豬胚胎冷凍保存的研究進展

摘 要: 胚胎冷凍保存對于胚胎的遠距離移植和遺傳資源的保護具有重要意義。然而,豬胚胎由于其胞質脂肪含量高,對低溫敏感,增加了其保存難度。研究表明,通過降低脂質含量、優化培養基成分、保護細胞骨架以及恢復線粒體功能等方式,能夠提高豬胚胎冷凍保存技術的效率,從而促進其廣泛應用。因此,本文介紹了胚胎冷凍保存技術,總結了提高胚胎冷凍效率的多種方法和措施。同時,筆者團隊將通過討論豬胚胎的內在特性以及低溫保存對轉錄組改變的影響,進一步關注豬胚胎低溫保存的機制,從而更好地了解和推進豬胚胎低溫保存的研究。
關鍵詞: 豬;胚胎;冷凍保存;玻璃化冷凍
中圖分類號:S828.3
文獻標志碼:A
文章編號:0366-6964(2024)11-4796-12
收稿日期:2024-04-15
基金項目:重慶地方豬胚胎冷凍保存技術研究與豬育種新材料創制(2022-CHQ-01-01);國家家養動物種質資源庫;中國農業科學院科技創新工程(ASTIP-IAS06)
作者簡介:董建華(2000-),男,吉林長春人,碩士生,主要從事動物繁殖研究,E-mail: 15143173829@163.com
*通信作者:趙學明,主要從事家畜胚胎生物技術研究,E-mail:zhaoxueming@caas.cn
Advances in Cryopreservation of Porcine Embryo
DONG" Jianhua1,3, FENG" Xiaoyi1, YANG" Baigao1, LI" Chongyang1, PAN" Hongmei2, L Lihua3,
ZHAO" Xueming1*
(1.Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193," China;
2.Chongqing Academy of Animal Science, Rongchang 402460;
3.College of Animal Science, Shanxi Agricultural University, Taigu 030801," China)
Abstract:" Cryopreservation of embryos is important for long-distance embryo transfer and conservation of genetic resources. However, porcine embryos are sensitive to low temperatures due to their high cytoplasmic lipid content, which increases the difficulty of their preservation. Studies have indicated that techniques such as reducing lipid content and optimizing medium composition can significantly enhance the efficiency of porcine embryo cryopreservation. Additionally, protecting the cytoskeleton and restoring mitochondrial function are also crucial strategies that contribute to this advancement. These improvements collectively foster the broader application and sustainable development of this critical technology.
Therefore, this article introduces embryo cryopreservation and summaries multiple methods and measures to improve embryo freezing efficiency.
Meanwhile, the writers’team will focus more on the mechanism of cryopreservation of porcine embryos by discussing the intrinsic properties of embryos and the effects of cryopreservation on transcriptome alteration to help people better understand and advance research on porcine embryo cryopreservation.
Key words: porcine; embryo; cryopreservation; vitrification
*Corresponding author: ZHAO Xueming, E-mail: zhaoxueming@caas.cn
胚胎低溫保存的意義在于胚胎冷凍保存技術可以提高雌性動物的繁殖能力,促進種質的跨地區交流,減少疾病引起的減產損失,構建種質資源庫,保護瀕危動物遺傳資源,提高動物福利,降低環境影響和運輸成本[1]。胚胎在冷卻后比卵母細胞更有可能完全脫水,在胚胎低溫保存過程中,雖然有些細胞受損或死亡,但剩余的細胞足以使胚胎正常發育[2]。此外,胚胎的膜脂組成比卵母細胞含有更多的多不飽和脂肪酸(polyunsaturated fatty acids),比卵母細胞更容易冷凍保存[2]。自20世紀70年代成功保存哺乳動物卵母細胞和胚胎以來,該項技術得到了廣泛應用[3-4]。
