葡萄VlCKX5基因的表達特性及轉錄調控分析
摘 " "要:【目的】對VlCKX5基因進行克隆及生物信息學、表達特異性和轉錄調控分析,探究其在葡萄坐果中的調控作用。【方法】克隆VlCKX5基因及其啟動子,對其進行生物信息學、表達特異性和轉錄調控分析。使用GUS組織化學染色法分析VlCKX5啟動子的活性。使用PlantTFDB、CIS-BP和JASPAR數據庫對VlCKX5的轉錄調控關系進行預測分析并篩選出關鍵轉錄因子。使用亞細胞定位、實時熒光定量(RT-qPCR)、酵母單雜交和雙熒光素酶驗證VlAGL6a對VlCKX5的調控作用。【結果】VlCKX5具有CKX家族典型特征的FAD結構域和細胞分裂素結合位點。VlCKX5在葡萄根和葉中高表達,其次是在花序中,CPPU處理后VlCKX5的表達量顯著降低。VlCKX5響應CPPU激素的處理。VlAGL6a是VlCKX5的關鍵轉錄因子,定位于細胞核中。VlAGL6a在花序中高表達,在CPPU處理后的表達模式與VlCKX5的一致。VlAGL6a可以與VlCKX5相互作用,并促進其表達。【結論】葡萄VlCKX5基因響應CPPU的信號,轉錄因子VlAGL6a特異性結合VlCKX5基因的啟動子并促進VlCKX5的轉錄,為進一步解析葡萄坐果機制提供了理論基礎。
關鍵詞:葡萄;CPPU;VlCKX5;VlAGL6a轉錄因子
中圖分類號:S663.1 文獻標志碼:A 文章編號:1009-9980(2025)01-0016-14
Expression characteristics and transcriptional regulation analysis of VlCKX5 gene in grape
LIU Yiting1, 2, WANG Ruxin1, 2, ZHANG Haimeng1, 2, JING Pengwei1, 2, SHI Qiaofang1, 2, ZHAO Xiao-chun1, 2, YU Yihe1, 2*
(1College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, Henan, China; 2Henan Provincial Engineering Research Center on Characteristic Berry Germplasm Innovation amp; Utilization, Luoyang 471023, Henan, China)
Abstract: 【Objective】 Grapes (Vitis vinifera L.) are an economically important fruit crop in the world, and severe berry drop can affect grape yield and quality. The synthetic cytokinin analog N-(2-chloro-4-pyridyl)-N'-phenylurea (CPPU) is known to enhance berry set in grapes. Cytokinin oxidase/dehydrogenase (CKX) enzymes, which are responsible for the irreversible degradation of cytokinin, are pivotal in modulating plant growth and development. In the present investigation, the cytokinin oxidase/dehydrogenase 5 (VlCKX5) gene and its promoter were cloned, and bioinformatic analysis, expression specificity and transcriptional regulation were performed to illustrate its role in grape berry setting. 【Methods】 In this study, we conducted experiments using Kyoho grapes (V. vinifera L. × V. labrusca L.) as the experimental material. The young berries were treated with 10 mg·L-1 of cytokinin-like growth regulator CPPU 5 days after anthesis. The treated berries were sampled at 1, 2, 4 and 8 days after treatment. Furthermore, at 13th day after anthesis, we systematically harvested roots, stems, leaves, inflorescences, tendrils and young berries from grapevines for subsequent tissue-specific analysis. The VlCKX5 gene region was amplified via polymerase chain reaction (PCR). Bioinformatic analysis of the VlCKX5 protein sequence, including various physicochemical properties, was performed using the Expasy web tool. The identification of conserved domains within VlCKX5 was conducted through the InterPro database. Furthermore, the phylogenetic relationship among VlCKX5 and its homologs was examined using MEGA software. Protein domain architecture of VlCKX5 and its orthologous proteins was examined utilizing the GeneDoc 2.7. Expression levels of VlCKX5 in grapevine tissues, including roots, canes, leaves, inflorescences, tendrils and young berries under natural growth conditions, as well as in young berries following treatment with the CPPU, were quantified using real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR). The activity of the VlCKX5 promoter was evaluated through histochemical staining with β-glucuronidase (GUS). To predict the transcriptional regulatory interactions involving VlCKX5, we utilized the PlantTFDB, CIS-BP and JASPAR databases to identify potential key transcription factors that may modulate its expression. The coding sequence (CDS) of VlAGL6, with the termination codon excised, was cloned into the 101LYFP vector. The construct was then transformed into Agrobacterium Competent Cells (GV3101), which were subsequently mixed with a selection marker and used to infiltrate Nicotiana benthamiana plants. At 72 hours post-transformation, the subcellular localization of fluorescence within N. benthamiana leaf cells was analyzed using laser scanning confocal microscopy. RT-qPCR was used to analyze the expression pattern of VlAGL6a in grape tissues after CPPU treatment. A fragment of the VlCKX5 promoter containing the VlAGL6a binding site, designated as P, was cloned into the pAbAi vector, generating the recombinant bait plasmid pAbAi-proVlCKX5/P. This plasmid was then transfected into the Y1HGold yeast strain. Subsequently, the VlAGL6a gene was cloned into the pGADT7 vector to create the recombinant prey plasmid pGADT7-VlAGL6a, which was transfected into a yeast strain positive for the bait genome to perform yeast one-hybrid (Y1H) interaction detection. A 1566 base pair segment of the VlCKX5 promoter, located in upstream of the ATG start codon, was cloned into the pGreenII0800-LUC vector to create a reporter construct. The VlAGL6a CDS was subcloned into the pSAK277 vector to produce an effector construct. Agrobacterium tumefaciens strains carrying these constructs were co-infiltrated into N. benthamiana leaves. The luciferase activity in the infiltrated samples was measured 48 hours post-infiltration using a dual-luciferase reporter assay system. 【Results】 VlCKX5 was 1641 bp in length and encoded 546 amino acids. The molecular weight of VlCKX5 was 61.516 62 ku, the isoelectric point was 8.36, the instability index was 36.64, the fat coefficient was 94.27, and the protein structure was stable. VlCKX5 had the closest homology to CKX amino acids in Chinese kiwifruit, and had a FAD domain and cytokinin binding site (CK-binding), belonging to a typical CKX family. VlCKX5 was highly expressed in roots and leaves, followed by inflorescences, and the expression of VlCKX5 was significantly reduced at 1, 2, 4 and 8 d after CPPU treatment. Prediction of the cis-acting elements of the VlCKX5 promoter revealed elements containing hormones responsive to IBA, SA and ABA. GUS chemical tissue staining test results showed that VlCKX5 activated its promoter activity in response to the treatment of CPPU, SA, IBA and ABA. Transcriptional regulation analysis showed that BPC, DOF, MADS and FLC family transcription factors may be involved in the transcriptional regulation of VlCKX5, and VlAGL6a was a key candidate transcription factor for VlCKX5. Subcellular localization assay verified that VlAGL6a was localized in the nucleus. The results of fluorescence quantification showed that VlAGL6a was the highest in inflorescences, followed by berries and tendrils, and the lowest in roots, stems and leaves. The RT-qPCR results after CPPU treatment showed that the expression levels of VlAGL6a were significantly reduced on the 1st, 2nd, 4th and 8th days, which was consistent with the expression pattern of VlCKX5. Y1H and double luciferase assay showed that VlAGL6a could interact with VlCKX5 and promote its expression. 【Conclusion】 The VlCKX5 gene of grape responded to the signal of CPPU, and the transcription factor VlAGL6a was specifically bound to the promoter of VlCKX5 gene and promoted the transcription of VlCKX5, which affected grape berry setting by regulating the level of cytokinin, which provides a theoretical basis for further analysis of the mechanism of grape berry set.
