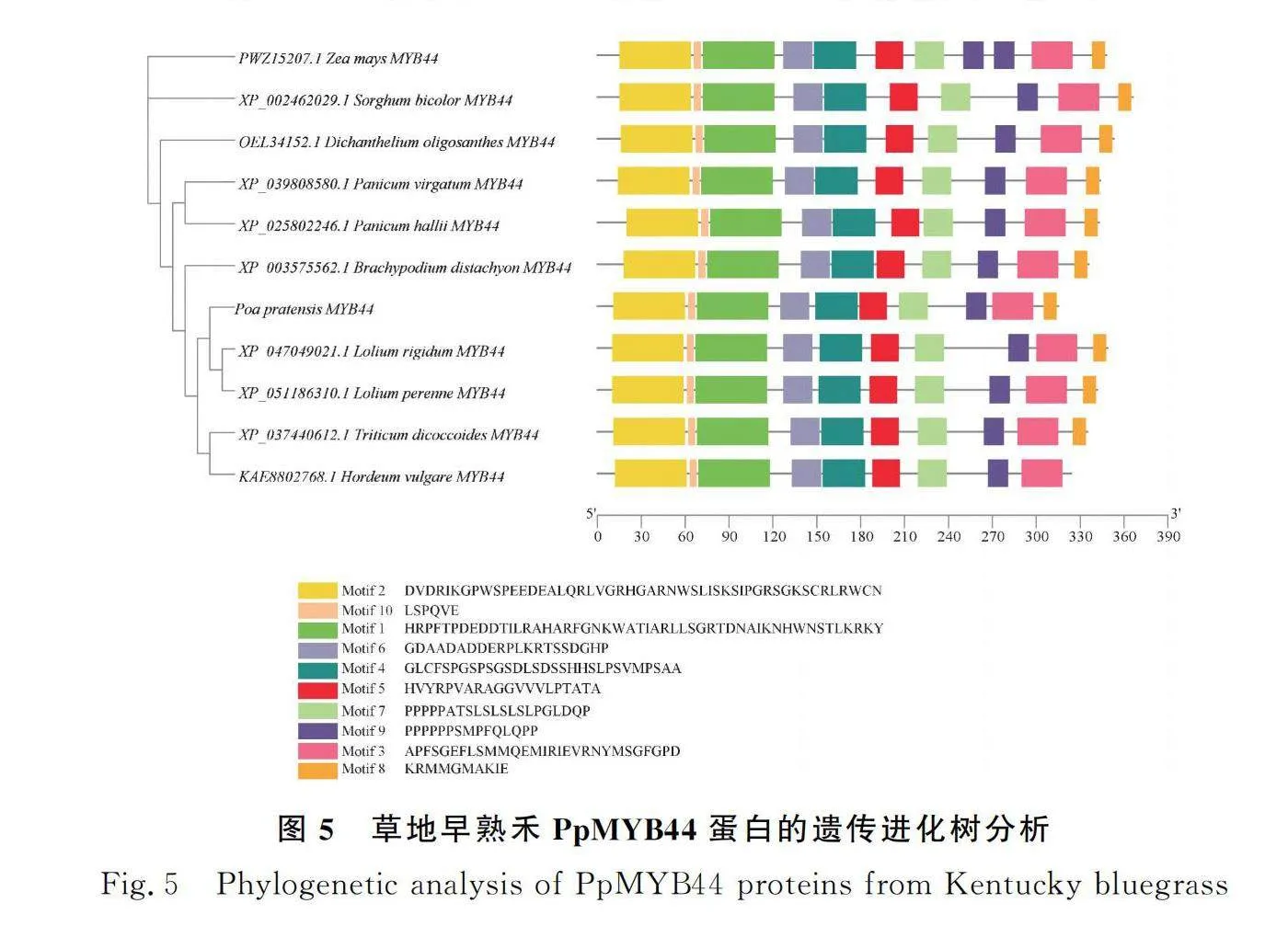
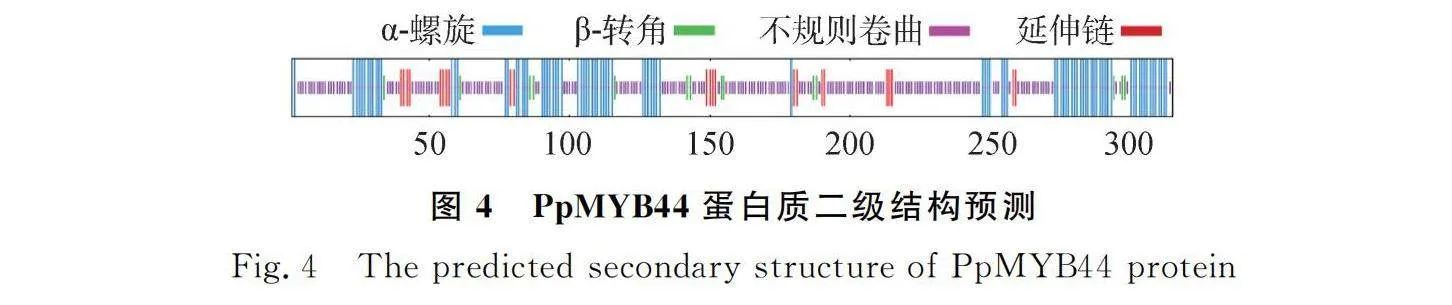
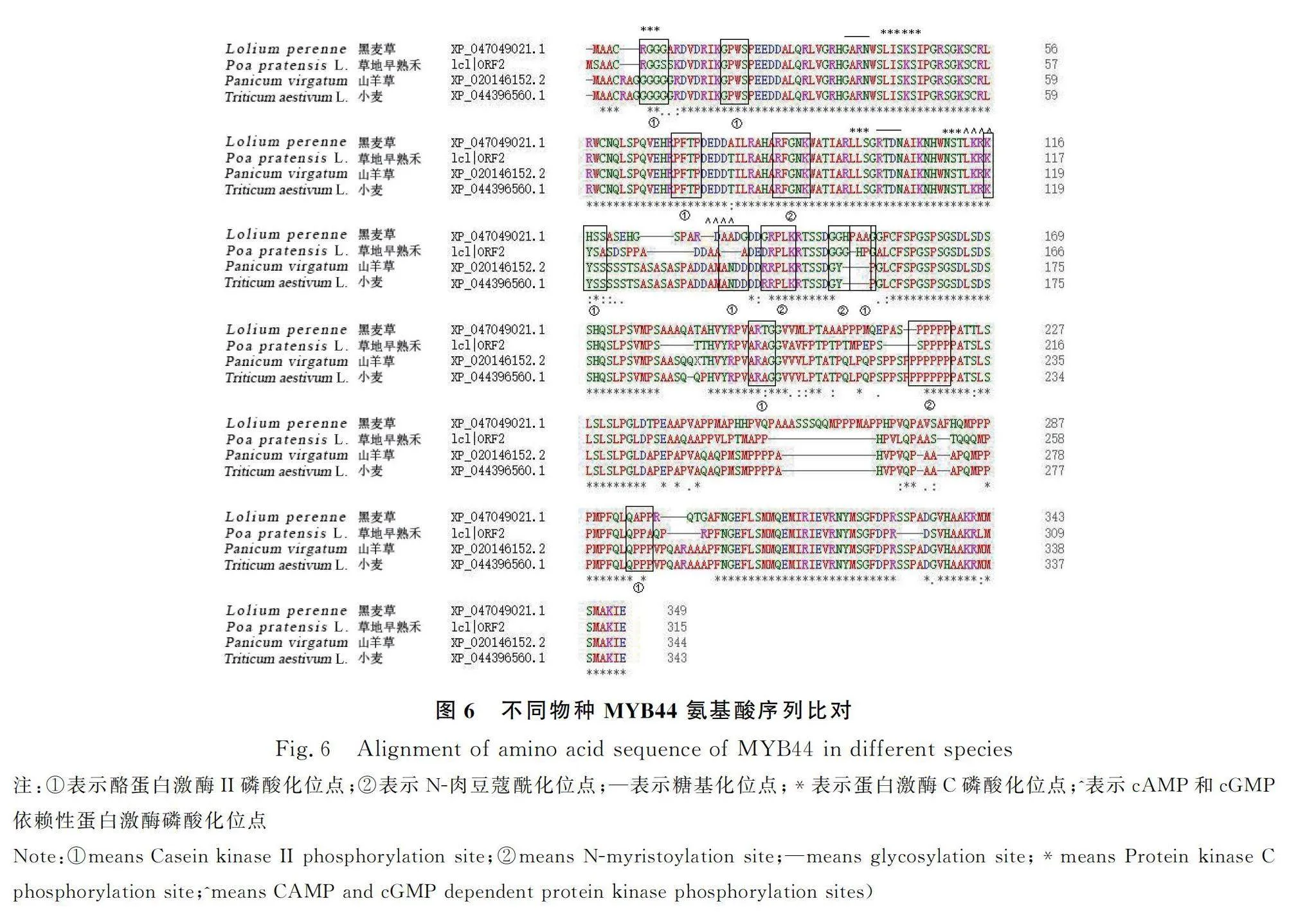
SMART分析草地早熟禾PpMYB44蛋白結構域,發現其含有2個MYB的DNA保守結構域SANT(圖3),這2個SANT結構域分別位于第16~65和第68~116位氨基酸,根據轉錄因子所含MYB結構域的個數及特征,可推測PpMYB44屬于R2R3-MYB轉錄因子家族成員。蛋白質理化性質分析的結果顯示PpMYB44分子量為33.99 KD,等電點為8.94,蛋白分子式為CHNOS。該基因編碼的氨基酸序列中脯氨酸含量最高,占比為13.3%。脂肪系數為60.19,平均親水性為-0.606,不穩定指數76.39,為親水性不穩定蛋白。通過Plant-mPloc在線預測,該蛋白定位于細胞核。使用SOPMA對PpMYB44基因編碼的蛋白質二級結構進行分析,結果顯示,該蛋白多肽鏈主要組成成分為α-螺旋、不規則卷曲、β-轉角和延伸鏈,共同形成其整體構象(圖4)。為確定PpMYB44與其他相關蛋白的同源性,利用MEME和TBtools軟件分析發現,草地早熟禾PpMYB44與黑麥草MYB44(XP_ 047049021.1)同源性最高(圖5)。利用Motif Scan比對草地早熟禾、黑麥草、山羊草(Panicum virgatum)、小麥MYB44氨基酸序列中的潛在蛋白質生物活性位點。結果顯示,PpMYB44在39~42 aa,111~114 aa含有2個糖基化位點;116~119 aa,138~141 aa含有2個cAMP和cGMP依賴性蛋白激酶磷酸化位點;9~12 aa,21~24 aa,73~76 aa,119~122 aa,140~143 aa,159~162 aa,200~203 aa,294~297 aa含有8個酪蛋白激酶II磷酸化位點;88~93 aa,145~150 aa,156~161 aa,223~228 aa含有4個N-肉豆蔻酰化位點以及9~11 aa,51~53 aa,54~56 aa,99~101 aa,113~115 aa含有5個蛋白激酶C磷酸化位點(圖6)。
2.2 草地早熟禾PpMYB44基因不同組織部位表達模式分析
利用qRT-PCR技術檢測PpMYB44基因在草地早熟禾不同組織中的表達情況,結果顯示(圖7),根、莖和葉中均檢測到PpMYB44基因的表達,其在根中表達量最高,是葉的6.20倍,在組織部位中PpMYB44相對表達量由高到底依次為根>莖>葉(P<0.05)。
2.3 草地早熟禾PpMYB44基因在不同非生物脅迫處理下的表達水平分析
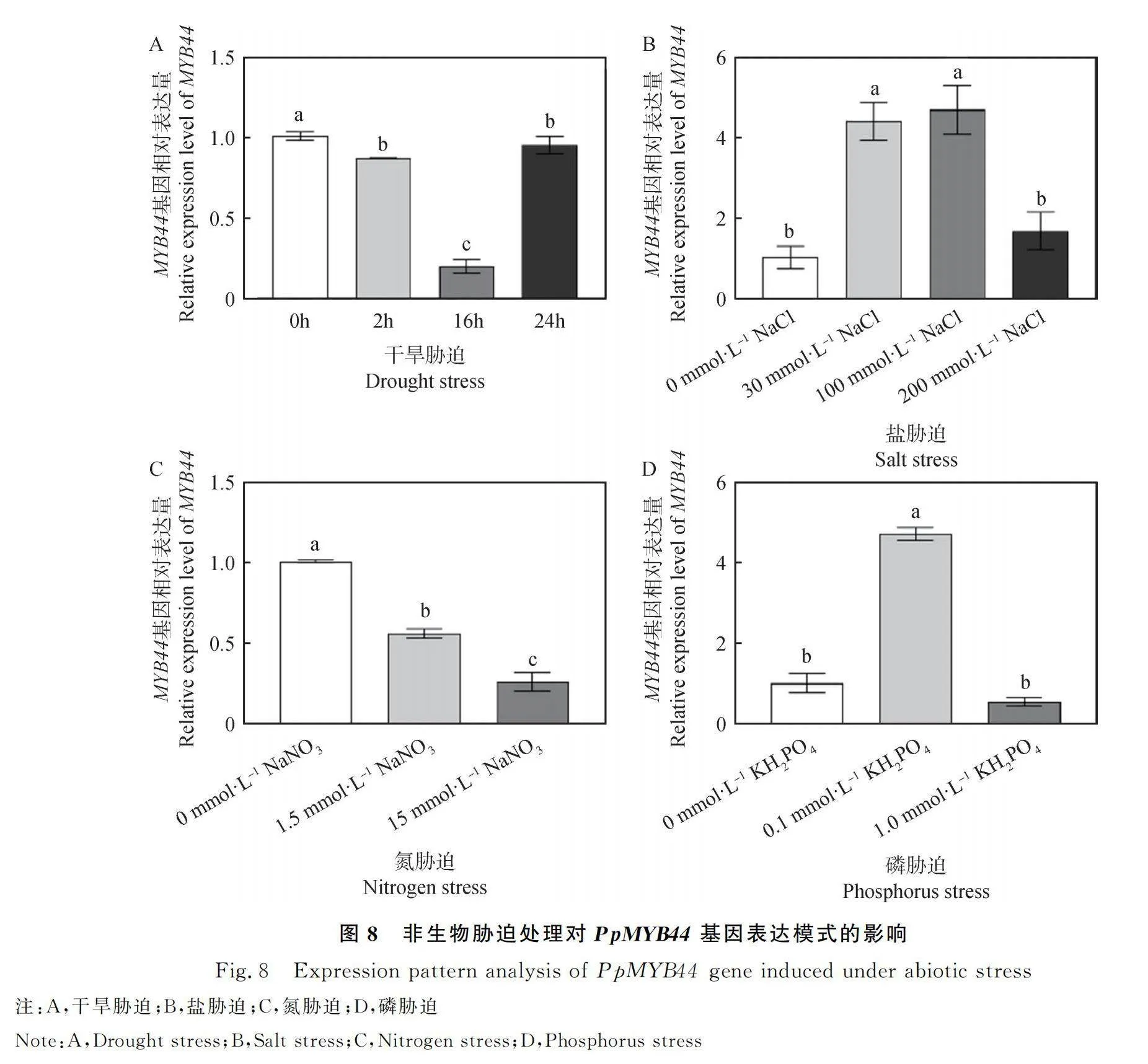
為驗證PpMYB44是否在草地早熟禾中響應逆境脅迫,通過qRT-PCR分析其在不同非生物脅迫處理下的表達水平。結果顯示,隨干旱時間的增加,PpMYB44相對表達量呈現先降低后升高的趨勢,16 h時表達水平最低,0 h相對表達量是16 h的5.05倍(P<0.05)(圖8A)。NaCl處理下PpMYB44相對表達量呈先升高后降低的趨勢,30 mmol·L-1及100 mmol·L-1NaCl濃度下其相對表達量較高,低、中鹽濃度促進其表達。隨著鹽濃度的增加,該基因的表達被抑制,200 mmol·L-1 NaCl相對表達量為100 mmol·L-1的35%(P<0.05)(圖8B)。由圖8C可知,PpMYB44積極響應氮脅迫,其隨NaNO濃度的增加呈現顯著降低(P<0.05)。磷處理下的表達趨勢不同于氮處理組,0.10 mmol·L-1 KHPO濃度時PpMYB44相對表達量最高,是0 mmol·L-1 KHPO和1.00 mmol·L-1 KHPO的4.61倍和7.31倍(圖8D),可見,低磷促進其表達,而無磷與適磷組間無顯著差異。