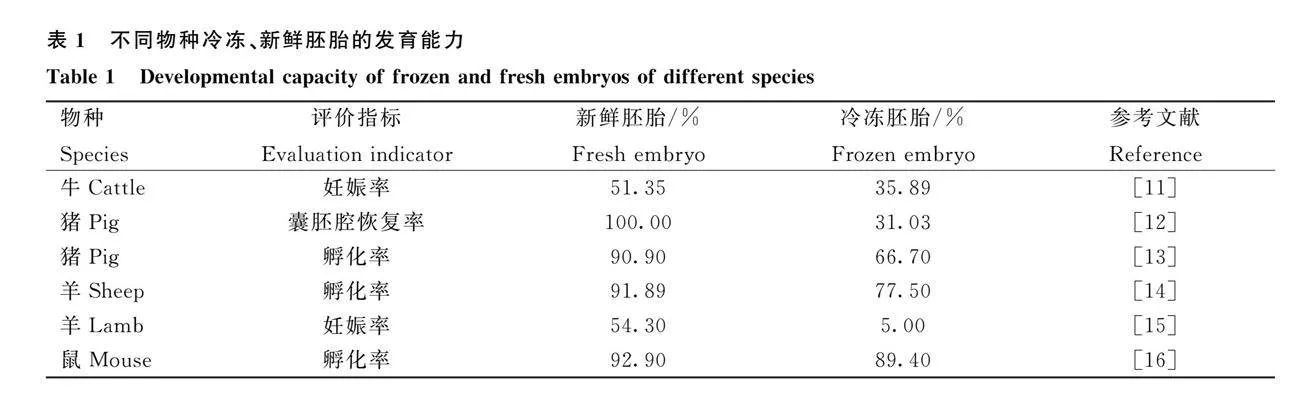
雖然胚胎冷凍保存技術已得到進一步的發展,但胚胎經冷凍后的存活率和后續發育潛能顯著低于新鮮胚胎[5]。相關研究表明,大家畜如牛、豬、羊等的冷凍后胚胎的發育能力明顯下降,如表1所示,造成了一定的經濟損失。豬胚胎的細胞質的脂質含量顯著高于綿羊[7]、牛[8]和小鼠胚胎[9],研究表明,胚胎中過高的脂質含量在冷凍保存過程中可能會增加脂質過氧化以及脂肪毒性的風險,冷凍還會引發細胞器受損,包括過氧化物酶體以及線粒體的形態異常[10]。
關于豬胚胎低溫保存的相關研究很多,但缺乏橫向和系統的比較。本文綜述了不同玻璃化方法和胚胎冷凍保存遇到的問題及解決措施。此外,還總結了一些可以提高冷凍豬胚胎存活率的技術,如去脂方法和減少氧化應激。同時,將通過討論胚胎的發育周期和低溫保存對轉錄組改變的影響,進一步關注豬胚胎低溫保存的機制,以幫助人們更好地了解和推進豬胚胎低溫保存的研究,以期為優化豬胚胎冷凍保存體系提供一定的參考。
1 豬胚胎主要冷凍保存方法
目前,豬的胚胎冷凍保存主要采用兩種方法:慢速冷凍和玻璃化冷凍。慢速冷凍作為一項發展較早的技術,通過逐步降低溫度實現細胞內水分的有序結晶,減少對胚胎細胞的損傷,但存在冷凍過程中形成無序冰晶損傷細胞結構和冷卻、解凍過程耗時較長等缺點[17]。相較之下,玻璃化冷凍技術致力于提高冷卻速率,產生類似玻璃樣的凝固,通過高濃度冷凍保護劑和極快的冷卻速率避免了冰晶的形成,減少了細胞損傷,同時縮短了冷凍和解凍的時間,有效提高了胚胎的存活率和發育潛力[18]。近年來,玻璃化冷凍在豬胚胎保存中的應用更為廣泛[19]。
1.1 慢速冷凍
慢速冷凍是胚胎冷凍保存的方法之一,通過使用可編程的冷凍儀控制溫度,使胚胎在預設的程序下緩慢降溫,封存在液氮罐中進行胚胎冷凍保存[20]。慢速冷凍可采用低毒性的低溫抗凍保護劑,如乙二醇(ethylene glycol,EG)、甘油(glycerol,Gly)、二甲基亞砜(dimethylsulfoxide,DMSO)等[20]。
雖然慢速冷凍是一種常見的豬胚胎保存方法,但仍存在一些局限性和缺陷。如需要高精密的降溫儀器、冷凍過程耗時較長,以及冷凍后胚胎發育率低等問題[21]。此外,在慢速冷凍過程中,細胞外液結冰和過度脫水可能會出現細胞外滲透壓增高的溶液效應[22],冷凍過程會導致冰晶形成,引起多種損傷,包括脂質過氧化(脂質氧化損傷)、線粒體結構的異常變化(線粒體變性)、核膜和質膜的破損、透明帶的厚度減少和破裂、細胞器解體以及細胞骨架的受損等[23]。這些損傷會顯著影響胚胎的存活率和孵化率,降低其發育潛力[23]。相對于未經冷凍的胚胎,慢速冷凍的胚胎妊娠率出現明顯的下降,囊胚凍融后的胚胎平均存活率((31.0±10.2)%)低于未冷凍胚胎((96.8±2.3)%)[24],這表明冷凍過程中的損傷對胚胎的發育能力造成了顯著的負面影響[25]。為提高胚胎冷凍效率,需要進一步研究和開發更先進的冷凍保存技術[26]。
1.2 玻璃化冷凍
玻璃化冷凍是一種經濟、快速且高效的豬胚胎冷凍保存方法,能夠替代傳統的慢速冷凍技術[26]。相較于慢速冷凍,玻璃化冷凍不需要使用昂貴的冷凍設備,冷凍時間也大大縮短[26]。玻璃化冷凍的主要特點是冷卻/升溫速度快,乙二醇、甘油、二甲基亞砜(DMSO)和1,2-丙二醇(PrOH)等可滲透的低溫保護劑能夠降低玻璃化冷凍過程中冰晶的形成[27]。糖類等不滲透的低溫保護劑的使用更是能調節細胞內外的滲透壓,促使玻璃化的形成[28]。這些物質有助于避免胚胎受到更多的冷凍損傷,從而提升胚胎的存活能力[27]。在豬胚胎冷凍過程中,相較于慢速冷凍,玻璃化冷凍具有更高的囊胚率、孵化率以及存活率等[26],如表2所示。
玻璃化冷凍技術雖然因其高效性和減少冰晶損傷的優勢而被廣泛應用,但仍存在一些不足之處[32],包括較高的操作要求、特定設備和耗材的成本、可能的胚胎損傷風險、基因表達和表觀遺傳狀態的影響[33]、批量處理的限制[34]、標準化難度[35]、冷凍保護劑的潛在毒性,以及法律和倫理方面的考量[36]。此外,對于長期效果和對后代健康的影響還需進一步研究,且不同胚胎對冷凍條件的適應性也需要個性化優化[39]。盡管面臨這些挑戰,通過不斷的技術創新和改進,許多問題有望得到克服,使玻璃化冷凍技術更加完善。
2 豬胚胎冷凍存在的問題
影響豬胚胎冷凍的相關機制涉及脂質相變、氧化應激、線粒體損傷、蛋白質變性、溶質濃度不平衡以及解凍過程等多個方面[43]。在實際應用中,需要綜合考慮這些因素,優化冷凍方案,提高豬胚胎冷凍的成功率。
2.1 脂質相變
在冷凍保存過程中,豬胚胎受損的主要原因有生物膜脂質相變(lipid phase transition,LPT)[43]、脂肪毒性以及細胞器損傷等[44]。這些因素會導致內質網應激[45]、蛋白質分泌減少[46]、線粒體活性降低[47]、細胞凋亡增加[48],最終影響囊胚發育能力。此外,冷凍還會引起脂滴及其相連細胞器的損傷,影響脂質代謝和細胞功能[49]。因此,在冷凍前降低脂滴含量可能有助于提高胚胎的發育能力。
2.2 氧化應激
高水平的活性氧(reactive oxygen species,ROS)會導致胚胎的發育潛力下降,從而降低胚胎的冷凍效率[50]。體外胚胎冷凍保存會對胚胎的線粒體造成損傷,包括破壞質膜完整性[51]、粗面內質網擴張[52]、滋養層細胞微絨毛數量減少和線粒體結構和功能的改變等[53],這些損傷可能導致體外胚胎解凍后存活率降低。冷凍保存過程中對線粒體的損傷可能包括線粒體腫脹、線粒體質膜功能受損[54]、基質電子密度低和線粒體嵴改變等[55],這些損傷會影響線粒體的功能和DNA完整性。因此,解凍后的體外胚胎可能出現線粒體數量減少、ATP含量顯著降低等現象,最終導致存活率降低[56]。