Key words: Grape; CPPU; VlCKX5; VlAGL6a transcription factor
葡萄(Vitis vinifera L.)作為重要的經濟水果作物,果實中富含花青素、黃酮醇及白藜蘆醇等多種生物活性成分有利于人體健康[1-2]。在葡萄的生長發育過程中,大多數幼果在開花后9~10 d發生嚴重的脫落[3]。坐果過程是決定葡萄產量和品質的關鍵階段,是由內部特定基因的調控和植物激素水平的變化共同驅動的[4]。細胞分裂素處理可以顯著提高坐果率和產量[5-7]。氯吡苯脲(CPPU)是一種細胞分裂素類的植物生長調節劑,已被證實能夠通過提高果實中碳水化合物水平來促進鱷梨坐果[8],此外CPPU還可以通過降低呼吸代謝和維持較高的能量電荷水平來促進葡萄果實坐果[9]。CPPU處理還可以促進甜瓜坐果和提高產量[10]。然而,關于CPPU促進葡萄果實坐果的分子機制尚不清楚。因此,深入研究CPPU在葡萄坐果中的作用機制,對提升葡萄果實的產量和品質具有重要的生產實踐意義。
CKX是一種不可逆降解細胞分裂素為腺嘌呤/腺苷的黃素酶,是細胞分裂素信號轉導中降解分支的關鍵酶[11-12]。CKX酶具有N端FAD和C端細胞分裂素結合位點(CK-binding)兩個保守結構域[13]。CKX基因家族成員不僅可以調控對非生物脅迫的響應[14]、根的形成[15]和果實發育[16],還可以調控植物細胞分裂素水平進而影響產量[17]。在水稻中,沉默OsCKX11可以提高旗葉中的細胞分裂素水平,增加分蘗數和穗粒數從而提高產量[18]。同樣,沉默OsCKX2可以增加水稻花序分生組織中細胞分裂素含量,提高鹽脅迫條件下的產量[19]。油菜中突變CKX3CKX5使細胞分裂素濃度增加,花和胚珠增加,產量提高[20]。然而VlCKX5基因在葡萄中的功能尚不清楚,還需進一步研究。
VlAGL6a也稱為VlMADS3,是MADS-box基因家族中的成員之一[21]。MADS-box基因分為Ⅰ型和Ⅱ型,Ⅰ型MADS-box基因被分為3個不同的進化群:Mα、Mβ和Mγ,Ⅱ型MADS-box基因包括動物和酵母中的MEF2-like基因以及植物中特有的MIKC型基因[22-23]。MIKC型基因又分為MIKCC型和MIKC*型,植物中大多數MIKC型基因為MIKCC型,包括12個亞家族SVP、FLC、TM3、AP1/FUL、SEP、AGL6、AG、AGL12、ANR1、AP3/PI、AGL15、BS[24-25]。MADS-box在植物發育中發揮重要作用,在番茄中異源過表達VvMADS45基因會使花、果和種子變大,而沉默其同源基因SlAGL104會使花、果實和種子變小[26]。在擬南芥中AGL6可以抑制FLC/MAF基因轉錄調控開花時間[27]。FveSEP3在草莓花發育中起主要作用,FveSEP3可以抑制單性果實生長和促進正常授粉的果實成熟[28]。HvMADS1直接調控細胞分裂素降解酶HvCKX3基因進而保持細胞分裂素的穩態,在高溫下維持無分支的穗結構[29]。
