多殺性巴氏桿菌的攝鐵機制研究進展
摘 要: 多殺性巴氏桿菌可廣泛感染多種動物,引起出血性敗血癥或傳染性肺炎。鐵是多殺性巴氏桿菌感染宿主過程中生長、定植和增殖必不可少的營養物質,競爭宿主鐵離子是該病原體感染致病的關鍵環節。近年來,多殺性巴氏桿菌的攝鐵系統及其發生與調控機制方面的研究取得了一系列重要進展。本文系統闡述了多殺性巴氏桿菌的轉鐵蛋白受體攝鐵機制、血紅素受體攝鐵機制、鐵載體攝鐵機制及其攝鐵系統的表達與調控機制,以期為多殺性巴氏桿菌攝鐵系統的分子致病機理研究提供系統的理論知識,為多殺性巴氏桿菌分子靶標藥物及亞單位疫苗的研發提供新思路。
關鍵詞: 多殺性巴氏桿菌;攝鐵機制;鐵載體;ExbB-ExbD-TonB;Fur
中圖分類號:Q935; S852.612
文獻標志碼:A
文章編號:0366-6964(2024)11-4852-11
收稿日期:2023-12-04
基金項目:國家自然科學基金項目(31672530;U1704117)
作者簡介:沈香香(1999-),女,河南柘城人,碩士生,主要從事家畜傳染病及其疫苗研究,E-mail:2939116494@qq.com
*通信作者:趙戰勤,主要從事家畜傳染病及其疫苗研究,E-mail:zhaozhanqin@126.com
Research Progress on Iron Uptake Mechanism of Pasteurella multocida
SHEN" Xiangxiang1, GUAN" Lijun1,2, ZHANG" Junfeng1, XUE" Yun1, SI" Lifang1,2, ZHAO" Zhanqin1,2*
(1.Key Lab of Animal Bacterial Infectious Disease Prevention and Control Technology,
College of Animal Science and Technology, Henan University of Science and Technology,
Luoyang 471003," China;
2.Key-Disciplines Lab of Safety of Environment and Animal Product, College of Animal Science and Technology, Henan University of
Science and Technology, Luoyang 471003," China)
Abstract:" Pasteurella multocida (P. multocida) can infect a wide range of animals, causing hemorrhagic septicemia or infectious pneumonia. Iron is an essential nutrient for growth, colonization, and proliferation of P. multocida during infection of the host, and competition for iron ions in the host is a critical link in the pathogenesis of this pathogen. In recent years, a series of significant research advances have progressed in the iron uptake system of P. multocida, as well as its occurrence and regulatory mechanisms.
The mechanisms of iron uptake by transferrin, heme receptors and siderophore, and the mechanism of expression and regulation of the P. multocida iron uptake system are all described in this paper.
Aiming to provide systematic theoretical knowledge for the study of the molecular pathogenesis of the P. multocida iron uptake system and to spark new ideas for the investigation and development of molecular target drugs and subunit vaccines of P. multocida.
Key words: Pasteurella multocida; iron uptake mechanism; siderophore; ExbB-ExbD-TonB; Fur