2.3 線粒體損傷
線粒體的質量是影響胚胎發育的重要因素[57]。胚胎中的線粒體參與維持多個生物化學過程,涉及降解、生物合成、融合和分裂等[58]。其主要功能是通過β-氧化途徑合成三磷酸腺苷(adenosine triphosphate,ATP)[59]。在細胞周期的關鍵時期,線粒體向高能量消耗區域的運動對于胚胎發育至關重要[60]。因此,線粒體在細胞質的分布模式與胚胎的質量和發育能力有關[61]。
冷凍會引起線粒體質膜完整性下降、粗面內質網擴張和滋養層細胞微絨毛減少等現象[62]。在冷凍胚胎擴張囊胚階段,線粒體的形態從具有最小脊的球形轉變為具有大量橫向脊的拉長,表明冷凍導致著床前胚胎發育異常[63]。線粒體膜電位(mitochondrial membrane potential,MMP)通常用來衡量植入前胚胎的線粒體功能和發育進程,有相關研究表明,冷凍顯著降低了胚胎線粒體膜電位水平[64]。冷凍過程會影響線粒體功能和遺傳物質完整性,導致解凍后胚胎線粒體數量下降、ATP含量明顯降低,顯著降低了胚胎存活率[65]。此外,冷凍還降低了胚胎對營養物質的吸收和利用,抑制了線粒體內膜解偶聯蛋白活性,從而降低了胚胎發育潛能[65]。
2.4 透明帶損傷
胚胎透明帶(zona pellucida,ZP)高度敏感于酶類物質,而透明帶損傷是導致胚胎在低溫條件下容易受損的一個重要因素[66]。冷凍保存過程中會導致透明帶硬化,通透性增加,降低解凍胚胎的發育能力[67]。同時,透明帶的病理調控功能會影響胚胎的發育能力,這對于預測存活率、解凍后的胚胎狀態以及提高冷凍胚胎的質量具有重要意義[66]。盡管透明帶在對抗病原體方面發揮積極的屏障功能,但當胚胎的營養來源來自外部環境時,透明帶會對胚胎吸收營養物質造成不利影響[68]。
2.5 基因表達異常
冷凍保存過程中高滲性冷凍保護劑和低溫會影響胚胎的基因表達[69],對玻璃化過程中基因表達變化的研究有助于人們更好地了解冷凍保存對胚胎的影響[70]。研究表明,玻璃化導致囊胚發育能力相關基因POU5F1表達下調,HSPA1A基因表達上調[71]。2020年的一項研究表明,玻璃化囊胚中細胞凋亡率增加,caspase、caspase-3、caspase-8和caspase-9活性也增加,但BCL-2和SOD-1 mRNA的相對豐度降低[72-73]。這些研究表明,冷凍保存會影響胚胎多能性、應激水平、過氧化物水平、凋亡水平和表觀遺傳修飾有關的基因表達[74]。
3 改善胚胎冷凍效率的研究
盡管相對于常規冷凍,玻璃化冷凍胚胎存活率較高,但解凍胚胎移植后的存活率依舊較低[75]。因此,研究并提出提高胚胎冷凍效率的措施,旨在提高解凍速率、增強胚胎質量和適應性,以及減少冰晶和保護劑傷害,從而達到增加可移植胚胎量的目的[76]。
3.1 去脂
減少細胞內脂質可提高胚胎冷凍存活率,以促進胚胎正常發育[77]。目前,顯微操作去脂和化學去脂等方法已被成功應用,并取得了良好的效果[78]。
3.1.1 顯微操作去脂
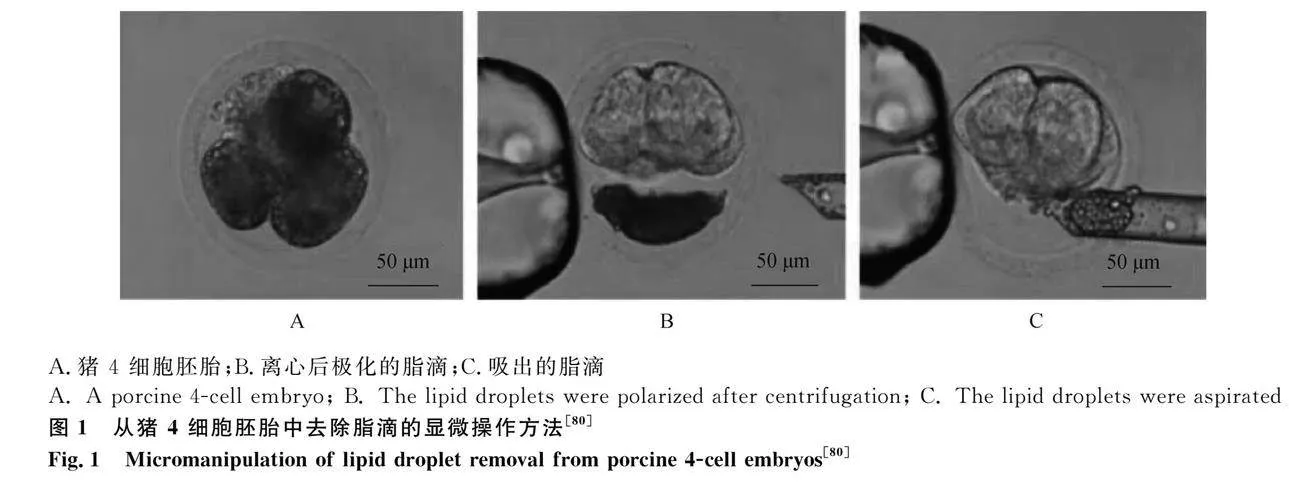
有研究顯示,采用離心后操作進行侵入式脫脂和使用4%胰蛋白酶結合離心進行非侵入式脫脂,均能顯著提高玻璃化胚胎的存活率[79]。圖1展示了Hara等[80]從胚胎細胞中通過高速離心使脂滴極化,再利用顯微打孔技術將脂滴吸出實現去脂的操作,在去脂后4細胞期胚胎凍后囊胚發育率提高了21.9%; 豬桑葚胚冷凍后存活率提高了42.1%并成功產出健康仔豬。根據已有研究,脫脂技術在囊胚階段的應用能夠有效地提高胚胎對低溫條件的耐受性[69]。這意味著通過去除脂質組分,囊胚可以更好地抵抗低溫引起的損傷,從而提高其在冷凍保存等過程中的存活率和發育成功率。
然而,進行顯微操作后,可能對胚胎的透明帶造成損傷,這可能會增加病原體傳播的風險[81]。因此,在進行顯微操作時,需要謹慎操作以減少透明帶受損,來提高胚胎的發育能力。
3.1.2 化學去脂
通過優化培養基來改變胚胎的脂質成分或降低胚胎的脂質含量,對胚胎造成的破壞較小[82]。有關胚胎去脂的研究表明,胚胎去脂后有利于其發育[83]。用胰蛋白酶使透明帶膨脹,以及通過高滲透壓濃縮胚胎體積,這兩種方法可打破透明帶周圍空間的橋狀結構,防止脂滴重新分布到胚胎中[84]。此外,Tatsumi等[85]開發了一種選擇性自噬系統,誘導噬脂,從而減少了脂滴(lipid droplet,LD)的大小和數量。
研究表明,在培養基中添加左旋肉堿(L-carnitine)[86]、福斯可林(forskolin)、黃連素(berberine)等化學物質[87],能夠有效減少脂質含量,促進細胞脂質代謝,增強胚胎對低溫的耐受性,并提升冷凍后的存活率[88]。在冷凍前24 h內用福斯可林處理2-4細胞胚胎能夠明顯提高其冷凍耐受性[89]。在培養過程中加入β腎上腺素受體激動劑異丙腎上腺素(ISO)可以提高cAMP水平,從而提高豬卵母細胞的成熟率[90]。左旋肉堿是一種脂質代謝增強劑,可影響卵母細胞中的β-氧化和t(ATP)水平[91]。研究表明,在受精前將卵母細胞在3 mmol·L-1左旋肉堿中培養1 h,可提高卵裂率,并提高囊胚的低溫耐受性[90-91]。在不干擾胚胎發育的前提下,左旋肉堿表現出脂質調節的活性,能夠促進脂質代謝,從而降低胚胎內的脂質含量[26]。