筆者課題組前期研究發現植物生長調節劑CPPU處理降低了葡萄內源細胞分裂素含量,顯著提高葡萄坐果率[30],但其潛在的分子機制尚不清楚。本研究在巨峰葡萄基因組中克隆VlCKX5基因及其啟動子,通過生物信息學分析、表達特異性分析、GUS組織化學染色、亞細胞定位、酵母單雜交和雙熒光素酶分析等方法,對VlCKX5的功能以及轉錄調控模式進行初步探索,為后續進一步研究VlCKX5調控細胞分裂素水平進而影響葡萄坐果的分子機制奠定基礎。
1 材料和方法
1.1 材料處理
植物材料取自中國河南省洛陽市偃師葡萄種植區種植的10年生巨峰葡萄(Vitis vinifera L.×Vitis labrusca L.)。在盛花后5 d,使用10 mg·L-1的CPPU溶液(含0.03% Silwet-L77表面活性劑)浸蘸葡萄幼果10 s,蒸餾水(含0.03% Silwet-L77表面活性劑)處理作為對照,具體處理參考Sun等[31]的方法。在CPPU處理和蒸餾水處理后1、2、4和8 d選擇長勢一致的整串葡萄幼果進行采樣。在盛花后13 d時,采集自然發育的根(1年生)、莖(1年生)、葉(1年生枝條從基部數起的第4至6枚葉)、花序(1年生枝上)、卷須(1年生枝上)和幼果(1年生枝上),用于基因的組織特異性表達分析。本氏煙草(Nicotiana benthamiana)在25 ℃培養室中生長,光照16 h/黑暗8 h。
1.2 序列分析
使用在線網站Ensembl Plants(https://plants.ensembl.org/index.html)查詢VlCKX5(Vitvi13g01614)的編碼序列(CDS)和蛋白質序列。使用在線網站Expasy(https://web.expasy.org/protparam/)查詢VlCKX5蛋白序列的各種物理和化學參數。使用在線網站Interpro(https://www.ebi.ac.uk/interpro/)查詢VlCKX5的保守結構域。在NCBI(https://www.ncbi.nlm.nih.gov/)數據庫中下載VlCKX5的同源氨基酸序列,使用MEGA11軟件對VlCKX5及其同源氨基酸序列進行比對,生成系統發育樹。利用GeneDoc 2.7對VlCKX5同源物進行氨基酸序列比對。
1.3 RNA提取與VlCKX5克隆
使用RNA提取試劑盒(諾唯贊,南京)提取巨峰葡萄的RNA。使用反轉錄試劑盒(雅禮,江蘇)獲得葡萄的全長cDNA,并以此為模板通過PCR擴增VlCKX5的編碼序列(CDS),擴增引物為pSAK277-VlCKX5-F:TAGTGGATCCAAAGAATTCCATGTTGAGGGGCTTCTGTCTTTGG,pSAK277-VlCKX5-R:CGAGAAGCTTTTTGAATTCGATCACAAGAA-