*Corresponding author: ZHAO Zhanqin,E-mail:zhaozhanqin@126.com
多殺性巴氏桿菌(Pasteurella multocida, Pm)是一種短桿狀的革蘭陰性條件致病菌[1]。根據莢膜抗原(K抗原)可將多殺性巴氏桿菌分為5種血清型(A、B、D、E和F)[2],根據菌體抗原(O抗原)可分為16種血清型(1~16)[3]。該菌廣泛感染多種動物(牛、羊、禽、豬、兔、犬、貓和人),其引起的巴氏桿菌病(Pasteurellosis)主要表現為傳染性肺炎和出血性敗血癥[4],給養殖業造成重大經濟損失。鐵離子是多殺性巴氏桿菌感染宿主過程中定植和增殖的必需元素因子[5],多殺性巴氏桿菌的攝鐵機制是研究其與宿主發生感染與免疫機制的重要內容,受到了研究學者的廣泛關注[6-7]。鐵參與DNA和蛋白質的生物合成、生物膜形成、氧化還原及電子轉移等過程,是微生物和動物生長代謝必不可少的營養物質[8-9]。在多殺性巴氏桿菌與宿主相互作用中,鐵是促進細菌生長、繁殖、黏附以及毒力因子表達[10-12]的重要因子,該菌獲取鐵的能力是影響其致病性的重要因素[13]。但由于宿主的營養免疫機制,多殺性巴氏桿菌在感染宿主過程中常面臨游離鐵匱乏的狀況[14-15],為此,該菌進化出多種攝鐵機制從宿主的轉鐵蛋白(transferrin, Tf)和血紅蛋白(haemoglobin, Hb)中奪取鐵離子。本文對多殺性巴氏桿菌三種獲取鐵離子的途徑、攝鐵相關蛋白功能和調控因子的研究進展進行綜述。系統掌握多殺性巴氏桿菌的攝鐵機制,將有助于從干擾細菌攝鐵這一視角為研發新型抗菌藥物及生物制品提供新思路。
1 鐵在細菌中的生物學功能
鐵是地球上含量最豐富的金屬元素之一,通常以氧化態(Fe3+)或還原態(Fe2+)形式存在,鐵離子的這種氧化還原狀態使其在生物系統中被廣泛利用。鐵離子通常與蛋白質結合或作為鐵硫簇或血紅素基團的一部分,參與微生物中多種重要的生物過程,包括有氧呼吸、ATP合成、電子轉移、DNA和蛋白質合成等過程[16]。因此,鐵是細菌實現自身代謝與功能必不可少的因子。
1.1 鐵硫簇在細菌中的功能
鐵硫簇是普遍存在于生物體內的最古老的生命物質之一,是具有重要調節功能或催化作用的蛋白質金屬簇。以多殺性巴氏桿菌為模式微生物進行鐵硫簇功能的研究較少,但是在其它細菌中已有一些了解。細菌中常見的鐵硫簇結構有[4Fe-4S]、[3Fe-4S]和[2Fe-2S],這些結構之間可以進行置換,從而發生電子轉移[17]。鐵硫簇能夠穩定細菌中蛋白質的特定功能,如[4Fe-4S]簇可使大腸桿菌核酸內切酶Ⅲ具有更穩定的DNA結合位點[18]。鐵硫簇還能夠保護蛋白質不被蛋白酶分解,如[4Fe-4S]簇可使枯草芽孢桿菌中氨基轉移酶具有穩定的結構和活性,鐵硫簇被破壞則會導致氨基轉移酶被降解[19]。鐵硫簇參與基因表達和調控,如根瘤菌鐵調節因子A(rhizobial iron regulator A, RirA)必須具備完整的[3Fe-4S]簇才能夠調節DNA的轉錄[20]。此外,大腸桿菌中的SoxR(superoxide response)蛋白作為一種信號傳遞因子和轉錄激活因子,其發揮功能和維持活性有賴于[2Fe-2S]簇[21]。
1.2 血紅素在細菌中的功能
血紅素(heme)是一類含有亞鐵離子的卟啉類化合物,存在于動物、植物和微生物中[22],充足的血紅素能夠促進多殺性巴氏桿菌的生長[23]。血紅素作為輔因子參與細菌氧化酶、過氧化物酶等酶類的生物合成,血紅素含量的高低決定了酶的活性[24-25]。氧化酶參與細菌有氧呼吸,過氧化物酶基因參與細菌代謝、生物膜形成和菌體運動,它們在細菌定植、感染宿主過程中發揮著重要作用[25-26]。此外,5-羥色氨酸是細菌中許多生物活性物質的化學前體,而血紅素能夠利用鐵離子的氧化還原能力,協同O2或H2O2使色氨酸的吲哚C5位置羥化形成5-羥色氨酸[27]。由此可見,鐵在細菌中發揮著十分重要的作用。
1.3 缺鐵對多殺性巴氏桿菌的影響
細菌生長所需的最佳鐵離子濃度約為10-6 mol·L-1,低于此濃度會造成“鐵饑餓”現象[28]。而鐵在pH=7時的溶解度為1.4×10-9 mol·L-1,宿主中的鐵離子濃度甚至更低,遠不能滿足細菌所需[29]。在多殺性巴氏桿菌中,鐵關系到該菌在宿主中的生長、黏附和毒力。低鐵條件下,多殺性巴氏桿菌的生長明顯減緩,細菌莢膜的厚度顯著降低,毒力明顯減弱。相反,脂多糖的合成在限鐵條件下顯著增加。脂多糖是巴氏桿菌科細菌黏附宿主的重要工具,多殺性巴氏桿菌在限鐵條件下發生的一系列變化使其更容易黏附于宿主[10-11]。多殺性巴氏桿菌外膜上的蛋白組分受鐵影響,在限鐵條件下表達出多種鐵調節外膜蛋白(iron-regulated outer membrane proteins, IROMPs)(如:轉鐵蛋白受體、血紅素受體等)奪取宿主的鐵離子,抵御缺鐵造成的不利影響[30]。
2 多殺性巴氏桿菌轉鐵蛋白受體攝鐵機制
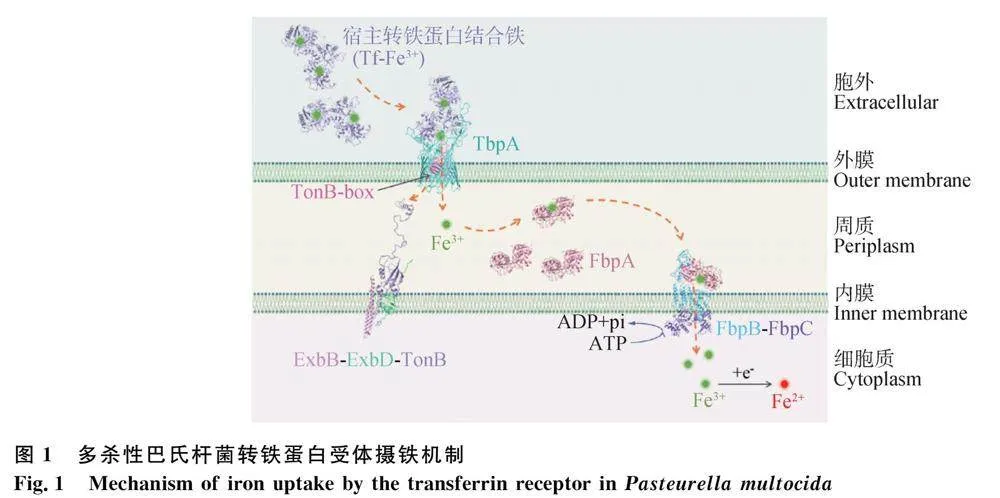