雖然這些去脂物質能夠有效減少胚胎脂質含量,但受到冷凍方法、藥物濃度、處理時間等因素的影響,這些去脂物質對胚胎低溫耐受性的改善效果差異較大[92],需要考慮冷凍方法、藥物濃度等因素[93]。這些去脂物質雖可提高冷凍后胚胎的存活率和囊胚再擴張率,但少有研究追蹤其后續發育,這些物質對胚胎后續發育是否具有持續的積極作用還有待深入研究。
3.2 添加抗氧化劑
培養系統會影響胚胎的發育效率,特別是含有血清的培養基會導致細胞中脂質成分的沉積,從而降低胚胎的耐寒性[94]。因此,為玻璃化過程開發一種有效的化學成分明確培養基(chemical defined medium)非常重要[95]。2016年,Cuello等[95]將泰羅德乳酸鹽-HEPES-聚乙烯醇培養基(TL-PVA)作為基本培養基用于玻璃化和升溫程序,獲得了較高的胚胎存活率(gt;90%)。因此,優化培養基可提高胚胎的冷凍保存耐受性[96]。
L-抗壞血酸(AC)是一種非酶抗氧化劑,可保護細胞免受有害氧化產物的傷害[97]。Castillo-Martín等[98]在培養基和玻璃化升溫培養基中添加了抗壞血酸,增加了SOD1和GPX1的基因表達,降低了HSPA1A的表達和過氧化物水平,從而提高了豬玻璃化囊胚的存活率。此外,用10 μmol·L-1褪黑素進行玻璃化解凍胚胎,表明可減少線粒體產熱和ROS水平,提高ATP水平,降低非整倍體率[99]。小鼠卵母細胞在IVM前3h用米立農(milrinone)孵育,可恢復ROS水平和線粒體膜電位,并恢復囊胚腔形成率[100]。玻璃化可誘導線粒體分布異常,而米力農作為一種cAMP降解抑制劑,可穩定線粒體功能活性和氧化應激,從而減少線粒體的冷凍損傷[101]。
培養基的基質和溫度也很重要[102]。相關研究發現,適當的溫度是獲得高存活率的關鍵因素,在升溫過程中分別將熱板溫度調至38.5℃與42℃,前者玻璃化卵母細胞的存活率比后者提高20%[103]。另一項研究表明,38℃時培養基產生的低溫保護劑毒性低于22℃時的毒性,提高了升溫后的存活率[104]。
3.3 增強線粒體功能
保持線粒體功能完整性是一種維持豬冷凍保存胚胎細胞發育能力的有效方法[105]。據報道,在玻璃化前用線粒體膜孔抑制劑環孢菌素A(cyclosporin A,CsA)處理卵母細胞可有效保護成熟豬卵母細胞的發育能力[106]。在較早的一篇報道中,將環孢菌素A在玻璃化豬卵母細胞中應用,有效提高了胚胎的存活率[96]。
冷凍保存不僅會損害線粒體,還會損害細胞骨架結構,例如微管[107],這些細胞骨架對豬胚胎細胞中線粒體的固定和分布很重要[108]。豬玻璃化胚胎細胞內線粒體分布異常,可以使用微管穩定劑紫杉醇(paclitaxel)對胚胎細胞進行預處理來緩解[108]。聯合使用環孢菌素A與紫杉醇可以提高玻璃化冷凍后卵母細胞的存活率、成熟率和囊胚率[107]。此外,1 μg·mL-1環孢菌素A提高胚胎囊胚期的發育效率較好,而4 μg·mL 對胚胎發育有害(對照組、1 μg·mL和4 μg·mL處理的囊胚率為 52.0%、61.0%和23.0%)[107]。
3.4 人工強制塌陷囊胚腔
降低胚胎囊胚腔內液體含量可以通過多種方法實現,包括微量移液[70]、微針穿刺[71]以及激光脈沖等技術[71]。2004年,采用最小體積冷卻(MVC)法對處于囊胚膨大期的生產(IVP)胚胎進行玻璃化處理,胚胎存活率為41.2%[27]。使用超細開放式細管法(SOPS)和Vit-Master對體外培養的囊胚進行玻璃化,冷卻速率為80,000℃·min-1,存活率為75%[109]。此外,通過孤雌激活(PA)和手工克隆從脫脂卵母細胞中獲得的豬囊胚也可通過Cryotop方法進行有效玻璃化[109]。Fujino等[110]使用金屬網玻璃化(metal mesh vitrification, MMV)方法對豬囊胚進行玻璃化,存活率為84.4%,可見使用金屬網的玻璃化方法比塑料板玻璃化方法的冷卻速度更快,塑料板玻璃化方法的存活率只有53.1%[110]。此外,使用pullulan膜對豬囊胚進行玻璃化,其耗氧量和細胞存活率與使用MVC玻璃化方法的胚胎相似[111]。pullulan膜可溶于溫水,因此玻璃化溶液可在吸管中稀釋,然后將胚胎直接移植到受體中[112],該方法能夠提高胚胎解凍效率。在玻璃化前塌陷囊胚腔,以避免囊胚腔內冰的形成,并提高囊胚期胚胎解凍后的存活率[113]。在豬胚胎中人工誘導囊腔塌陷可提高Cryotop玻璃化后囊胚的再膨脹率。
4 小 結
盡管對于豬胚胎冷凍保存的研究在不斷深入,但為了優化和標準化豬胚胎冷凍保存的體系,未來仍需進行更多凍存方案的探索。未來研究中,深入探討胚胎在冷凍及復蘇過程中的代謝調控機制和細胞損傷的分子機制將是關鍵方向。同時,目前使用的經典冷凍保護劑自20世紀50年代被發現以來未有大的突破。因此,開發新型的抗凍有機物質,例如新型納米材料,抗凍蛋白等,以減少對胚胎的毒理作用并增強膜的通透性,將成為未來研究的一個有前景的領域。
玻璃化冷凍技術因其在提高胚胎存活率和發育率方面的潛力,在胚胎冷凍保存中扮演著至關重要的角色。隨著技術的進步,通過優化玻璃化方法來進一步提升胚胎的保存效果將具有深遠的意義。預計隨著冷凍保存技術的持續發展,它將在生物遺傳育種、種質資源保護等更廣泛的領域中受到更多的關注,并發揮更加重要的作用。
參考文獻(References):
[1] LPEZ A,DUCOLOMB Y,CASAS E,et al.Effects of porcine immature oocyte vitrification on actin microfilament distribution and chromatin integrity during early embryo development in vitro[J].Front Cell Dev Biol,2021,9:636765.