GGGTGTCGCCTTTC。使用膠回收試劑盒(康為世紀,北京)回收目標片段。將連接產物轉化入大腸桿菌感受態(Trans5α),使用質粒提取試劑盒(聚合美,北京)提取pSAK277-VlCKX5質粒,并送至蘇州金唯智生物科技有限公司進行測序得到pSAK277-VlCKX5序列。測序結果正確后將質粒轉至農桿菌感受態(GV3101)中。
1.4 實時熒光定量(RT-qPCR)
使用反轉錄試劑盒(雅禮,江蘇)進行反轉錄獲取片段cDNA,經內參基因Ubiqutin1(GenBank:CA808925)檢測后在-20 ℃保存備用。采用2-ΔΔCT法計算基因的相對表達量,并使用Excel 2016和Graphpad Prism 8軟件對所得數據進行分析。構建載體所用引物序列為VlCKX5-F:GCATTCGTTTC-
ATAGCAAGC,VlCKX5-R:AATGCCCGTCAACA-
GAAAGT;VlAGL6a-F:ACTTTCTGTGCTTTGTGATGCT,VlAGL6a-R:TGATACCGCTCTAGGGT-
TTTG。
1.5 啟動子的克隆與GUS組織化學染色
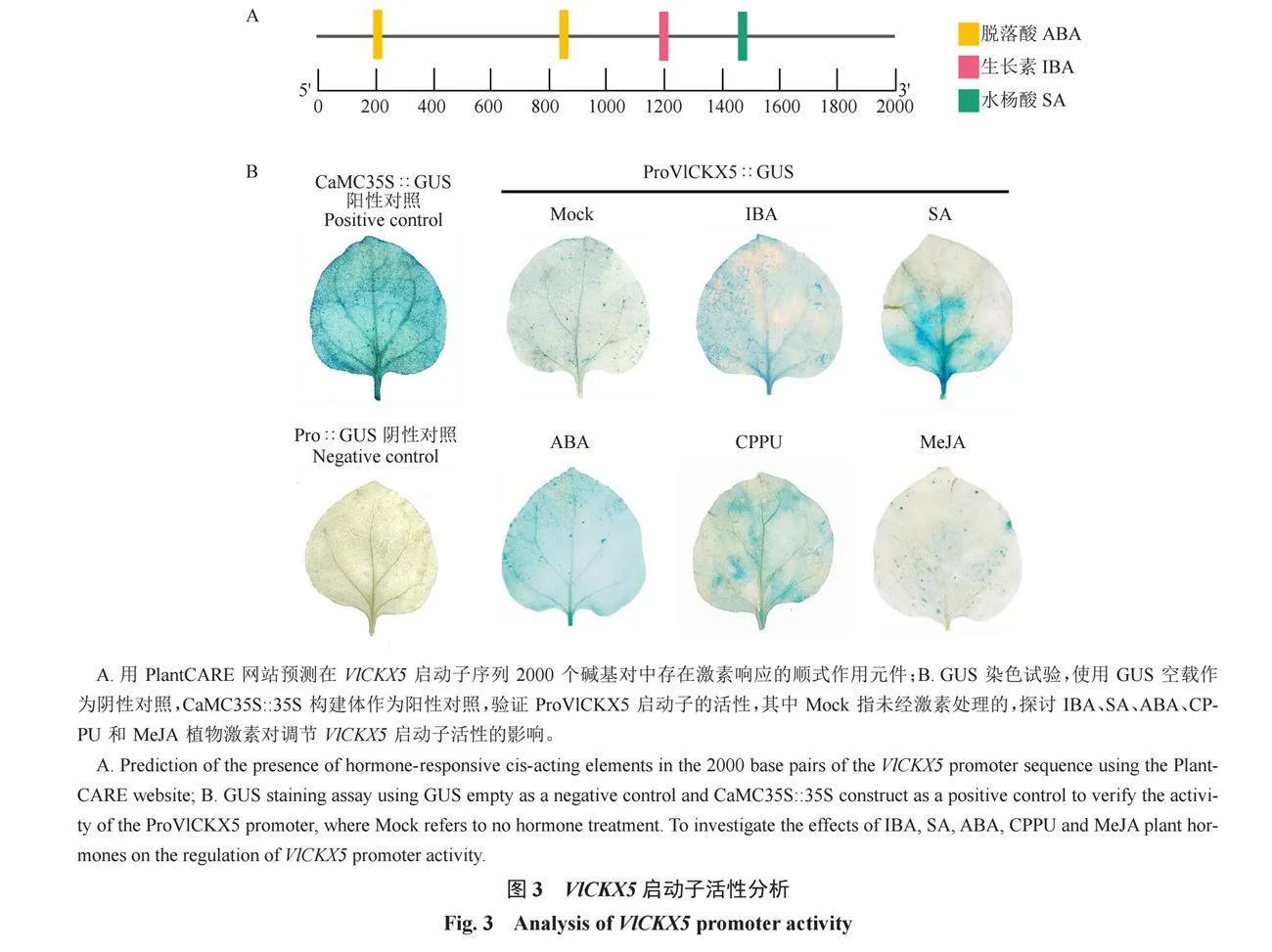
使用DNA提取試劑盒(雅禮,江蘇)提取葡萄果實的DNA。使用添加同源臂的引物以DNA為模板使用高保真酶Primer STAR Max Premix(TaKaRa,大連)克隆VlCKX5的5′端上游1566 bp序列,連接GUS載體,獲得pC0390-GUS-proVlCKX5質粒。將其質粒轉到GV3101中,獲得農桿菌菌液。使用PlantCARE網站(https://bioinformatics.psb.ugent.be/webtools/plantcare/html/)預測VlCKX5啟動子的順式作用元件。使用真空滲透方法進行煙草葉片的瞬時轉化,GUS組織化學染色試驗的具體方法參考GUS染色試劑盒(酷來搏,北京)的說明書。使用生長素(IBA)(100 μmol·L-1)、水楊酸(SA)(100 μmol·L-1)、脫落酸(ABA)(100 μmol·L-1)、氯吡苯脲(CPPU)(40 μmol·L-1)和茉莉酸甲酯(MeJA)(100 μmol·L-1)溶液噴施浸染pC0390-GUS-proVlCKX5菌液后的煙草葉片,噴施葉片的正反面,用濕潤的脫脂棉包裹住葉柄,放入托盤中正常光照培養24 h。以pC0390-GUS載體為陰性對照,帶有35S啟動子的GUS載體作為陽性對照。將培養好的煙草葉片放進一次性培養皿中,加入GUS染色劑,使葉片完全浸入,放在37 ℃培養箱中過夜培養,其間使用封口膜將容器封閉避免GUS染色液揮發,使用70%乙醇脫色2~3次,至綠色完全脫去,肉眼或顯微鏡下觀察到白色背景上的藍色小點即為GUS表達位點。觀察GUS染色情況并拍照記錄。構建載體所用引物序列為GUS-proVlCKX5-F:TGGGCCCGGCGCGCCAAGCTTG-