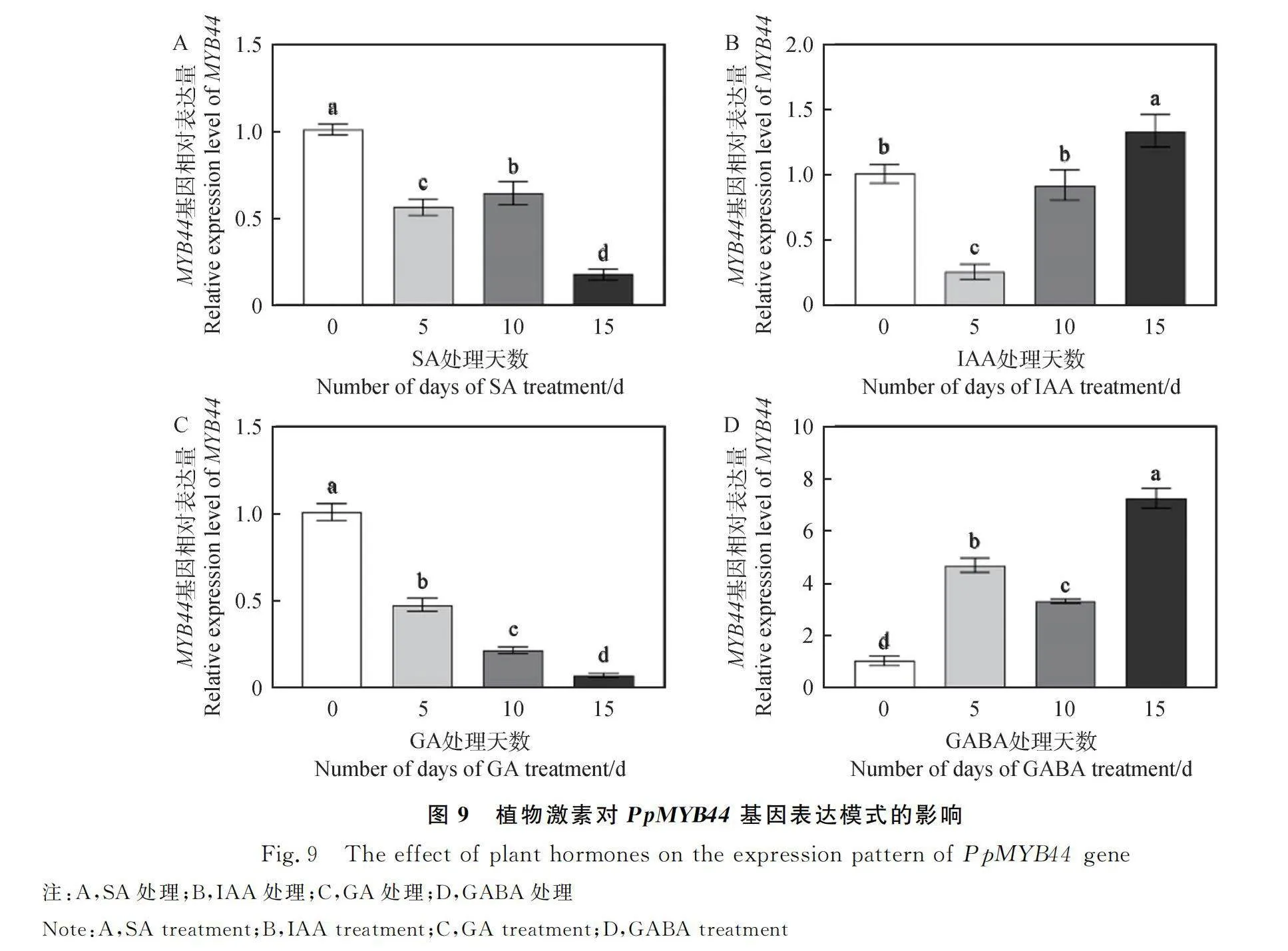
2.4 草地早熟禾PpMYB44基因在不同外源激素處理下的表達水平分析
植物激素在植物生長發育的不同時期相互作用,共同調節植物生長發育及逆境脅迫下的各種生理過程[22],通過qRT-PCR分析植物激素對草地早熟禾葉部PpMYB44表達水平的影響。結果顯示,SA激素處理抑制PpMYB44基因的表達,隨著處理時間的增加PpMYB44表達量呈先降后升再降的變化趨勢,15 d時該基因相對表達量僅為0 d時的17%(P<0.05)(圖9A)。由圖9B可知,IAA誘導處理下PpMYB44相對表達量呈先降后升趨勢,在第5 d時表達量最低,隨著處理時間的延長,該基因表達量逐漸上升,在15 d時較0 d增加了30%(P<0.05)。外源噴施GA對PpMYB44表達呈負調控模式,15 d的相對表達量僅為0 d的7% (P<0.05),其表達趨勢與SA處理組相似(圖9C)。由圖9D可知,GABA處理顯著促進PpMYB44基因表達,15 d的相對表達量達到峰值,是0 d的7.1倍(P<0.05)。綜上可見,不同植物激素顯著影響PpMYB44的表達模式,其中SA和GA處理抑制PpMYB44基因表達,GABA顯著促進其表達。
3 討論
MYB轉錄因子是植物轉錄因子家族,其特征是含有與DNA結合的MYB結構域該結構域由1~3個串聯且不完全重復的R結構組成,每個R結構約含50 ~55個氨基酸從而形成螺旋-轉角-螺旋(Helix-tuen-helix,HTH)的結構[23-24]。HTH結構使得MYB轉錄因子能夠插入到靶DNA的主凹槽中,促進其與特定靶基因精確結合[25]。Grune等[26]研究表明,SANT結構與MYB的DNA結合結構域整體高度相似,兩者為同系物。擬南芥AtMYB44與茄子(Solanum melongena L.)SmMYB44氨基酸序列均包含HTH結構,是典型R2R3-MYB類轉錄因子[27-28]。這與本研究結果相似,PpMYB44的N端含有2個SANT結構域,具有HTH構型,屬于典型R2R3-MYB類轉錄因子。MYB44在植物中存在組織特異性。文冠果XsMYB44在葉中高度表達,根和莖中低表達[16]。但花生(Arachis hypogaea L.)AhMYB44在根部及葉中均高度表達[29]。煙草(Nicotina tabacum)NtMYB44在根中表達量最高,其次是莖和葉[30],這與本研究結果相似,MYB44存在組織特異性。
R2R3-MYB轉錄因子MYB44積極響應非生物脅迫[31]。干旱促進核桃(Juglans regia L.)葉和根JrMYB44基因的表達[32]。干旱顯著抑制油菜(Brassicanapus)根中BnMYB44以及蘋果(Malus domestica Borkh.)葉中MdMYB44的表達[33-34]。本研究中干旱顯著抑制葉部PpMYB44表達。過表達AtMYB44可使蛋白磷酸酶2C(Protein phosphatase 2C,PP2C)活性降低,耐鹽性增強[35]。草莓(Fragaria vesca)FvMYB44的表達受鹽濃度的影響,高鹽下FvMYB44表達顯著上調[36]。