多殺性巴氏桿菌的外膜上存在轉鐵蛋白受體(transferrin receptor),即轉鐵蛋白結合蛋白A(transferrin binding protein A, TbpA),能夠從宿主的轉鐵蛋白結合鐵(Tf-Fe3+)中攝取鐵離子。由于細菌的外膜缺乏能量,鐵離子需要在ExbB-ExbD-TonB系統提供能量的情況下經TbpA進入細胞周質,再通過ABC轉運系統(ATP-binding cassette transporter)進入細胞質,供多殺性巴氏桿菌進行生長、繁殖和代謝等生命活動。多殺性巴氏桿菌從宿主Tf-Fe3+中攝取鐵離子的過程大致分為三步:1)Tf-Fe3+與TbpA復合物的形成;2)Fe3+進入細胞周質的過程;3)Fe3+經ABC轉運系統進入細胞質的過程。
2.1 Tf-Fe3+與TbpA復合物的形成
多殺性巴氏桿菌在缺乏游離鐵的環境下,利用自身細胞外膜的TbpA攝取宿主轉鐵蛋白上結合的鐵離子,即Tf-Fe3+。Tf是一種主要由宿主肝細胞合成的糖蛋白,能夠與Fe3+可逆性結合[31]。TbpA是一種多殺性巴氏桿菌的TonB依賴性跨膜受體蛋白[32-33],其C端為由22條反向平行的β-鏈組成的桶狀結構域(β-桶),N端為鑲嵌在“β-桶”內的“塞子”結構域[34]。多殺性巴氏桿菌TbpA的C端“β-桶”結構域能夠特異性識別宿主Tf-Fe3+,并結合形成跨膜Tf-Fe3+-TbpA復合物[35-36];同時,TbpA N端塞狀結構域的“TonB盒(TonB-box)”保守區域發生構象改變,延伸到周質中,并與內膜上的TonB蛋白結合獲取能量,促使Tf-Fe3+-TbpA復合物的Fe3+向周質轉移(圖1)[37-38]。
2.2 Fe3+進入細胞周質的過程
多殺性巴氏桿菌細胞內膜上的TonB蛋白通過N端疏水性α-螺旋結構錨定在細胞內膜上,與ExbB-ExbD蛋白結合形成ExbB-ExbD-TonB復合物[39],該復合物能夠提供細菌進行外膜物質交換的能量,參與鐵離子等多種營養物質的跨膜轉運[34]。在ExbB-ExbD-TonB復合物中,TonB通過N端的α-螺旋結構與ExbB-ExbD結合,而ExbB-ExbD的跨膜結構域共同構成質子通道,將內膜上的質子動力勢能傳遞給TonB。另外,內膜上的ExbB-ExbD通過“旋轉運動”促使TonB的長鞭狀區域(包括可伸縮的間隔區和C末端結構域)在周質中發生擺動,并與暴露在周質中的TbpA N端的TonB-box區域結合,從而將能量傳遞給TbpA,導致其“塞子”結構域打開,并在桶狀結構域中形成一個通道,供Fe3+經該通道進入細胞周質中(圖1)[34,40-41]。
2.3 Fe3+經ABC轉運系統進入細胞質的過程
ABC轉運系統(ATP-binding cassette transporter),即腺苷三磷酸結合盒轉運蛋白,可利用ATP水解的能量來逆濃度梯度將鐵離子等底物轉運進入細胞中。多殺性巴氏桿菌通過TbpA捕獲的Fe3+進入細胞周質后,再通過ABC轉運系統(FbpABC)將其輸送到細胞質中。該過程中,鐵結合蛋白A(ferric binding protein A, FbpA)在自然條件下以單體形式游離于周質中,捕獲周質中的Fe3+[42-44];鐵結合蛋白B(ferric binding protein B, FbpB)是細胞內膜上的一種疏水性跨膜蛋白,能夠接收FbpA遞呈的Fe3+;鐵結合蛋白C(ferric binding protein C, FbpC)是位于細菌內膜內側的親水性ATP結合蛋白,其與FbpB結合形成二聚體,為FbpB提供轉運Fe3+所需的能量[45]。Fe3+通過FbpABC進入細胞質后,被還原為Fe2+供多殺性巴氏桿菌代謝利用(圖1)。
3 多殺性巴氏桿菌血紅素受體攝鐵機制
動物體內約有70%的鐵儲存在血紅素(heme)中[46]。血紅素可與珠蛋白(globin)結合形成血紅蛋白(haemoglobin, Hb)、肌紅蛋白(myoglobin, Mb)和細胞色素(cytochromes, Cyt)等[47-48]。其中,Hb是細菌攝取血紅素的主要來源[49]。多殺性巴氏桿通過血紅素轉運系統從宿主血紅素結合蛋白中攝取血紅素,并將其轉運至細胞質中分解,獲取血紅素中的Fe2+[7]。血紅素轉運方式通常分為兩種:1)直接轉運,即細菌利用外膜的血紅素受體直接攝取宿主的血紅素;2)間接轉運,即細菌分泌高親和力的血紅素載體蛋白到細胞外,奪取宿主血紅素,并遞呈給外膜上的血紅素受體[50]。
3.1 血紅素直接轉運系統
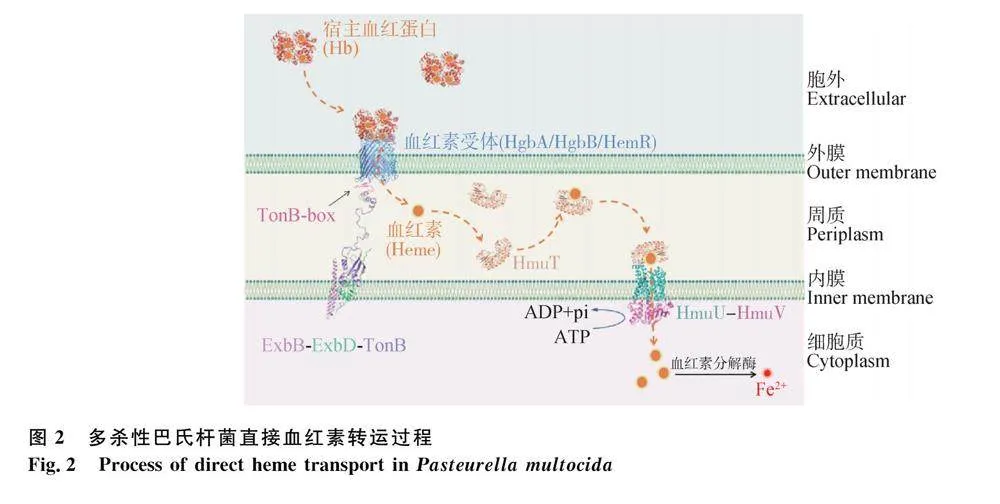
多殺性巴氏桿菌在限鐵條件下表達血紅蛋白結合蛋白A(hemoglobin-binding protein A, HgbA)、血紅蛋白結合蛋白B(hemoglobin-binding protein B, HgbB)和血紅素-血凝素受體(heme-hemopexin receptor, HemR)等血紅素受體。這些血紅素受體與TbpA的結構相似,能夠直接識別宿主的血紅素[51]。血紅素受體的C端“β-桶”結構域與宿主Hb結合后,其N端“塞子”結構域的TonB-box區域發生構象改變,延伸到多殺性巴氏桿菌的細胞周質中,與TonB C末端結構域結合,獲得ExbB-ExbD-TonB復合物傳遞的能量,導致其“塞子”結構域打開并形成通道,將血紅素轉運到細胞周質中(圖2)[52]。