[2] MARTINEZ E A,CUELLO C,PARRILLA I,et al.Design,development,and application of a non-surgical deep uterine embryo transfer technique in pigs[J].Anim Front,2013,3(4):40-47.
[3] BLOCKEEL C,CAMPBELL A,COTICCHIO G,et al.Should we still perform fresh embryo transfers in ART?[J].Hum Reprod, 2019, 34(12):2319-2329.
[4] CHEN Z J,SHI Y H,SUN Y,et al.Fresh versus frozen embryos for infertility in the polycystic ovary syndrome[J].N Engl J Med,2016,375(6):523-533.
[5] 高 峰,何琪富,吳盛輝,等.哺乳動物配子冷凍保存并應用于珍稀瀕危動物保護的技術策略[J].畜牧獸醫學報,2022, 53(8): 2479-2489.
GAO F,HE Q F,WU S H,et al.Mammalian gametes cryopreserved and applied to technical strategies for the protection of rare and endangered animals[J].Acta Veterinaria et Zootechnica Sinica,2022,53(8):2479-2489.(in Chinese)
[6] ZENG X Z,LI S Y,LIU L,et al.Role of functional fatty acids in modulation of reproductive potential in livestock[J].J Anim Sci Biotechnol,2023,14(1):24.
VINING L M,ZAK L J,HARVEY S C, et al.The role of apoptosis in cryopreserved animal oocytes and embryos[J]. Theriogenology,2021,173:93-101.
[7] ZHUAN Q, LI J, DU X, et al. Antioxidant procyanidin B2 protects oocytes against cryoinjuries via mitochondria regulated cortical tension. J Anim Sci Biotechnol. 2022;13(1):95.
[8] KERE M,LIU P C,CHEN Y K,et al.Ultrastructural characterization of porcine growing and in vitro matured oocytes[J].Animals (Basel),2020,10(4):664.
[9] CHEN J S,TSAI L K,YEH T Y,et al.Effects of electromagnetic waves on oocyte maturation and embryonic development in pigs[J].J Reprod Dev,2021,67(6):392-401.
[10] CAO L H,YANG T,HUANG S H,et al.Expression patterns of ZO-1/2 and their effects on porcine oocyte in vitro maturation and early embryonic development[J].Theriogenology,2021,161:262-270.
[11] ABEDPOUR N,SHOOREI H,RAJAEI F.Detrimental effects of vitrification on integrin genes (α9 and β1) and in vitro fertilization in mouse oocytes[J].Mol Biol Rep,2023,50(6):4823-4829.
[12] GONZALEZ-PLAZA A,CAMBRA J M,PARRILLA I,et al.The open Cryotop system is effective for the simultaneous vitrification of a large number of porcine embryos at different developmental stages[J].Front Vet Sci,2022,9:936753.
[13] TAJIMA S,UCHIKURA K,KURITA T,et al.Insemination of recipient sows improves the survival to term of vitrified and warmed porcine expanded blastocysts transferred non-surgically[J].Anim Sci J,2020,91(1):e13453.
[14] QIU J,MATSUKAWA K,KOSHIMOTO C,et al.Equilibrium vitrification of mouse embryos at various developmental stages using low concentrations of cryoprotectants[J].J Reprod Dev,2021,67(2):109-114.
[15] MARTNEZ-RODERO I,GARCIA-MARTNEZ T,ORDEZ-LEN E A,et al.A shorter equilibration period improves post-warming outcomes after vitrification and in straw dilution of in vitro-produced bovine embryos[J].Biology (Basel),2021,10(2): 142.
[16] SKRZYPEK K,NIBBELINK M C,LIEFERS-VISSER J,et al.A high cell-bearing capacity multibore hollow fiber device for macroencapsulation of islets of Langerhans[J].Macromol Biosci,2020,20(8):2000021.
[17] ALMIANA C,DUBUISSON F,BAUERSACHS S,et al.Unveiling how vitrification affects the porcine blastocyst:clues from a transcriptomic study[J].J Anim Sci Biotechnol,2022,13(1):46.
[18] ALMUBARAK A,LEE S,YU I J,et al.Effects of Nobiletin supplementation on the freezing diluent on porcine sperm cryo-survival and subsequent in vitro embryo development[J].Theriogenology,2023,214:314-322.
[19] ABDELHADY A W,MITTAN-MOREAU D W,CRANE P L,et al.Ice formation and its elimination in cryopreservation of oocytes[J].Sci Rep,2024,14:18809.
[20] CUELLO C, GONZLEZ-PLAZA A, CAMBRA J M, et al. Vitrification of pig embryos dysregulates the microRNA transcriptome profile[J]. Theriogenology. 2024;226:243-252.
[21] NAKAGAWA Y,KANEKO T.Improvement of survivability and developmental ability in vitrified rat oocytes[J].Cryobiology, 2024,115:104882.
[22] DI GUARDO F,RACCA A,COTICCHIO G,et al.Impact of cell loss after warming of human vitrified day 3 embryos on obstetric outcome in single frozen embryo transfers[J].J Assist Reprod Genet,2022,39(9):2069-2075.
[23] HOCHI S.Cryodevices developed for minimum volume cooling vitrification of bovine oocytes[J].Anim Sci J,2022,93(1):e13683.
[24] MORADO S,APARICIO A,PINCHETTI D,et al.Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming[J].Open Vet J,2023,13(11):1416-1424.
[25] MARCANTONINI G,BARTOLINI D,ZATINI L,et al.Natural cryoprotective and cytoprotective agents in cryopreservation:a focus on melatonin[J].Molecules,2022,27(10):3254.
[26] GONZALEZ-PLAZA A,CAMBRA J M,GARCIA-CANOVAS M,et al.Cryotop vitrification of large batches of pig embryos simultaneously provides excellent postwarming survival rates and minimal interference with gene expression[J].Theriogenology, 2023,206:1-10.
[27] VAN NGUYEN T,DO L T K,NGUYEN N A T,et al.The effects of an in vitro oocyte maturation system and chlorogenic acid supplementation during embryo culture on the development of porcine cloned embryos derived from native vietnamese ban pigs[J].Vet Med Int,2023,2023(1):5702970.
[28] WIESAK T,GORYSZEWSKA-SZCZUREK E.Effect of vitrification on the expression of genes in porcine blastocysts derived from in vitro matured oocytes[J].Syst Biol Reprod Med,2022,68(4):239-246.
[29] RIENZI L,GRACIA C,MAGGIULLI R,et al.Oocyte,embryo and blastocyst cryopreservation in ART:systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance[J].Hum Reprod Update,2017,23(2):139-155.