本研究與上述結果有所不同,低鹽促進PpMYB44表達,但高鹽抑制其表達。MYB轉錄因子積極響應氮濃度變化[37]。陸地棉GhMYB44在適氮脅迫中上調表達,而低氮、高氮脅迫抑制其表達[19]。本研究中無氮、低氮環境促進PpMYB44基因表達,氮素濃度顯著影響其表達。MYB家族是調節磷酸鹽饑餓反應,擬南芥AtMYB44啟動子區域存在響應磷脅迫應激的P1BS基序,其在磷饑餓信號通路下游發揮作用,AtMYB44在磷饑餓條件下上調表達,而磷充足時則抑制其表達[38],這與本研究結果相似,低磷促進PpMYB44誘導表達。
MYB44轉錄因子通過調控植物激素SA、IAA和GA等信號通路,使植物對逆境脅迫做出應答[39]。擬南芥AtMYB44通過調控AtWRKY70的表達,從而調節SA和JA信號通路之間的拮抗作用[40]。Jeky等[41]研究發現,外源SA的施用導致了珍珠粟(Pennisetum glaucum L.)PgMYB44表達下調,這與本研究結果相似。PYL9蛋白與MYB44相互作用從而調控IAA的應答來促進植物側根的生長[42]。油菜BnMYB44啟動子中存在多種激素響應順式調控元件,這些元件與IAA密切相關[33],IAA可誘導AhMYB44上調表達[43],這與本研究結果相似。GA對種子萌發和幼苗發育具有拮抗作用,GA可通過上調AtMYB44的表達來抑制種子萌發[44],但本研究中外源噴施GA降低PpMYB44的表達量。GABA與植物激素IAA、GA等具有相互調控的作用,外源施用GABA可顯著提高植物IAA、GA等內源激素的含量[45]。本研究中,外源GABA可顯著促進PpMYB44的表達。可見,外源SA、IAA、GA和GABA可顯著誘導PpMYB44的應答,該基因可能參與植物激素信號網絡。
4 結論
本研究克隆得到草地早熟禾PpMYB44基因,包含典型的結構域MYB的DNA保守結構域,其與黑麥草同源性較高。草地早熟禾PpMYB44組織特異性顯著,能積極響應干旱、鹽、磷、氮及植物激素等多種非生物脅迫。
參考文獻
[1]岑慧芳,錢文武,朱慧森,等. 干旱脅迫對草地早熟禾葉片顯微結構和光合特征的影響[J]. 草地學報,2023,31(5):1368-1377
[2]LI J L,HAN G L,SUN C F,et al. Research advances of MYB transcription factors in plant stress resistance and breeding[J]. Plant Signaling and Behavior,2019,14(8):1613131
[3]ZHANG Z X,CHENG J,WANG W X,et al. Transcription factors dealing with Iron-deficiency stress in plants:focus on the bHLH transcription factor family[J]. Physiologia Plantarum,2023,175(6):14091
[4]GAO H C,LI Z Z,HAN F F,et al. Cloning,subcellular location and expression analysis of grape MYB gene[J]. Bangladesh Journal of Botany,2023,51(3):499-506