關于多殺性巴氏桿菌通過ABC轉運系統(HmuTUV)將血紅素轉運到細胞質中的過程尚不明確。但鼠疫耶爾森菌血紅素ABC轉運系統(HmuTUV)的相關研究已經基本清晰(圖2):血紅素被轉運到細胞周質后,被血紅素結合蛋白HmuT捕獲,并遞呈給細胞內膜上的跨膜蛋白HmuU;而ATP結合蛋白HmuV水解ATP為HmuU提供能量,將血紅素轉運到細胞質中[53-54];進入細胞質的血紅素被血紅素分解酶分解,從而釋放Fe2+供細菌代謝利用[55]。GenBank登錄的多殺性巴氏桿菌基因組(MAPT01000007.1、MANI01000004.1、MAPS01000002.1等)均具有同源的HmuTUV蛋白基因簇,其注釋信息預測它們具有相同的ABC轉運系統功能(圖2)。
3.2 血紅素間接轉運系統
血紅素間接轉運系統是由血紅素載體(Hemophore)介導的一種血紅素轉運方式,其比直接轉運系統的效率更高。血紅素載體是由革蘭陰性菌分泌到細胞外的一類蛋白質,迄今已在銅綠假單胞菌、鼠疫耶爾森菌和黏質沙雷菌等多種細菌中發現的血紅素載體主要有HasA、HusA、HxuA、HphA等[56-60]。這類蛋白質對血紅素的親和力極高,能夠競爭宿主血紅蛋白(Hb)、肌紅蛋白(Mb)和細胞色素(Cyt)中的血紅素,并遞呈給細菌外膜上的特異性血紅素受體(不同的血紅素載體對應的受體不同)[61]。血紅素受體獲得ExbB-ExbD-TonB復合物提供的能量后,導致其“塞子”結構域打開并形成通道,將血紅素轉運到細胞周質中,再由ABC轉運系統(HmuTUV)將血紅素轉運到細胞質中供細菌代謝利用,該過程與血紅素直接轉運系統相同。雖然關于多殺性巴氏桿菌血紅素載體的相關研究尚不明確,但在該菌中已發現了HasA的受體,即血紅素獲取系統受體(Heme acquisition system receptor, HasR)[62]。在鼠疫耶爾森菌中,HasR可特異性識別并結合HasA,接收HasA從宿主Hb中獲取的血紅素[63]。
4 多殺性巴氏桿菌鐵載體攝鐵機制
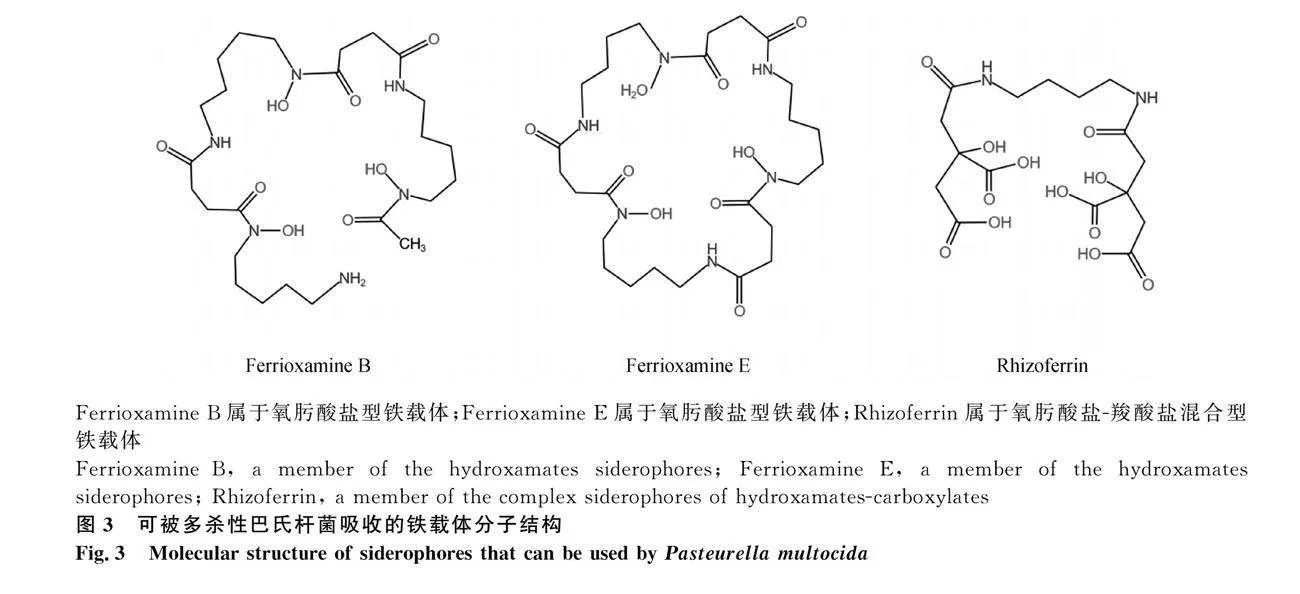
鐵載體(Siderophore)是由微生物產生的高親和力、低分子量的金屬螯合劑,主要是與宿主或環境中的Fe3+結合形成Fe3+-Siderophore螯合物,但它也能與其他金屬元素(例如:鉬、錳、鈷和鎳)形成螯合物[64-65]。根據化學性質的差異,可將鐵載體分為氧肟酸鹽型(Hydroxamates;由酰化和羥化的烷胺組成)、兒茶酚鹽型(Phenolates;
由兒茶酚酸酯和羥基組成)和羧酸鹽型(Carboxylates;由檸檬酸或β-羥基天冬氨酸組成)三類。除上述類型外,某些鐵載體也可歸為混合型,多為氧肟酸鹽-兒茶酚鹽或氧肟酸鹽-羧酸鹽的組合[66]。其中,兒茶酚鹽型鐵載體與鐵結合的能力最強,羧酸鹽型鐵載體與鐵結合的能力最弱[67]。
多殺性巴氏桿菌A血清型菌株在限鐵條件下分泌一種羧酸鹽型鐵載體,被命名為multocidin[68],目前僅知其受體蛋白為76、84和94 ku的鐵相關外膜蛋白[69],關于該鐵載體詳細的攝取機制尚不清楚。多殺性巴氏桿菌不產生氧肟酸"" 鹽型鐵載體,但卻可以吸收氧肟酸鹽型鐵載體ferrioxamine B和ferrioxamine E(圖3);該菌也不產生兒茶酚鹽型鐵載體,但可以利用一些酚鹽型鐵載體的代謝中間產物如二羥基苯甲酸(DHBA)[70]。另外,尚未證實多殺性巴氏桿菌是否產生混合型鐵載體,但已證實該菌可吸收混合型鐵載體rhizoferrin(圖3)[70]。
已有研究發現,多殺性巴氏桿菌外膜上存在一種鐵載體受體FecA[7]。在大腸桿菌的研究中,FecA具有與轉鐵蛋白受體TbpA、血紅素受體HgbA等相似的結構[71],由C端“β-桶”結構域和N端“塞子”結構域組成。FecA能夠識別并結合檸檬酸鐵,在獲得ExbB-ExbD-TonB復合物提供的能量后,其“塞子”結構域打開并形成通道,將檸檬酸鐵轉運到細胞周質中,再經鐵載體ABC轉運系統(FecBDE)轉運到細胞質中[72-73]。Fe3+在細胞質中被還原為Fe2+后從鐵載體中脫離,供細菌代謝利用,鐵載體則被降解或排出細胞外[74-75]。由于多殺性巴氏桿菌攜帶有與大腸桿菌同源的鐵載體受體FecA,因此推測,多殺性巴氏桿菌FecA介導的鐵載體轉運系統與大腸桿菌類似。
5 細菌攝鐵調節因子
缺鐵不利于細菌生長繁殖,但細菌攝入過量Fe2+會引起芬頓反應(Fenton reaction),產生活性氧和活性氮,造成氨基酸殘基氧化、蛋白及DNA損傷,最終導致細菌死亡[76]。因此,細菌細胞內的鐵離子濃度必須受到嚴格的調控。細菌進化出鐵相關調節因子維持鐵穩態,這些鐵調節因子包括:鐵攝取調節因子(ferric uptake regulator, Fur)[20]、鐵依賴性調節因子(iron-dependent regulator, IdeR)、鐵應答調節因子(iron response regulator, Irr)[77]、根瘤菌鐵調節因子A(rhizobial iron regulator A, RirA)[78]、鐵硫簇調節因子(iron sulfur cluster regulator, IscR)[79]。目前,僅有Fur在多殺性巴氏桿菌中被報道。Fur是維持細菌鐵穩態最主要的轉錄調節因子,其C端為二聚化的金屬離子結合結構域,N端為DNA結合結構域,通過感知細菌胞內Fe2+水平,抑制或激活鐵相關基因的轉錄[80]。