[30] XIANG D C,JIA B Y,GUO J X,et al.Transcriptome analysis of mRNAs and long non-coding RNAs during subsequent embryo development of porcine cloned zygotes after vitrification[J].Front Genet,2021,12:753327.
[31] SOMFAI T,HARAGUCHI S,DANG-NGUYEN T Q,et al.Vitrification of porcine immature oocytes and zygotes results in different levels of DNA damage which reflects developmental competence to the blastocyst stage[J].PLoS One,2023,18(3):e0282959.
[32] GOMIS J,CUELLO C,SANCHEZ-OSORIO J,et al.Forskolin improves the cryosurvival of in vivo-derived porcine embryos at very early stages using two vitrification methods[J].Cryobiology,2013,66(2):144-150.
[33] XU H X,WANG X G,TAO R X,et al.Optimal stage for cryotop vitrification of porcine embryos[J].Cell Reprogram, 2022, 24(3):132-141.
[34] USHIJIMA H,YOSHIOKA H,ESAKI R,et al.Improved survival of vitrified in vivo-derived porcine embryos[J].J Reprod Dev,2004,50(4):481-486.
[35] HOCHI S, IDE M, UENO S, et al. High survival of bovine mature oocytes after nylon mesh vitrification, as assessed by intracytoplasmic sperm injection.[J]. J Reprod Dev. 2022;68(5):335-339.
[36] CUELLO C,MARTINEZ C A,CAMBRA J M,et al.Effects of vitrification on the blastocyst gene expression profile in a porcine model[J].Int J Mol Sci,2021,22(3):1222.
[37] TU C F,PENG S H,CHUANG C K,et al.Reproductive technologies needed for the generation of precise gene-edited pigs in the pathways from laboratory to farm[J].Anim Biosci,2023,36(2):339-349.
[38] LPEZ A,BETANCOURT M,DUCOLOMB Y,et al.DNA damage in cumulus cells generated after the vitrification of in vitro matured porcine oocytes and its impact on fertilization and embryo development[J].Porc Health Manag,2021,7(1):56.
[39] KAWAKAMI M,KATO Y,TSUNODA Y.The effects of time of first cleavage,developmental stage,and delipidation of nuclear-transferred porcine blastocysts on survival following vitrification[J].Anim Reprod Sci,2008,106(3-4):402-411.
[40] GAO M,CHEN M J,CHEN Q Z,et al.Integration of parallel metabolomics and transcriptomics reveals metabolic patterns in porcine oocytes during maturation[J].Front Endocrinol (Lausanne),2023,14:1131256.
[41] MARCO-JIMNEZ F, GARCIA-DOMINGUEZ X, GARCA-VALERO L,et al. A 3D-Printed Large Holding Capacity Device for Minimum Volume Cooling Vitrification of Embryos in Prolific Livestock Species[J]. Animals (Basel). 2023;13(5):791.
[42] HLKER M, PETERSEN B, HASSEL P, et al. Duration of in vitro maturation of recipient oocytes affects blastocyst development of cloned porcine embryos[J]. Cloning Stem Cells. 2005;7(1):35-44.
[43] TAJIMA S, MOTOYAMA S, WAKIYA Y, et al. Piglet production by non-surgical transfer of vitrified embryos, transported to commercial swine farms and warmed on site[J]. Anim Sci J. 2021 Dec;92(1):e13588.
[44] NGUYEN V K,VU H,NGUYEN H T T,et al.Comparison of the microdrop and minimum volume cooling methods for vitrification of porcine in vitro-produced zygotes and blastocysts after equilibration in low concentrations of cryoprotectant agents[J].J Reprod Dev,2018,64(5):457-462.
[45] NAJAFZADEH V,SECHER J B M,PIHL M,et al.Vitrification yields higher cryo-survival rate than slow freezing in biopsied bovine in vitro produced blastocysts[J].Theriogenology,2021,171:44-54.
[46] KAJDASZ A,WARZYCH E,DEREBECKA N,et al.Lipid stores and lipid metabolism associated gene expression in porcine and bovine parthenogenetic embryos revealed by fluorescent staining and RNA-seq[J].Int J Mol Sci,2020,21(18):6488.
[47] IBAYASHI M,AIZAWA R,MITSUI J,et al.Homeostatic regulation of lipid droplet content in mammalian oocytes and embryos[J].Reproduction,2021,162(6):R99-R109.
[48] DUNNING K R,RUSSELL D L,ROBKER R L.Lipids and oocyte developmental competence:the role of fatty acids and β-oxidation[J].Reproduction,2014,148(1):R15-R27.
[49] WANG C,NIU Y,CHI D,et al.Influence of delipation on the energy metabolism in pig parthenogenetically activated embryos[J]. Reprod Domest Anim,2015,50(5):826-833.
[50] RAZA S H A,ABD EL-AZIZ A H,ABDELNOUR S A,et al.The role of forskolin as a lipolytic stimulator during in vitro oocyte maturation and the in vitro embryo production of livestock[J].Reprod Domest Anim,2021,56(12):1486-1496.
[51] AMSTISLAVSKY S,MOKROUSOVA V,BRUSENTSEV E,et al.Influence of cellular lipids on cryopreservation of mammalian oocytes and preimplantation embryos:a review[J].Biopreserv Biobank,2019,17(1):76-83.
[52] CASTILLO-MARTN M,BONET S,MORAT R,et al.Supplementing culture and vitrification-warming media with L-ascorbic acid enhances survival rates and redox status of IVP porcine blastocysts via induction of GPX1 and SOD1 expression[J].Cryobiology, 2014,68(3):451-458.
[53] LI J, XIONG S, ZHAO Y, et al. Effect of the re-vitrification of embryos at different stages on embryonic developmental potential[J]. Front Endocrinol (Lausanne). 2021;12:653310.
[54] MENEZO Y,CLEMENT P,DALE B,et al.Modulating oxidative stress and epigenetic homeostasis in preimplantation IVF embryos[J].Zygote,2022,30(2):149-158.
[55] MENEZO Y,ELDER K,CLEMENT P,et al.Biochemical hazards during three phases of assisted reproductive technology:repercussions associated with epigenesis and imprinting[J].Int J Mol Sci,2022,23(16):8916.
[56] PAVANELI A P P,RECUERO S,CHAVES B R,et al.The presence of seminal plasma during liquid storage of pig spermatozoa at 17 ℃ modulates their ability to elicit in vitro capacitation and trigger acrosomal exocytosis[J].Int J Mol Sci,2020,21(12):4520.
[57] HARDY M L M,DAY M L,MORRIS M B.Redox regulation and oxidative stress in mammalian oocytes and embryos developed in vivo and in vitro[J].Int J Environ Res Public Health,2021,18(21):11374.