[5]WANG X P,NIU Y L,ZHENG Y,et al. Multiple functions of MYB transcription factors in abiotic stress responses[J]. International Journal of Molecular Sciences,2021,22(11):6125
[6]FANG Q,WANG X Q,WANG H Y,et al. The poplar R2R3 MYB transcription factor PtrMYB94 coordinates with abscisic acid signaling to improve drought tolerance in plants[J]. Tree Physiology,2020,40(1):46-59
[7]MATSUI K,UMEMURA Y,OHME-TAKAGI M. AtMYBL2,a protein with a single MYB domain,acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis[J]. Plant Journal,2010,55(6):954-967
[8]YUAN Y D,ZHANG J C,LIU X,et al. Tissue-specific transcriptome for Dendrobium officinale reveals genes involved in flavonoid biosynthesis[J]. Genomics,2020,112(2):1781-1794
[9]JIANG C K,RAO G Y. Insights into the diversification and evolution of R2R3 MYB transcription factors in plants[J]. Plant Physiology,2020,183(2):637-655
[10]TOMBULOGLU H. Genome-wide identification and expression analysis of R2R3,3R-and 4R-MYB transcription factors during lignin biosynthesis in flax (Linum usitatissimum) [J]. Genomics,2020,112(1):782-795
[11]PERSAK H,PITZSCHKE A. Dominant repression by Arabidopsis transcription factor MYB44 causes oxidative damage and hypersensitivity to abiotic stress[J]. International Journal of Molecular Sciences,2014,15(2):2517-2537
[12]WEI Z Z,HU K D,ZHAO D L,et al. MYB44 competitively inhibits the formation of the MYB340-bHLH2-NAC56 complex to regulate anthocyanin biosynthesis in purple-fleshed sweet potato[J]. BMC Plant Biology,2020,20(1):258