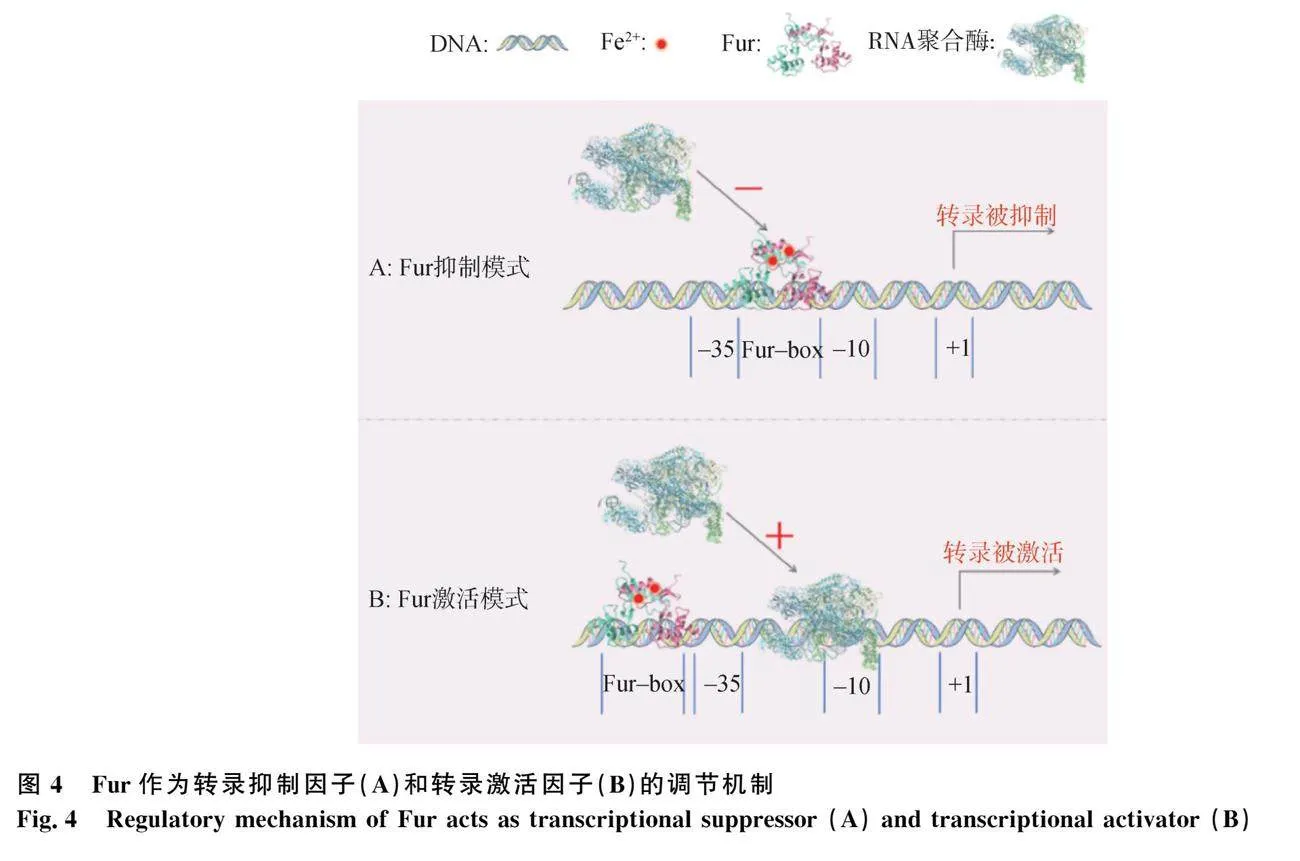
5.1 Fur作為轉錄抑制因子的調控機制
當細菌細胞內Fe2+水平過高時,Fur能夠識別并結合DNA上的一段共有序列“Fur盒(Fur-box)”,若DNA上的啟動子與Fur-box位置重合,啟動子區域則被Fur占據,導致RNA聚合酶無法結合DNA,其轉錄受到抑制[81-82](圖4A)。如:在大腸桿菌中,Fur C端與Fe2+結合形成Fur-Fe2+復合物,促使Fur N端的DNA結合結構域與大腸桿菌鐵載體合成相關基因簇iucABCD上的Fur-box結合,導致RNA聚合酶無法聚集到iucABCD的啟動子上,從而抑制鐵載體合成,阻礙細菌攝取鐵離子[83-84]。關于多殺性巴氏桿菌Fur的探索始于2001年[85],研究相對滯后。隨后,研究學者在多殺性巴氏桿菌的鐵相關基因tbpA、hgbA和hgbB中發現了共有序列Fur-box,這些基因的表達受Fur抑制[86-88]。此外,多殺性巴氏桿菌鐵載體的合成也受到Fur的負調控[89]。
5.2 Fur作為轉錄激活因子的調控機制
當Fur-box位于轉錄起始位點上游-100 bp左右時,Fur能夠促進RNA聚合酶在DNA的-10和-35區聚集,激活DNA的轉錄[82,90](圖4B)。Fur可促進儲鐵相關蛋白——細菌鐵蛋白(bacterioferritin, Bfr)的表達[91]。Bfr能夠將Fe2+氧化為Fe3+,并將Fe3+儲存起來,避免Fe2+過多對細菌造成損傷[92]。Bfr分為兩種類型,即不含血紅素的鐵蛋白A(ferritin A, FtnA)和含血紅素的細菌鐵蛋白B(bacterioferritin B, BfrB)[93]。以大腸桿菌為例,Fur與Fe2+形成Fur-Fe2+復合物,與細菌鐵蛋白基因ftnA上游-84 bp處的Fur-box結合,激活ftnA轉錄,從而將細胞質中冗余的游離鐵儲存起來[94]。在多殺性巴氏桿菌中也存在表達FtnA的同源基因[7],因此,在多殺性巴氏桿菌中可能也存在Fur的轉錄激活調節功能。
6 總結與展望
巴氏桿菌病是多種動物共患的一種重要細菌性病原菌。本文系統闡述了多殺性巴氏桿菌的三種攝鐵機制,為全面了解多殺性巴氏桿菌攝鐵系統的分子致病機理研究提供了系統的理論知識,也為多殺性巴氏桿菌分子靶標藥物及亞單位疫苗的研發提供了新思路。通過回顧多殺性巴氏桿菌的攝鐵系統及其發生與調控機制方面的重要研究進展,可以發現仍有許多問題亟待解決,總結歸納如下:
6.1 鐵載體在許多細菌攝鐵過程中發揮著重要作用,對其化學結構的解析在大腸桿菌、根瘤菌中已取得較大進展。目前,在多殺性巴氏桿菌中僅發現了羧酸鹽型鐵載體multocidin,尚需對其他鐵載體進行篩選鑒定;同時,關于鐵載體multocidin的化學結構、合成分泌通路及其特異性外膜受體方面仍有很大的知識空缺,急需深入研究闡明。
6.2 由血紅素載體介導的血紅素間接轉運系統轉運效率遠超血紅素直接轉運系統,但關于多殺性巴氏桿菌血紅素載體的研究極為匱乏,血紅素載體介導從宿主血紅素結合蛋白中攝取血紅素的詳細機制仍需進一步研究。
6.3 TbpA、HgbA和HgbB等外膜受體在不同地區、不同宿主以及不同血清型的多殺性巴氏桿菌毒株中的分布程度具有較大差異[95-97]。有必要對其分子流行病學進行系統分析,以便精準定位,找到具有普適性的藥物或疫苗靶點。
6.4 雖然fur基因在許多細菌中被廣泛研究,也有越來越多受Fur調控的基因被發現,但目前仍有許多分子機制和調節方式尚未完全闡釋清楚。此外,多殺性巴氏桿菌fur基因缺失后對毒力的影響遠不如其它細菌明顯[89],因此推測,該菌中存在其它的調節因子,有待進一步探究。
另外,在細菌攝鐵系統的應用研究與開發方面,當前比較重要的方向包括:1)通過抑制致病菌攝鐵系統相關蛋白的合成或阻斷攝鐵通路,開發出新的靶點藥物;2)人工設計合成鐵載體并與藥物耦合,載運藥物進入致病菌細胞內,提高治療多耐藥性細菌感染;3)設計轉鐵蛋白受體、血紅素受體和鐵載體受體等多種抗原成分的亞單位疫苗或多表位疫苗;4)將攝鐵相關基因作為缺失靶基因,設計新型的基因缺失弱毒疫苗。
參考文獻(References):
[1] BETHE A,WIELER L H,SELBITZ H J,et al.Genetic diversity of porcine Pasteurella multocida strains from the respiratory tract of healthy and diseased swine[J].Vet Microbiol,2009,139(1-2):97-105.