GGAGCCACCTTGAGCATCTC,GUS-proVlCKX5-R:GGTGGACTCCTCTTAGAATTCCATGGGTCTA-
GGGAAAGGAGCAG。
1.6 靶向VlCKX5的轉錄因子調控預測
通過CIS-BP數據庫(https://cisbp.ccbr.utoronto.ca/)、JASPAR數據庫(https://jaspar.elixir.no/)和PlantTFDB數據庫(http://planttfdb.gao-lab.org)得到葡萄轉錄因子信息,并使用TB-tools的GTF/GFF3 Sequences Extractor插件提取VlCKX5啟動子上游2000 bp序列,根據PlantTFDB、CIS-BP和JASPAR數據庫公布的轉錄因子結合位點信息,使用Find Individual Motif Occurences(FIMO)在線網站(https://meme-suite.org/meme/tools/fimo)在VlCKX5啟動子上進行TFBS預測,依據10-5截取閾值作為篩選預測結果。通過GENIE3預測轉錄因子與VlCKX5之間可能存在的共表達調控關系,依據weigh>0.1截取閾值作為篩選預測結果。根據GENIE3預測得到的共表達調控關系,通過Gephi0.10軟件將得到的預測信息進行可視化,使用Fruchterman Reingold布局,繪制共表達調控網絡圖。
1.7 亞細胞定位
去除VlAGL6a(Vitvi15g00776)編碼區的終止密碼子后,將其插入到101LYFP載體中,形成101LYFP-VlAGL6a重組質粒。將重組質粒轉化到GV3101中,將核標記物(VirD2NLS-mCherry)與轉化后的菌液混合,轉化到煙葉中瞬時表達。25 ℃下暗處理2 d,正常培養1 d后,用激光共聚焦顯微鏡(奧林巴斯,日本)觀察熒光信號。空白101LYFP作為對照。構建載體所用引物序列為101LYFP-VlAGL6a-F:ATGGGATCTACTAGTGAATTCATG-
GGGAGAGGAAGAGTGGAGC,101LYFP-VlAGL-
6a-R:GGGGGTACCGTCGACGGATCCAAGAACC-
CACCCTTGGATGAAG。
1.8 酵母單雜交(Y1H)
克隆VlCKX5啟動子中VlAGL6a的結合片段P并插入pAbAi載體中,構建重組餌料質粒pAbAi-proVlCKX5/P。將重組誘餌質粒pAbAi-proVlCKX5/P用BstbⅠ線性化,采用PEG/LiAc轉化方法將其整合到酵母菌株Y1H Gold中。轉化后的酵母菌株在SD/-Ura缺陷型培養基上培養3~5 d,將培養基放置于30 ℃培養箱中。通過加入不同濃度的金擔子素A(AbA)的SD/-Ura培養基篩選抗性濃度。隨后,將重組獵物質粒pGADT7-VlAGL6a轉化到陽性酵母菌株(包含誘餌基因組)中,并涂布在SD/-Leu缺陷型培養基上,在30 ℃培養箱中倒置培養3~5 d,以pGADT7空載體轉化到陽性酵母菌株(包含誘餌基因組)中作為對照。在SD/-Leu/AbA培養基上培養共轉化酵母細胞,檢測VlCKX5與VlAGL6a的相互作用。構建載體所用引物序列為pAbAi-proVlCKX5/P-F:TTGAATTCGAGCTCGGTACCC-