[13]ZHANG X J,HU Y F,GU B,et al. VaMYB44 transcription factor from Chinese wild Vitis amurensis negatively regulates cold tolerance in transgenic Arabidopsis thaliana and V. vinifera[J]. Plant Cell Reports,2022,41(8):1673-1691
[14]LIU W K,CHEN G L,HE M M,et al. ABI5 promotes heat stress-induced chlorophyll degradation by modulating the stability of MYB44 in cucumber[J]. Horticulture Research,2023,10(6):89-93
[15]SEO J S,SOHN H B,NOH K,et al. Expression of the Arabidopsis AtMYB44 gene confers drought/salt-stress tolerance in transgenic soybean[J]. Molecular Breeding,2012,29(3):601-608
[16]LI J B,ZHAO S,YU X,et al. Role of Xanthoceras sorbifolium MYB44 in tolerance to combined drought and heat stress via modulation of stomatal closure and ROS homeostasis [J]. Plant Physiology and Biochemistry,2021,162(0):410-420
[17]HAO Y L,WANG J J,HU C M,et al. Regulation of BcMYB44 on anthocyanin synthesis and drought tolerance in non-heading Chinese cabbage (Brassica campestris ssp. chinensis Makino) [J]. Horticulturae,2022,8(5):351
[18]QIN B,FAN S L,YU H Y,et al. HbMYB44,a rubber tree MYB transcription factor with versatile functions in modulating multiple phytohormone signaling and abiotic stress responses[J]. Frontiers in Plant Science,2022,13(5):893896
[19]CHEN J,WANG ZBA,LIU S D,et al. Nitrogen stress inhibits root growth by regulating cell wall and hormone changes in cotton (Gossypium hirsutum L.) [J]. Journal of Agronomy and Crop Science,2021,207(6):1006-1023