[2] CARTER G R.Studies on Pasteurella multocida.I.A hemagglutination test for the identification of serological types[J].Am J Vet Res,1955,16(60):481-484.
[3] HEDDLESTON K L,GALLAGHER J E,REBERS P A.Fowl cholera:gel diffusion precipitin test for serotyping Pasteurella multocida from avian species[J].Avian Dis,1972,16(4):925-936.
[4] HARPER M,BOYCE J D,ADLER B.Pasteurella multocida pathogenesis:125 years after Pasteur[J].FEMS Microbiol Lett,2006,265(1):1-10.
[5] FLOSSMANN K D,MLLER G,HEILMANN P,et al.Effect of iron on Pasteurella multocida[J].Zentralbl Bakteriol Mikrobiol Hyg A,1984,258(1):80-93.
[6] VARSHNEY R,VARSHNEY R,CHATURVEDI V K,et al.Development of novel iron-regulated Pasteurella multocida B:2 bacterin and refinement of vaccine quality in terms of minimum variation in particle size and distribution vis-a-vis critical level of iron in media[J].Microb Pathog,2020,147:104375.
[7] JATUPONWIPHAT T,CHUMNANPUEN P,OTHMAN S,et al.Iron-associated protein interaction networks reveal the key functional modules related to survival and virulence of Pasteurella multocida[J].Microb Pathog,2019,127:257-266.
[8] POGOUTSE A K,MORAES T F.Iron acquisition through the bacterial transferrin receptor[J].Crit Rev Biochem Mol Biol,2017,52(3):314-326.
[9] JAKUBOVICS N S,JENKINSON H F.Out of the iron age:new insights into the critical role of manganese homeostasis in bacteria[J].Microbiology,2001,147(Pt 7):1709-1718.
[10] HE F,QIN X B,XU N,et al.Pasteurella multocida Pm0442 affects virulence gene expression and targets TLR2 to induce inflammatory responses[J].Front Microbiol,2020,11:e1972.
[11] JACQUES M,BLANGER M,DIARRA M S,et al.Modulation of Pasteurella multocida capsular polysaccharide during growth under iron-restricted conditions and in vivo[J].Microbiology,1994,140(Pt 2):263-270.
[12] VARSHNEY R,CHATURVEDI V,AGRAWAL A,et al.Growth kinetics of Pasteurella multocida B:2 in iron controlled conditions in broth media[J].Int J Livest Res,2018,8(5):205-212.
[13] FLOSSMANN K D,ROSNER H,GRNKE U,et al.Modification of virulence and immunogenicity of Pasteurella multocida by iron in vitro[J].Z Allg Mikrobiol,1984,24(4):231-7.
[14] GKOUVATSOS K,PAPANIKOLAOU G,PANTOPOULOS K.Regulation of iron transport and the role of transferrin[J].Biochim Biophys Acta Gen Subj,2012,1820(3):188-202.
[15] PAUSTIAN M L,MAY B J,KAPUR V.Pasteurella multocida gene expression in response to iron limitation[J].Infect Immun,2001,69(6):4109-4115.
[16] PALMER L D,SKAAR E P.Transition metals and virulence in bacteria[J].Annu Rev Genet,2016,50:67-91.
[17] BEINERT H,HOLM R H,MNCK E.Iron-sulfur clusters:nature’s modular,multipurpose structures[J].Science,1997,277(5326):653-659.
[18] THAYER M M,AHERN H,XING D,et al.Novel DNA binding motifs in the DNA repair enzyme endonuclease III crystal structure[J].EMBO J,1995,14(16):4108-4120.
[19] GRANDONI J A,SWITZER R L,MAKAROFF C A,et al.Evidence that the iron-sulfur cluster of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase determines stability of the enzyme to degradation in vivo[J].J Biol Chem,1989,264(11):6058-6064.
[20] BEHRINGER M,PLTZKY L,BAABE D,et al.RirA of Dinoroseobacter shibae senses iron via a [3Fe-4S]1+ cluster co-ordinated by three cysteine residues[J].Biochem J,2020,477(1):191-212.
[21] GAUDU P,WEISS B.SoxR,a [2Fe-2S] transcription factor,is active only in its oxidized form[J].Proc Natl Acad Sci U S A,1996,93(19):10094-10098.
[22] STOJILJKOVIC I,PERKINS-BALDING D.Processing of heme and heme-containing proteins by bacteria[J].DNA Cell Biol,2002,21(4):281-295.
[23] JORDAN R M M.The nutrition of Pasteurella septica.I.The action of haematin[J].Br J Exp Pathol,1952,33(1):27-35.
[24] 潘 梅.大腸桿菌血紅素合成調節及其對血紅素過氧化物酶的影響[D].無錫:江南大學,2020.
PAN M.Regulation of heme synthesis and its effect on heme peroxidase in Escherichia coli[D].Wuxi:Jiangnan University,2020.(in Chinese)
[25] HAMMER N D,RENIERE M L,CASSAT J E,et al.Two heme-dependent terminal oxidases power Staphylococcus aureus organ-specific colonization of the vertebrate host[J].mBio,2013,4(4):e00241-13.
[26] 劉 琪.大腸桿菌過氧化物酶EfeB的生理功能及其對血紅素代謝的影響[D].無錫:江南大學,2022.
LIU Q.Physiological function of Escherichia coli peroxidase EfeB and its effect on heme metabolism[D].Wuxi:Jiangnan University,2022.(in Chinese)
[27] SHI X J,ZHAO G Y,LI H,et al.Hydroxytryptophan biosynthesis by a family of heme-dependent enzymes in bacteria[J].Nat Chem Biol,2023,19(11):1415-1422.
[28] MIETHKE M,MARAHIEL M A.Siderophore-based iron acquisition and pathogen control[J].Microbiol Mol Biol Rev,2007,71(3):413-451.
[29] RATLEDGE C,DOVER L G.Iron metabolism in pathogenic bacteria[J].Annu Rev Microbiol,2000,54:881-941.
[30] PENG Z,WANG X R,ZHOU R,et al.Pasteurella multocida:genotypes and genomics[J].Microbiol Mol Biol Rev,2019,83(4):e00014-19.
[31] SUN Y,WANG X H,GONG Q W,et al.Extraintestinal pathogenic Escherichia coli utilizes surface-located elongation factor g to acquire iron from holo-transferrin[J].Microbiol Spectr,2022,10(2):e0166221.
[32] SHIVACHANDRA S B,KUMAR A A,AMARANATH J,et al.Cloning and characterization of tbpA gene encoding transferrin-binding protein (TbpA) from Pasteurella multocida serogroup B:2 (strain P52)[J].Vet Res Commun,2005,29(6):537-542.