[58] SOTO-HERAS S,PARAMIO M T.Impact of oxidative stress on oocyte competence for in vitro embryo production programs[J].Res Vet Sci,2020,132:342-350.
[59] JIANG Y,HE Y T,PAN X C,et al.Advances in oocyte maturation in vivo and in vitro in mammals[J].Int J Mol Sci,2023,24(10):9059.
[60] NIU Y J,ZHOU W J,NIE Z W,et al.Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos[J].J Pineal Res,2020,68(2):e12627.
[61] SUN M H,JIANG W J,LI X H,et al.ATF6 aggravates apoptosis in early porcine embryonic development by regulating organelle homeostasis under high-temperature conditions[J].Zool Res,2023,44(5):848-859.
[62] KAGEYAMA M,ITO J,SHIRASUNA K,et al.Mitochondrial reactive oxygen species regulate mitochondrial biogenesis in porcine embryos[J].J Reprod Dev,2021,67(2):141-147.
[63] YANG S G,BAE J W,PARK H J,et al.Mito-TEMPO protects preimplantation porcine embryos against mitochondrial fission-driven apoptosis through DRP1/PINK1-mediated mitophagy[J].Life Sci,2023,315:121333.
[64] LIU R P,WANG J,WANG X Q,et al.Xanthoangelol promotes early embryonic development of porcine embryos by relieving endoplasmic reticulum stress and enhancing mitochondrial function[J].Reprod BioMed Online,2023,47(2):103211.
[65] ZHOU D J,SUN M H,LEE S H,et al.ROMO1 is required for mitochondrial metabolism during preimplantation embryo development in pigs[J].Cell Div,2021,16(1):7.
[66] WANG C R,JI H W,HE S Y,et al.Chrysoeriol improves in vitro porcine embryo development by reducing oxidative stress and autophagy[J].Vet Sci,2023,10(2):143.
[67] NIU Y J,WANG C F,XIONG Q,et al.Distribution and content of lipid droplets and mitochondria in pig parthenogenetically activated embryos after delipation[J].Theriogenology,2015,83(1):131-138.
[68] TOSTI E,MNZO Y.Gamete activation:basic knowledge and clinical applications[J].Hum Reprod Update,2016,22(4):420-439.
[69] WASSARMAN P M,LITSCHER E S.Zona pellucida genes and proteins:essential players in mammalian oogenesis and fertility[J].Genes (Basel),2021,12(8):1266.
[70] NGUYEN N T,HIRATA M,TANIHARA F,et al.In vitro development of zona pellucida-free porcine zygotes cultured individually after vitrification[J].Cryo Letters,2020,41(2):86-91.
[71] SCIORIO R,MANNA C,FAUQUE P,et al.Can cryopreservation in Assisted Reproductive Technology (ART) induce epigenetic changes to gametes and embryos?[J].J Clin Med,2023,12(13):4444.
[72] ESTUDILLO E, JIMNEZ A,BUSTAMANTE-NIEVES P E,et al.Cryopreservation of gametes and embryos and their molecular changes[J].Int J Mol Sci,2021,22(19):10864.
[73] TIRGAR P,SARMADI F,NAJAFI M,et al.Toward embryo cryopreservation-on-a-chip:a standalone microfluidic platform for gradual loading of cryoprotectants to minimize cryoinjuries[J].Biomicrofluidics,2021,15(3):034104.
[74] ZHANG L,QI X,NING W,et al.Single-cell transcriptome profiling revealed that vitrification of somatic cloned porcine blastocysts causes substantial perturbations in gene expression[J].Front Genet,2020,11:640.
[75] PERO M E,ZULLO G,ESPOSITO L,et al.Inhibition of apoptosis by caspase inhibitor Z-VAD-FMK improves cryotolerance of in vitro derived bovine embryos[J].Theriogenology,2018,108:127-135.
[76] DENG D M,XIE J,TIAN Y,et al.Effects of meiotic stage-specific oocyte vitrification on mouse oocyte quality and developmental competence[J].Front Endocrinol (Lausanne),2023,14:1200051.
[77] TAJIMA S,MOTOYAMA S,WAKIYA Y,et al.Piglet production by non-surgical transfer of vitrified embryos,transported to commercial swine farms and warmed on site[J].Anim Sci J,2020,91(1):e13476.
[78] LEE K, UH K, FARRELL K. Current progress of genome editing in livestock[J]. Theriogenology. 2020;150:229-235.
[79] CHEN P R,REDEL B K,KERNS K C,et al.Challenges and considerations during in vitro production of porcine embryos[J].Cells,2021,10(10):2770.
[80] KHAJEDEHI N,FATHI R,AKBARINEJAD V,et al.Oocyte vitrification reduces its capability to repair sperm DNA fragmentation and impairs embryonic development[J].Reprod Sci,2024,31(5):1256-1267.
HARA K, ABEE Y, KUMADA N, et al. Extrusion and removal of lipid from the cytoplasm of porcine oocytes at the germinal vesicle stage: centrifugation under hypertonic conditions influences vitrification[J]. Cryobiology, 2005,50(2):216-222.
[81] OECKL J,BAST-HABERSBRUNNER A,FROMME T,et al.Isolation,culture,and functional analysis of murine thermogenic adipocytes[J].STAR Protoc,2020,1(3):100118.
[82] TATSUMI T,TAKAYAMA K,ISHII S,et al.Forced lipophagy reveals that lipid droplets are required for early embryonic development in mouse[J].Development,2018,145(4):dev161893.
[83] TIBBO A J,MIKA D,DOBI S,et al.Phosphodiesterase type 4 anchoring regulates cAMP signaling to Popeye domain-containing proteins[J].J Mol Cell Cardiol,2022,165:86-102.
[84] ISA T,SOMFAI T,OYADOMARI M,et al.Production of Agu piglets after transfer of embryos produced in vitro[J].Anim Sci J,2022,93(1):e13685.
[85] NAGASHIMA H,HIRUMA K,SAITO H,et al.Production of live piglets following cryopreservation of embryos derived from in vitro-matured oocytes[J].Biol Reprod,2007,76(5):900-905.
[86] AIZAWA R,IBAYASHI M,TATSUMI T,et al.Synthesis and maintenance of lipid droplets are essential for mouse preimplantation embryonic development[J].Development,2019,146(22):dev181925.
[87] LI R,LIU Y,PEDERSEN H S,et al.Development and quality of porcine parthenogenetically activated embryos after removal of zona pellucida[J].Theriogenology,2013,80(1):58-64.
[85] TATSUMI T, TAKAYAMA K, ISHII S, et al. Forced lipophagy reveals that lipid droplets are required for early embryonicdevelopment in mouse[J]. Development,2018,145(4):dev161893.
[86] EL-SOKARY M M M,EL-NABY A A H, HAMEED A R A E,et al. Impact of L-carnitine supplementation on the in vitro developmental competence and cryotolerance of buffalo embryos[J]. Vet World, 2021,14(12):3164-3169.