[20]ZHOU X J,ZHA M R,HUANG J,et al. StMYB44 negatively regulates phosphate transport by suppressing expression of PHOSPHATE1 in potato[J]. Journal of Experimental Botany,2017,68(5):1265-1281
[21]金一鋒,陳陽,高巖松,等. 草地早熟禾SnRK2.2基因克隆及非生物脅迫響應分析[J]. 草地學報,2022,30(6):1659-1667
[22]KIM J I,MURPHY A S,BAEK D,et al. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana[J]. Journal of Experimental Botany,2011,62(11):3981-3992
[23]THAKUR S,VASUDEV P G. MYB transcription factors and their role in medicinal plants[J]. Molecular Biology Reports,2022,49(11):10995-11008
[24]ZHU K K,FAN P H,MO Z H,et al. Identification,expression and co-expression analysis of R2R3-MYB family genes involved in graft union formation in pecan (Carya illinoinensis) [J]. Forests,2020,11(9):917
[25]ZHOU L,HE Y,LI J,et al. CBFs function in anthocyanin biosynthesis by interacting with MYB113 in eggplant (Solanum melongena L.) [J]. Plant and Cell Physiology,2020,61(2):416-426
[26]GRUNE T,BRZESKI J,EBERHARTER A,et al. Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI[J]. Molecular Cell,2003,12(2):449-460
[27]ZHAO Y,XING L,WANG X G,et al. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of Auxin-Responsive genes[J]. Science Signaling,2014,7(328):2005021
[28]QIU Z K,YAN S S,XIA B,et al. The eggplant transcription factor MYB44 enhances resistance to bacterial wilt by activating the expression of spermidine synthase[J]. Journal of Experimental Botany,2019,70(19):5343-5354
[29]LIU Y H,SHEN Y,LIANG M. Identification of peanut AhMYB44 transcription factor and their multiple roles in drought stress responses[J]. Plants-Basel,2022,11(24):3522