[33] CORNELISSEN C N,HOLLANDER A.TonB-dependent transporters expressed by Neisseria gonorrhoeae[J].Front Microbiol,2011,2:117.
[34] SAMANTARRAI D,LAKSHMAN SAGAR A,GUDLA R,et al.TonB-dependent transporters in Sphingomonads:unraveling their distribution and function in environmental adaptation[J].Microorganisms,2020,8(3):359.
[35] VEKEN J W,OUDEGA B,LUIRINK J,et al.Binding of bovine transferrin by Pasteurella multocida serotype B:2,5,a strain which causes haemorrhagic septicaemia in buffalo and cattle[J].FEMS Microbiol Lett,1994,115(2-3):253-257.
[36] BOSHUIZEN M,VAN DER PLOEG K,VON BONSDORFF L,et al.Therapeutic use of transferrin to modulate anemia and conditions of iron toxicity[J].Blood Rev,2017,31(6):400-405.
[37] MOECK G S,COULTON J W.TonB-dependent iron acquisition:mechanisms of siderophore-mediated active transport[J].Mol Microbiol,1998,28(4):675-681.
[38] TUCKMAN M,OSBURNE M S.In vivo inhibition of TonB-dependent processes by a TonB box consensus pentapeptide[J].J Bacteriol,1992,174(1):320-323.
[39] SILALE A,VAN DEN BERG B.TonB-dependent transport across the bacterial outer membrane[J].Annu Rev Microbiol,2023,77:67-88.
[40] KLEBBA P E.ROSET Model of TonB action in gram-negative bacterial iron acquisition[J].J Bacteriol,2016,198(7):1013-1021.
[41] LARSEN R A,DECKERT G E,KASTEAD K A,et al.His20 provides the sole functionally significant side chain in the essential TonB transmembrane domain[J].J Bacteriol,2007,189(7):2825-2833.
[42] BELZER C A,TABATABAI L B,FRANK G H.Purification and characterization of the Pasteurella haemolytica 35 kilodalton periplasmic iron-regulated protein[J].Prep Biochem Biotechnol,2000,30(4):343-355.
[43] CHAN C,NG D,FRASER M E,et al.Structural and functional insights into iron acquisition from lactoferrin and transferrin in Gram-negative bacterial pathogens[J].Biometals,2023,36(3):683-702.
[44] SHOULDICE S R,DOUGAN D R,WILLIAMS P A,et al.Crystal structure of Pasteurella haemolytica ferric ion-binding protein A reveals a novel class of bacterial iron-binding proteins[J].J Biol Chem,2003,278(42):41093-41098.
[45] ADHIKARI P,BERISH S A,NOWALK A J,et al.The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron[J].J Bacteriol,1996,178(7):2145-2149.
[46] 李紅敏,龍章彪,韓 冰.鐵穩態的維持及鐵代謝相關疾病[J].中華血液學雜志,2018,39(9):790-792.
LI H M,LONG Z B,HAN B.Iron homeostasis and iron-related disorders[J].Chinese Journal of Hematology,2018,39(9):790-792.(in Chinese)
[47] TOLOSANO E,FAGOONEE S,MORELLO N,et al.Heme scavenging and the other facets of hemopexin[J].Antioxid Redox Signal,2010,12(2):305-320.
[48] PAUSTIAN M L,MAY B J,CAO D W,et al.Transcriptional response of Pasteurella multocida to defined iron sources[J].J Bacteriol,2002,184(23):6714-6720.
[49] GELL D A.Structure and function of haemoglobins[J].Blood Cells Mol Dis,2018,70:13-42.
[50] 程興軍,劉馬峰,程安春.革蘭氏陰性菌血紅素轉運系統結構及功能特點[J].中國生物化學與分子生物學報,2014,30(9):848-855.
CHENG X J,LIU M F,CHENG A C.Structural and functional properties of the Heme acquisition system in gram-negative bacteria[J].Chinese Journal of Biochemistry and Molecular Biology,2014,30(9):848-855.(in Chinese)
[51] PRASANNAVADHANA A,KUMAR S,THOMAS P,et al.Outer membrane proteome analysis of Indian strain of Pasteurella multocida serotype B:2 by MALDI-TOF/MS analysis[J].Sci World J,2014,2014:617034.
[52] SRIKUMAR R,MIKAEL L G,PAWELEK P D,et al.Molecular cloning of haemoglobin-binding protein HgbA in the outer membrane of Actinobacillus pleuropneumoniae[J].Microbiology,2004,150(Pt 6):1723-1734.
[53] QASEM-ABDULLAH H,PERACH M,LIVNAT-LEVANON N,et al.ATP binding and hydrolysis disrupt the high-affinity interaction between the heme ABC transporter HmuUV and its cognate substrate-binding protein[J].J Biol Chem,2017,292(35):14617-14624.
[54] THOMPSON J M,JONES H A,PERRY R D.Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization[J].Infect Immun,1999,67(8):3879-3892.
[55] EXNER T E,BECKER S,BECKER S,et al.Binding of HasA by its transmembrane receptor HasR follows a conformational funnel mechanism[J].Eur Biophys J,2020,49(1):39-57.
[56] ARNOUX P,HASER R,IZADI N,et al.The crystal structure of HasA,a hemophore secreted by Serratia marcescens[J].Nat Struct Biol,1999,6(6):516-520.
[57] GAO J L,NGUYEN K A,HUNTER N.Characterization of a hemophore-like protein from Porphyromonas gingivalis[J].J Biol Chem,2010,285(51):40028-40038.
[58] HANSON M S,PELZEL S E,LATIMER J,et al.Identification of a genetic locus of Haemophilus influenzae type b necessary for the binding and utilization of heme bound to human hemopexin[J].Proc Natl Acad Sci U S A,1992,89(5):1973-1977.
[59] SMALLEY J W,OLCZAK T.Heme acquisition mechanisms of Porphyromonas gingivalis-strategies used in a polymicrobial community in a heme-limited host environment[J].Mol Oral Microbiol,2017,32(1):1-23.
[60] BATEMAN T J,SHAH M,HO T P,et al.A Slam-dependent hemophore contributes to heme acquisition in the bacterial pathogen Acinetobacter baumannii[J].Nat Commun,2021,12(1):6270.
[61] CESCAU S,CWERMAN H,LTOFF S,et al.Heme acquisition by hemophores[J].Biometals,2007,20(3-4):603-613.
[62] GU H W,LU C P.Selection of immunodominant mimics of IROMP-99 of rabbit Pasteurella multocida from a random 12-peptide library[J].Vet Microbiol,2006,115(4):339-348.
[63] ROSSI M S,FETHERSTON J D,LTOFF S,et al.Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis[J].Infect Immun,2001,69(11):6707-6717.
[64] HOLDEN V I,BACHMAN M A.Diverging roles of bacterial siderophores during infection[J].Metallomics,2015,7(6):986-995.
[65] SAHA M,SARKAR S,SARKAR B,et al.Microbial siderophores and their potential applications:a review[J].Environ Sci Pollut Res Int,2016,23(5):3984-3999.