CATGACCGAGTCCTCGTTATTTTG,pAbAi-proVlCKX5/P-R:TACAGAGCACATGCCTCGAGGAGCAACACCTAATCCTCCTCTC;pGADT7-VlAGL6a-F:GCCATGGAGGCCAGTGAATTCATGGGGAGAGGAAGAGTGGAGC,pGADT7-VlAGL6a-R:CAG-
CTCGAGCTCGATGGATCCTCAAAGAACCCAC-
CCTTGGATGAAG。
1.9 雙熒光素酶測定
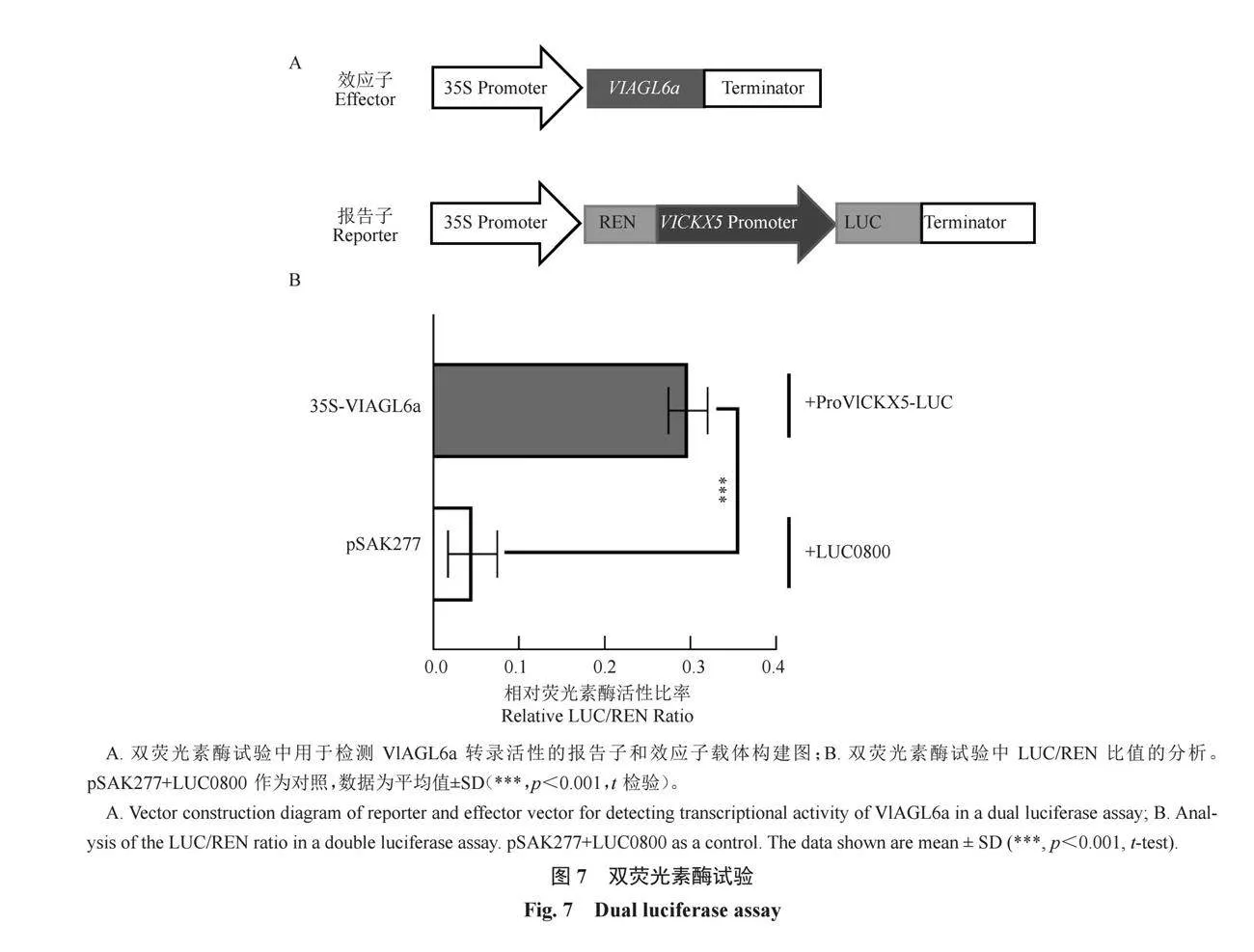
從巨峰葡萄DNA中PCR擴增出VlCKX5的ATG起始密碼子上游1566 bp的DNA序列,并插到pGreenII0800-LUC載體中。所生成的質粒ProVlCKX5-LUC作為報告子。將VlAGL6a的CDS編碼區插入載體pSAK277中,生成作為效應子的35S-VlAGL6a。未插入啟動子的載體pGreenII0800-LUC和未插入VlAGL6a的載體pSAK277作為陰性對照。將效應子和報告子分別導入農桿菌GV3101中,報告質粒與效應質粒以1∶9混合共轉化到本氏煙草葉片中[10]。48 h后使用雙熒光素酶報告基因檢測系統(Promega,美國)測定每個樣品的熒光素酶活性。構建載體所用引物序列為LUC-VlCKX5-F:GTCGACGGTATCGATAAGCTTGGGAGCCACCTTGAGCA-
TCTC,LUC-VlCKX5-R:CGCTCTAGAACTAGTGGATCCCATGGGTCTAGGGAAAGGAGCAG,pSA-
K277-VlAGL6a-F:TAGTGGATCCAAAGAATTCC-
ATGGGGAGAGGAAGAGTGGAGC,pSAK277-Vl-
AGL6a-R:CGAGAAGCTTTTTGAATTCGATCAA-
AGAACCCACCCTTGGATGAAG。
2 結果與分析
2.1 VlCKX5克隆及序列分析
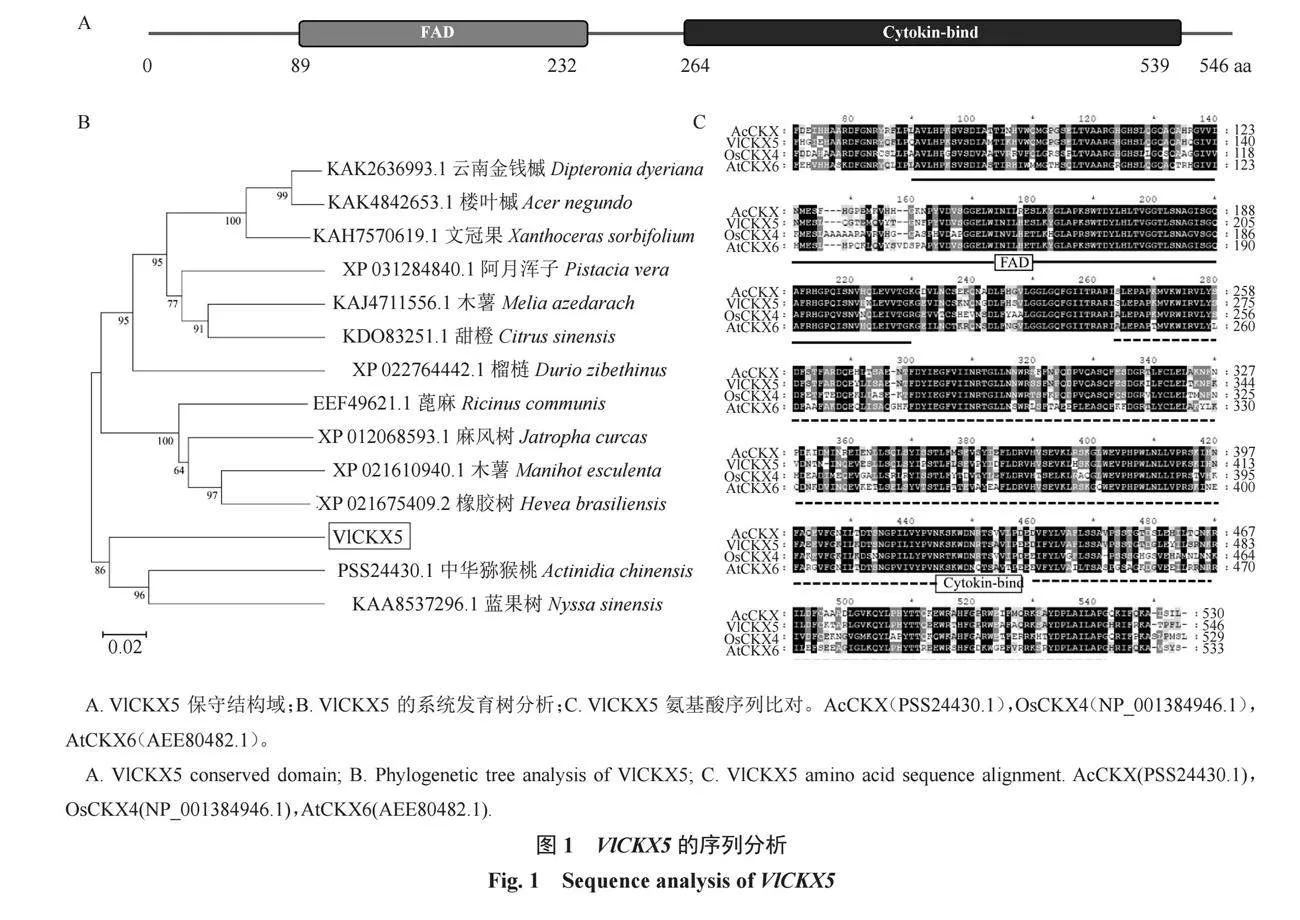
為進一步對VlCKX5的功能進行探究,克隆到VlCKX5(Vitvi13g01614)。VlCKX5的CDS長度為1641 bp,編碼546個氨基酸,分子質量為61.516 62 kDa,等電點為8.36,不穩定系數和脂肪系數分別為36.64和94.27,蛋白質穩定。VlCKX5具有CKXs特征結構域-甲酚甲基羥化酶(PCMH)型黃素腺嘌呤二核苷酸(FAD)結合域和細胞分裂素結合位點(CK-binding)(圖1-A)。系統發育樹顯示,VlCKX5與中華獼猴桃的同源關系最近(圖1-B)。VlCKX5與AcCKX(中華獼猴桃)、OsCKX4(水稻)和AtCKX6(擬南芥)蛋白的多序列比對結果顯示VlCKX5含有FAD和Cytokin-bind的核心保守結構域(圖1-C)。