[30]余婧,郭玉雙,雷波,等. 煙草NtMYB44b轉錄因子基因克隆以及生物信息學和表達分析[J]. 植物生理學報,2020,56(2):200-208
[31]JIANG C K,RAO G Y. Insights into the diversification and evolution of R2R3-MYB transcription factors in plants[J]. Plant Physiology,2020,183(2):637-655
[32]LI D P,PENG S B,CHEN S W,et al. Identification and characterization of 5 walnut MYB genes in response to drought stress involved in ABA signaling[J]. Physiology and Molecular Biology of Plants,2021,27(6):1323-1335
[33]SHAMLOO-DASHTPAGERDI R,RAZI H,EBRAHIMIE E,et al. Molecular characterization of Brassicanapus stress related transcription factors,BnMYB44 and BnVIP1,selected based on comparative analysis of Arabidopsis thaliana and Eutrema salsugineum transcriptomes[J]. Molecular Biology Reports,2018,45(5):1111-1124
[34]WU R,WANG Y,WU T,et al. Functional characterization of MdMYB44 as a negative regulator in the response to cold and salt stress in apple calli[J]. Journal of Horticultural Science and Biotechnology,2018,93(4):347-355
[35]WANG X P,NIU Y L,ZHENG Y. Multiple functions of MYB transcription factors in abiotic stress responses[J]. International Journal of Molecular Sciences,2021,22(11):6125
[36]LI W H,WEI Y F,ZHANG L H,et al. FvMYB44,a strawberry R2R3-MYB transcription factor,improved salt and cold stress tolerance in transgenic Arabidopsis[J]. Agronomy-Basel,2023,13(4):1051
[37]GAUDINIER A,RODRIGUEZ-MEDINA,ZHANG L F,et al. Transcriptional regulation of nitrogen-associated metabolism and growth[J]. Nature,2018,563(7730):259