[66] KHAN A,SINGH P,SRIVASTAVA A.Synthesis,nature and utility of universal iron chelator - siderophore:a review[J].Microbiol Res,2018,212-213:103-111.
[67] 殷奧杰,王 齊,葛淼淼,等.微生物鐵載體的應用研究進展[J].環境保護與循環經濟,2021,41(7):20-24,69.
YIN A J,WANG Q,GE M M,et al.The application and research progress of siderophore[J].Environmental Protection and Circular Economy,2021,41(7):20-24,69.(in Chinese)
[68] HU S P,FELICE L J,SIVANANDAN V,et al.Siderophore production by Pasteurella multocida[J].Infect Immun,1986,54(3):804-810.
[69] CHOI-KIM K,MAHESWARAN S K,FELICE L J,et al.Relationship between the iron regulated outer membrane proteins and the outer membrane proteins of in vivo grown Pasteurella multocida[J].Vet Microbiol,1991,28(1):75-92.
[70] REISSBRODT R,ERLER W,WINKELMANN G.Iron supply of Pasteurella multocia and Pasteurella haemolotica[J].J Basic Microbiol,1994,34(1):61-63.
[71] OGIERMAN M,BRAUN V.Interactions between the outer membrane ferric citrate transporter FecA and TonB:studies of the FecA TonB box[J].J Bacteriol,2003,185(6):1870-1885.
[72] NOINAJ N,GUILLIER M,BARNARD T J,et al.TonB-dependent transporters:regulation,structure,and function[J].Annu Rev Microbiol,2010,64:43-60.
[73] SUN Y,ZHANG Y,HOLLIBAUGH J T,et al.Ecotype diversification of an abundant Roseobacter lineage[J].Environ Microbiol,2017,19(4):1625-1638.
[74] AHMED E,HOLMSTRM S J M.Siderophores in environmental research:roles and applications[J].Microb Biotechnol,2014,7(3):196-208.
[75] WILSON B R,BOGDAN A R,MIYAZAWA M,et al.Siderophores in iron metabolism:from mechanism to therapy potential[J].Trends Mol Med,2016,22(12):1077-1090.
[76] CORNELIS P,WEI Q,ANDREWS S C,et al.Iron homeostasis and management of oxidative stress response in bacteria[J].Metallomics,2011,3(6):540-549.
[77] COSTA D,AMARELLE V,VALVERDE C,et al.The irr and RirA proteins participate in a complex regulatory circuit and act in concert to modulate bacterioferritin expression in Ensifer meliloti 1021[J].Appl Environ Microbiol,2017,83(16):e00895-17.
[78] O’BRIAN M R.Perception and homeostatic control of iron in the rhizobia and related bacteria[J].Annu Rev Microbiol,2015,69:229-245.
[79] NONOYAMA S,KISHIDA K,SAKAI K,et al.A transcriptional regulator,IscR,of Burkholderia multivorans acts as both repressor and activator for transcription of iron-sulfur cluster-biosynthetic isc operon[J].Res Microbiol,2020,171(8):319-330.
[80] POHL E,HALLER J C,MIJOVILOVICH A,et al.Architecture of a protein central to iron homeostasis:crystal structure and spectroscopic analysis of the ferric uptake regulator[J].Mol Microbiol,2003,47(4):903-915.
[81] BAICHOO N,HELMANN J D.Recognition of DNA by Fur:a reinterpretation of the Fur box consensus sequence[J].J Bacteriol,2002,184(21):5826-5832.
[82] DELANY I,RAPPUOLI R,SCARLATO V.Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis[J].Mol Microbiol,2004,52(4):1081-1090.
[83] ESCOLAR L,DE LORENZO V,PREZ-MARTN J.Metalloregulation in vitro of the aerobactin promoter of Escherichia coli by the Fur (ferric uptake regulation) protein[J].Mol Microbiol,1997,26(4):799-808.
[84] TROXELL B,HASSAN H M.Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria[J].Front Cell Infect Microbiol,2013,3:59.
[85] BOSCH M,TARRAG R,GARRIDO M E,et al.Expression of the Pasteurella multocida ompH gene is negatively regulated by the Fur protein[J].FEMS Microbiol Lett,2001,203(1):35-40.
[86] EKINS A,NIVEN D F.Identification of fur and fldA homologs and a Pasteurella multocida tbpA homolog in Histophilus ovis and effects of iron availability on their transcription[J].J Bacteriol,2002,184(9):2539-2542.
[87] BAHRAMI F,NIVEN D F.Iron acquisition by Actinobacillus suis:identification and characterization of a single-component haemoglobin receptor and encoding gene[J].Microb Pathog,2005,39(1-2):45-51.
[88] COX A J,HUNT M L,BOYCE J D,et al.Functional characterization of HgbB,a new hemoglobin binding protein of Pasteurella multocida[J].Microb Pathog,2003,34(6):287-296.
[89] LIU Q,HU Y L,LI P,et al.Identification of Fur in Pasteurella multocida and the potential of its mutant as an attenuated live vaccine[J].Front Vet Sci,2019,6:5.
[90] TEIXID L,CARRASCO B,ALONSO J C,et al.Fur activates the expression of Salmonella enterica pathogenicity island 1 by directly interacting with the hilD operator in vivo and in vitro[J].PLoS One,2011,6(5):e19711.
[91] BERESWILL S,GREINER S,VAN VLIET A H M,et al.Regulation of ferritin-mediated cytoplasmic iron storage by the ferric uptake regulator homolog (Fur) of Helicobacter pylori[J].J Bacteriol,2000,182(21):5948-5953.
[92] GUO M L,GAO M M,LIU J J,et al.Bacterioferritin nanocage:Structure,biological function,catalytic mechanism,self-assembly and potential applications[J].Biotechnol Adv,2022,61:108057.
[93] YAO H L,SOLDANO A,FONTENOT L,et al.Pseudomonas aeruginosa bacterioferritin is assembled from FtnA and BfrB subunits with the relative proportions dependent on the environmental oxygen availability[J].Biomolecules,2022,12(3):366.
[94] NANDAL A,HUGGINS C C O,WOODHALL M R,et al.Induction of the ferritin gene (ftnA) of Escherichia coli by Fe2+-Fur is mediated by reversal of H-NS silencing and is RyhB independent[J].Mol Microbiol,2010,75(3):637-657.
[95] EWERS C,LBKE-BECKER A,BETHE A,et al.Virulence genotype of Pasteurella multocida strains isolated from different hosts with various disease status[J].Vet Microbiol,2006,114(3-4):304-317.
[96] KATSUDA K,HOSHINOO K,UENO Y,et al.Virulence genes and antimicrobial susceptibility in Pasteurella multocida isolates from calves[J].Vet Microbiol,2013,167(3-4):737-741.
[97] LIU S T,LIN L,YANG H,et al.Pasteurella multocida capsular:lipopolysaccharide types D:L6 and A:L3 remain to be the main epidemic genotypes of pigs in China[J].Anim Dis,2021,1(1):26.
(編輯 范子娟)