2.2 VlCKX5基因表達模式分析
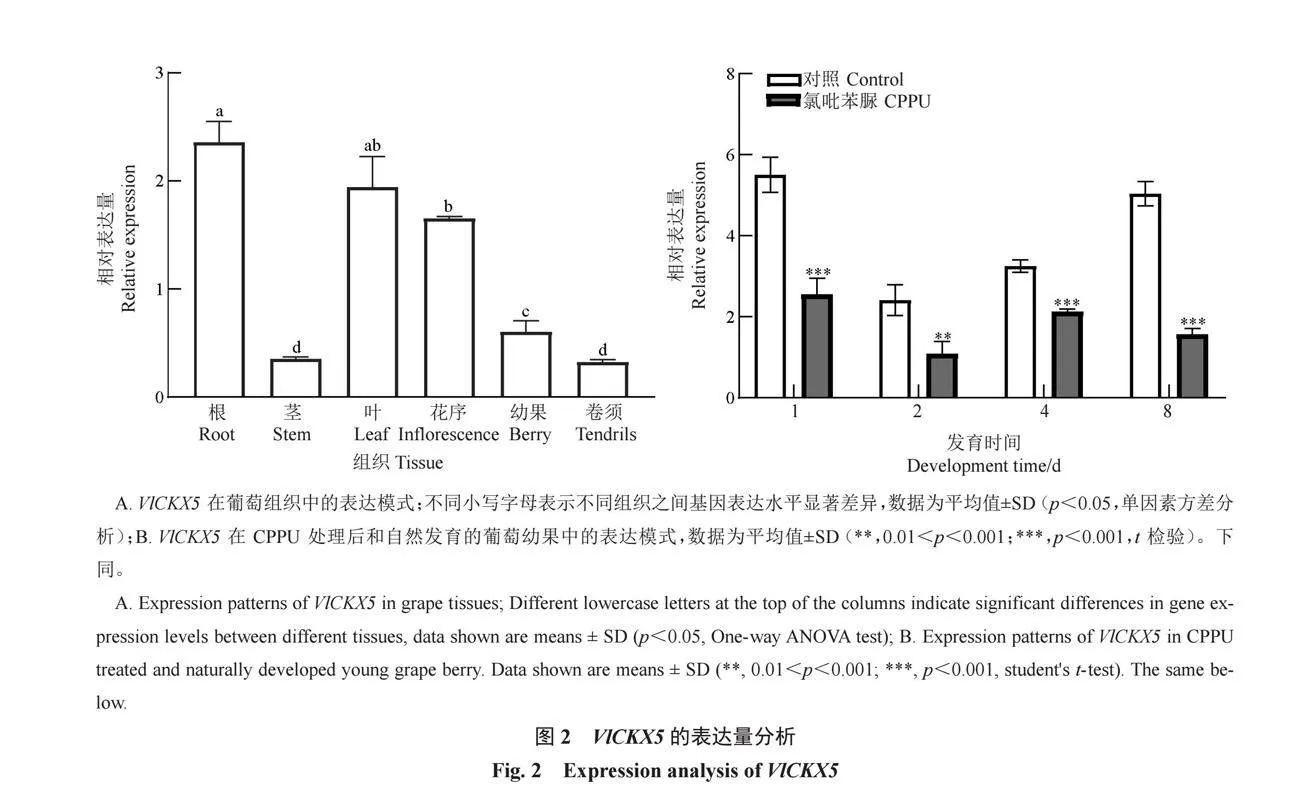
通過RT-qPCR檢測巨峰根、莖、葉、花序、幼果、卷須中VlCKX5的表達量,結果顯示VlCKX5基因在葡萄根和葉中相對表達量最高,其次是在花序中,在莖和卷須中的相對表達量最低(圖2-A)。檢測CPPU處理后不同時期VlCKX5的表達量,VlCKX5在CPPU處理后表達受到顯著抑制,在1、2、4、8 d的表達量顯著降低(圖2-B)。
2.3 VlCKX5啟動子克隆及分析
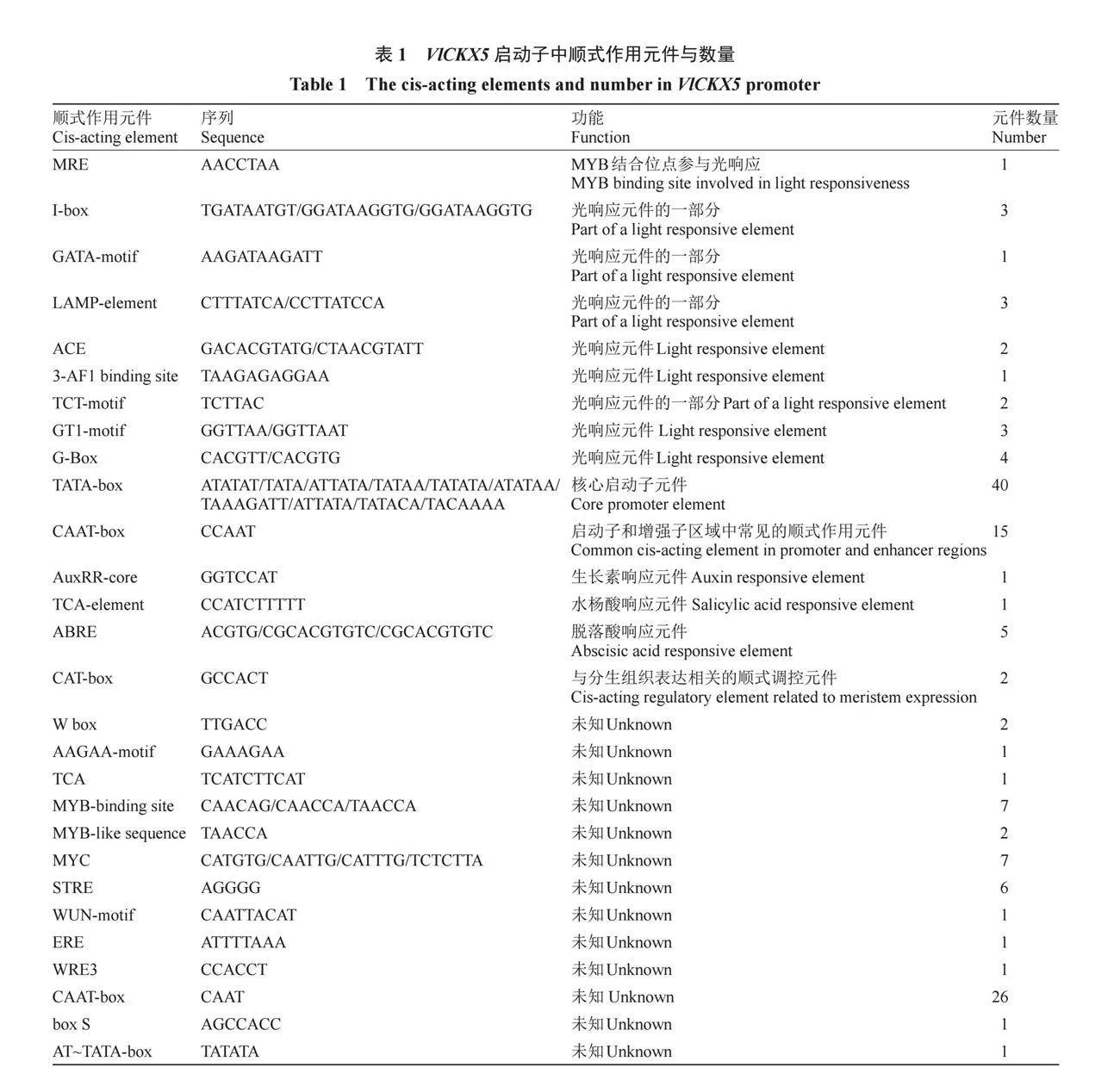
對VlCKX5啟動子序列的順式作用元件進行預測,結果顯示VlCKX5啟動子區域除了啟動子和增強子區域中常見的順式作用元件CAAT-box和核心啟動子元件TATA-box外,還包括光、分生組織表達和激素的響應元件(表1)。在VlCKX5的啟動子區域發現生長素、水楊酸和脫落酸的植物激素響應元件(圖3-A)。克隆VlCKX5的5′端上游1566 bp序列并進行GUS染色試驗,與陰性對照相比,Mock處理的煙草葉片明顯呈藍色,ProVlCKX5顯著增強了GUS基因的活性。此外,VlCKX5啟動子對IBA、SA、ABA和CPPU均表現出激素應答信號,其中IBA、SA、ABA和CPPU處理與Mock處理的葉片顏色相比,表現出更深的藍色,MeJA處理與Mock處理的葉片顏色相比沒有明顯的差別,對VlCKX5啟動子激活效果不明顯(圖3-B)。
2.4 靶向調控VlCKX5的轉錄因子預測及分析
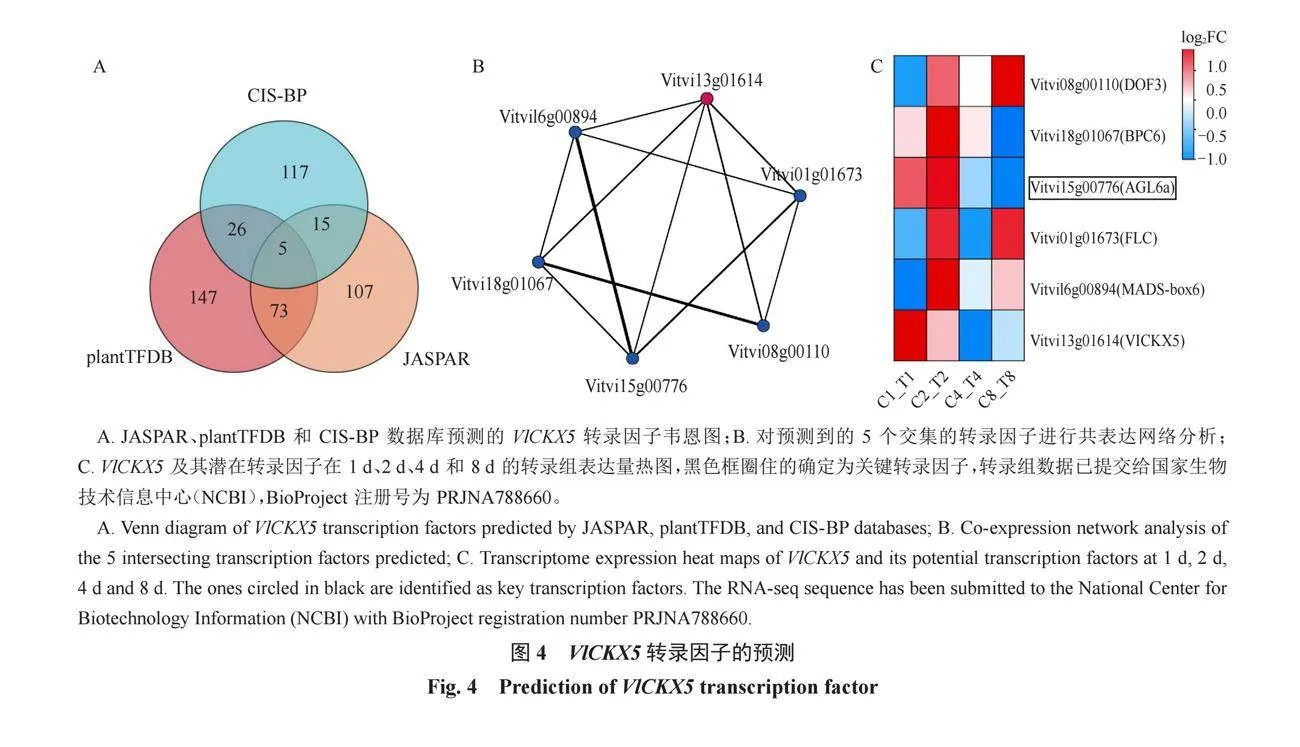
預測了可能靶向VlCKX5的轉錄因子,在PlantTFDB、CIS-BP和JASPAR數據庫中發現了共同靶向VlCKX5的5個轉錄因子(圖4-A)。通過Gephi0.10軟件對這5個轉錄因子以及VlCKX5之間的共表達網絡關系進行可視化,結果可以看出VlCKX5和這5個轉錄因子之間都存在靶向關系(圖4-B)。對轉錄因子進行功能注釋發現這5個轉錄因子屬于BPC、DOF、MADS和FLC家族,結合VlCKX5的表達量分析和轉錄因子熱圖(圖2-B,圖4-C),挑選出與VlCKX5表達趨勢同為下調的AGL6a作為關鍵轉錄因子。
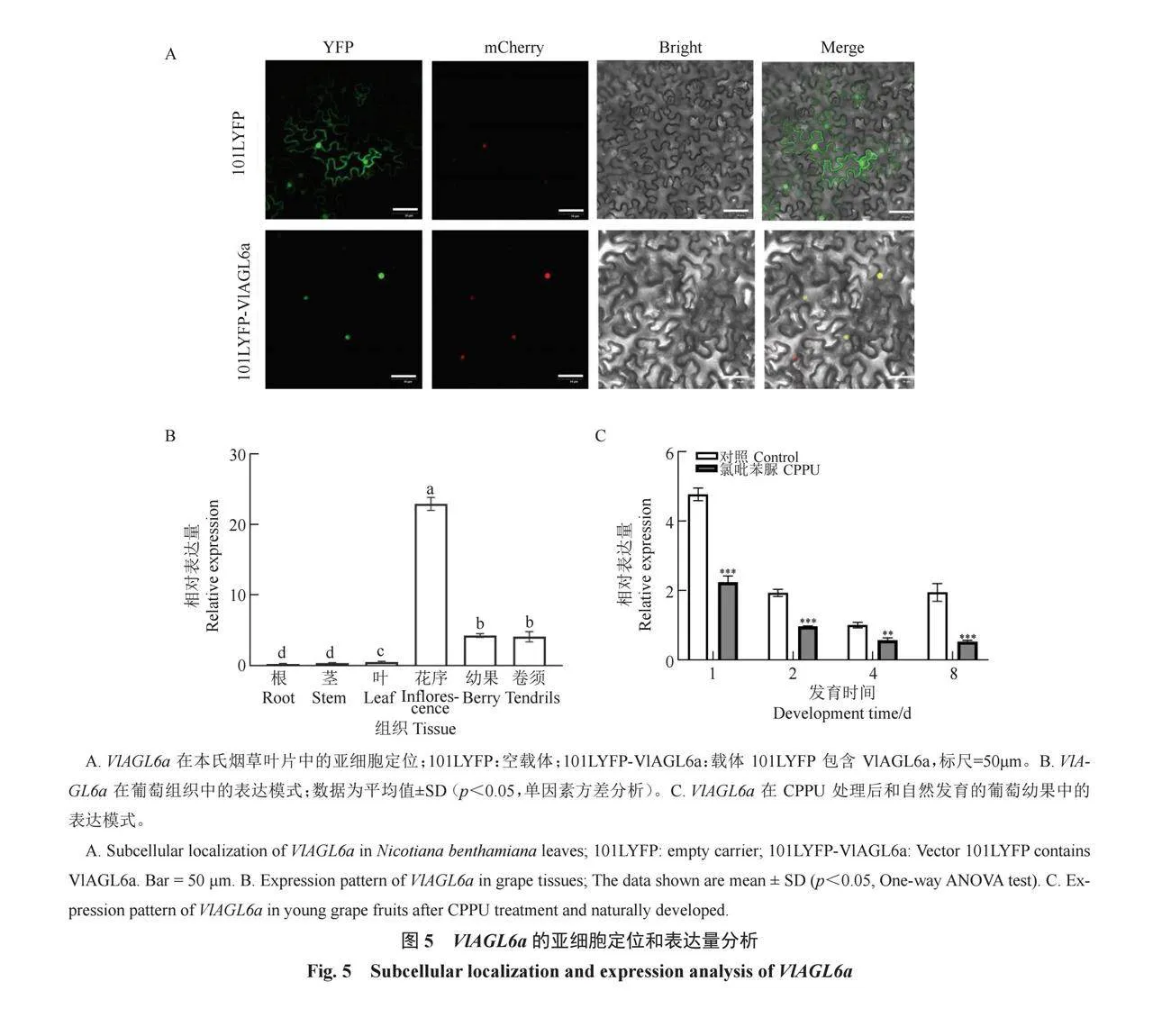
2.5 VlAGL6a的亞細胞定位及RT-qPCR
亞細胞定位試驗結果顯示,101LYFP-VlAGL6a標記的熒光與mcherry標記的熒光在細胞核內共定位(圖5-A)。RT-qPCR結果顯示,在巨峰葡萄的根、莖、葉、花序、幼果和卷須組織的表達模式中,VlAGL6a在花序中相對表達量最高,其次是在果實和卷須中,在根、莖和葉中的相對表達量最低(圖5-B)。CPPU處理后,VlAGL6a在1、2、4和8 d的表達量顯著降低(圖5-C)。