[38]OLUKAYODE T,CHEN J Y,ZHAO Y. Phloem-mobile MYB44 negatively regulates expression of phosphate transporter 1 in Arabidopsis roots[J]. Plants-Basel,2023,12(20):3617
[39]MULLER R,BORGHI L,KWIATKOWSKA D,et al. Dynamic and compensatory responses of Arabidopsis shoot and floral meristems to CLV3 signaling[J]. Plant Cell,2006,18(5):1188-1198
[40]SHIM J S,JUNG C,LEE S,et al. AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling[J]. Plant Journal,2013,73(3):483-495
[41]CHANWALA J,KHADANGA B,JHA D K,et al. MYB transcription factor family in pearl millet:genome-wide identification,evolutionary progression and expression analysis under abiotic stress and phytohormone treatments[J]. Plants-Basel,2023,12(2):355
[42]XING L,ZHAO Y,GAO J H,et al. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth[J]. Scientific Reports,2016,6(1):27177
[43]TIEN N Q D,KHA H,LOC N H. Identification,characterization and expression of two transcription factors MYB44 and WRKY22 from drought tolerant peanut cultivar l14[J]. Applied Biochemistry and Microbiology,2024,60(1):26-37
[44]NGUYEN X C,MY H T H,KIM H S,et al. Phosphorylation of the transcriptional regulator MYB44 by mitogen activated protein kinase regulates Arabidopsis seed germination[J]. Biochemical and Biophysical Research Communications,2012,423(4):703-708
[45]JUNG C J,HUR Y Y,MOON J S,et al. Pre-bloom application of gibberellin in ‘Tamnara’ grape increases γ-aminobutyric acid (GABA) production at full bloom[J]. Horticulture Environment and Biotechnology,2017,58(6):568-575
(責任編輯 劉婷婷)