[87] RAKHMANOVA T, MOKROUSOVA V, OKOTRUB S, et al. Effects of forskolin on cryopreservation and embryo development in the domestic cat[J]. Theriogenology, 2023,210:192-198.
[88] NAMULA Z,HIRATA M,LE Q A,et al.Zona pellucida treatment before CRISPR/Cas9-mediated genome editing of porcine zygotes[J].Vet Med Sci,2022,8(1):164-169.
[89] ROMEK M,GAJDA B,KRZYSZTOFOWICZ E,et al.New technique to quantify the lipid composition of lipid droplets in porcine oocytes and pre-implantation embryos using Nile Red fluorescent probe[J].Theriogenology,2011,75(1):42-54.
[90] CAMBRA J M, GIL M A, CUELLO C, et al. Cytokine profile in peripheral blood mononuclear cells differs between embryo donor and potential recipient sows[J]. Front Vet Sci. 2024;11:1333941.
[91] FU X W,WU G Q,LI J J,et al.Positive effects of Forskolin (stimulator of lipolysis) treatment on cryosurvival of in vitro matured porcine oocytes[J].Theriogenology,2011,75(2):268-275.
[92] YANG C X, LIANG H, WU Z W, et al. Identification of lncRNAs involved in maternal-to-zygotic transition of in vitro-produced porcine embryos by single-cell RNA-seq[J]. Reprod Domest Anim. 2022;57(1):111-122.
[93] ZHUAN Q,MA H J,CHEN J,et al.Cytoplasm lipids can be modulated through hormone-sensitive lipase and are related to mitochondrial function in porcine IVM oocytes[J].Reprod Fertil Dev,2020,32(7):667-675.
[94] LI J Y,XIONG S,ZHAO Y H,et al.Effect of the re-vitrification of embryos at different stages on embryonic developmental potential[J].Front Endocrinol (Lausanne),2021,12:653310.
[95] SHI X Y,JIN X H,LIN J Y,et al.Idebenone relieves the damage of heat stress on the maturation and developmental competence of porcine oocytes[J].Reprod Domest Anim,2022,57(4):418-428.
CUELLO C, MARTINEZ C A, NOHALEZ A, et al. Effective vitrification and warming of porcine embryos using a pH-stable,chemically defined medium[J]. Sci Rep, 2016,6:33915.
[96] SANCHEZ-OSORIO J,CUELLO C,GIL M A,et al.Vitrification and warming of in vivo-derived porcine embryos in a chemically defined medium[J].Theriogenology,2010,73(3):300-308.
[97] MARTINEZ C A,CUELLO C,PARRILLA I,et al.Exogenous melatonin in the culture medium does not affect the development of in vivo-derived pig embryos but substantially improves the quality of in vitro-produced embryos[J].Antioxidants (Basel),2022, 11(6):1177.
[98] CUELLO C,MARTINEZ C A,CAMBRA J M,et al.Vitrification effects on the transcriptome of in vivo-derived porcine morulae[J].Front Vet Sci,2021,8:771996.
[99] XIANG D C,JIA B Y,QUAN G B,et al.Effect of knockout serum replacement during postwarming recovery culture on the development and quality of vitrified parthenogenetic porcine blastocysts[J].Biopreserv Biobank,2019,17(4):342-351.
[99] CUELLO C,MARTINEZ C A,CAMBRA J M,et al.Vitrification effects on the transcriptome of in vivo-derived porcine morulae[J].Front Vet Sci,2021,8:771996.
[100] BUDANI M C,TIBONI G M.Effects of supplementation with natural antioxidants on oocytes and preimplantation embryos[J]. Antioxidants (Basel),2020,9(7):612.
[101] GAO L,DU M,ZHUAN Q,et al.Melatonin rescues the aneuploidy in mice vitrified oocytes by regulating mitochondrial heat product[J].Cryobiology,2019,89:68-75.
[102] YOON S Y,EUM J H,CHA S K,et al.Prematuration culture with phosphodiesterase inhibitors after vitrification may induce recovery of mitochondrial activity in vitrified mouse immature oocytes[J].Biopreserv Biobank,2018,16(4):296-303.
[103] PAN B,QAZI I H,GUO S C,et al.Melatonin improves the first cleavage of parthenogenetic embryos from vitrified-warmed mouse oocytes potentially by promoting cell cycle progression[J].J Anim Sci Biotechnol,2021,12(1):84.
[104] LIN Q Y,LE Q A,TAKEBAYASHI K,et al.Short-term preservation of porcine zygotes at ambient temperature using a chemically defined medium[J].Anim Sci J,2022,93(1):e13711.
[105] KIKUCHI K,KASHIWAZAKI N,NAGAI T,et al.Selected aspects of advanced porcine reproductive technology[J].Reprod Domest Anim,2008,43(S2):401-406.
[106] WHALEY D,DAMYAR K,WITEK R P,et al.Cryopreservation:an overview of principles and cell-specific considerations[J].Cell Transplant,2021,30:963689721999617.
[107] CZERNIK M,WINIARCZYK D,SAMPINO S,et al.Author correction:mitochondrial function and intracellular distribution is severely affected in in vitro cultured mouse embryos[J].Sci Rep,2022,12(1):21276.
[108] NGUYEN H T,NGUYEN N T,NGUYEN L V,et al.The effects of pretreatment with Cyclosporin A and Docetaxel before vitrification of porcine immature oocytes on subsequent embryo development[J].Reprod Biol,2023,23(4):100798.
[109] DUARTE F V,CIAMPI D,DUARTE C B.Mitochondria as central hubs in synaptic modulation[J].Cell Mol Life Sci,2023,80(6):173.
[110] FUJINO Y, KOJIMA T, NAKAMURA Y, et al. Metal mesh vitrification (MMV) method for cryopreservation of porcine embryos[J]. Theriogenology, 2008,70(5):809-817.
[110] ZHAN L,HAN Z H,SHAO Q,et al.Rapid joule heating improves vitrification based cryopreservation[J].Nat Commun,2022, 13(1):6017.
[111] PANG Y W, AN L, WANG P, et al. Treatment of porcine donor cells and reconstructed embryos with the antioxidant melatonin enhances cloning efficiency[J]. J Pineal Res. 2013;54(4):389-397.
[112] JOE S Y,YANG S G,LEE J H,et al.Stabilization of F-actin cytoskeleton by paclitaxel improves the blastocyst developmental competence through P38 MAPK activity in porcine embryos[J].Biomedicines,2022,10(8):1867.
[113] PARRILLA I,GIL M A,CUELLO C,et al.Immunological uterine response to pig embryos before and during implantation[J]. Reprod Domest Anim,2022,57(S5):4-13.
(編輯 郭云雁)