2.6 VlAGL6a與VlCKX5相互作用
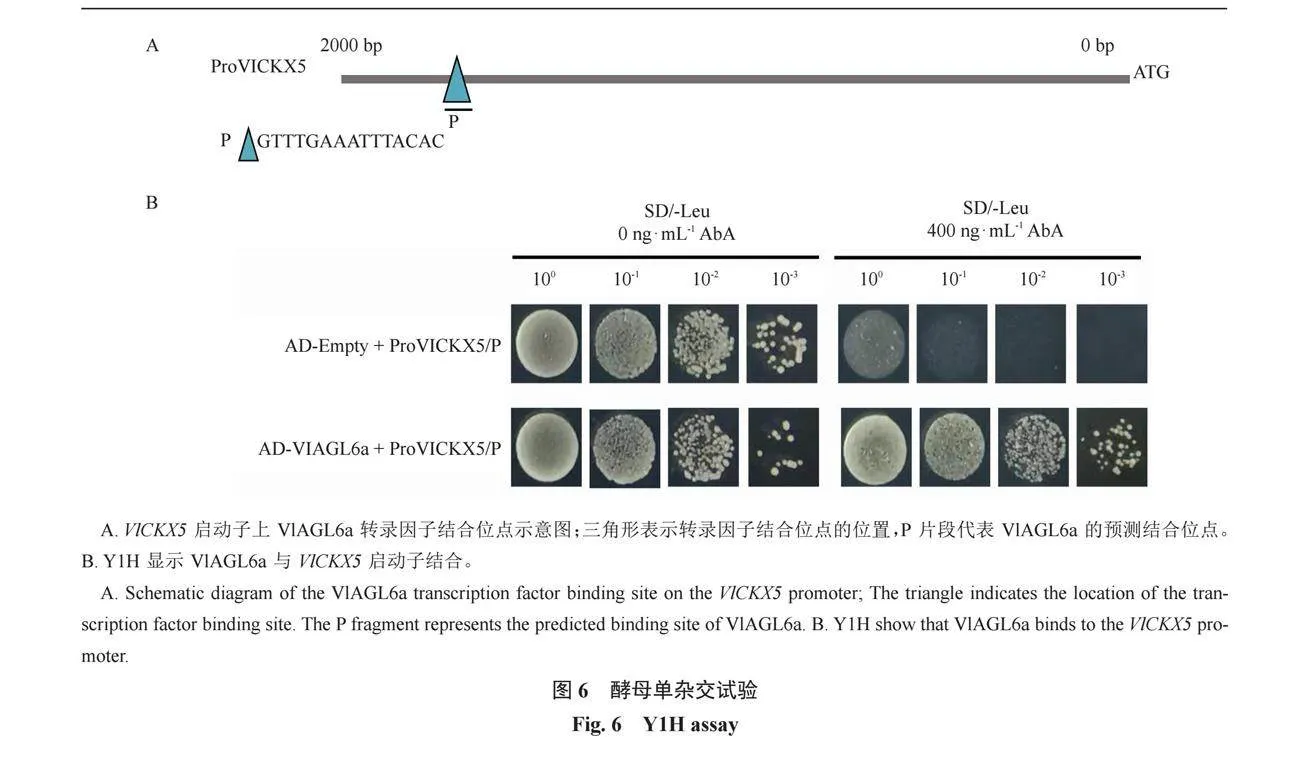
在VlCKX5啟動子區域預測出VlAGL6a的結合位點,標記結合位點片段為P(圖6-A)。利用酵母單雜交技術,將P片段插入pAbAi載體并作為誘餌導入Y1HGold酵母菌株中。并將重組獵物質粒pGADT7-VlAGL6a轉化到陽性酵母菌株(包含誘餌基因)中,以驗證VlCKX5與VlAGL6a的相互作用。結果顯示只有同時含有pGADT7-VlAGL6a和pAbAi-proVlCKX5/P的酵母菌落才可以在濃度為400 ng·mL-1的AbA篩選培養基上正常生長,而pGADT7-VlAGL6a和pAbAi-VlCKX5/P的酵母菌落400 ng·mL-1的AbA篩選培養基上不能正常生長(圖6-B)。
為了進一步研究VlAGL6a在調控VlCKX5表達中的功能,進行了雙熒光素酶試驗。構建了雙熒光素酶載體(圖7-A)。設計了一個實驗組:ProVlCKX5-LUC+35S-VlAGL6a,以及一個空白對照組:LUC0800+pSAK277。在煙草葉片中注入等量菌液瞬時表達,用LUC/REN比值計算熒光素酶的相對活性。結果顯示,與對照組相比,35S-VlAGL6a試驗組的LUC/REN比率顯著增加(圖7-B)。酵母單雜交和雙熒光素酶試驗證明了VlAGL6a可以與VlCKX5啟動子內P段基序特異性結合,且VlAGL6a作為VlCKX5的正調控轉錄因子,促進了VlCKX5基因的表達。
3 討 論
細胞分裂素是影響植物生長發育的一類重要激素,可以促進細胞分裂、調控根生長、延緩葉片衰老和調節作物產量等[32],CKXs可以通過切割不飽和的類異戊二烯側鏈促進生物活性細胞分裂素的不可逆降解。筆者在本研究中對VlCKX5進行生物信息學分析的結果顯示,VlCKX5具有黃素腺嘌呤二核苷酸(FAD)結合域和細胞分裂素結合位點(CK-binding),這與谷子[33]、白菜[34]、鴿豆[35]等CKX基因的研究結果相同,完全符合CKX基因的結構特征。VlCKX5基因的特異性表達分析顯示,VlCKX5在巨峰葡萄的根、莖、葉、花序、果實和卷須中均能表達,其中VlCKX5基因在根和葉中相對表達量較高,在花序中的表達量次之,在莖和卷須中的表達量最低。VlCKX5的表達具有組織特異性,這說明VlCKX5可能在葡萄的營養生長和生殖生長過程中都發揮著重要作用。與此一致的是,在水稻中,OsCKX基因家族成員也表現出組織特異性的表達模式,其中OsCKX4在根部表達顯著,OsCKX9在葉片和腋芽中表達量較高,而OsCKX5則在所有檢測的組織中均表現出高水平的表達,ckx4和ckx9雙突變體會影響水稻的分蘗數和穗長[36]。在本試驗中,經CPPU處理后,VlCKX5的表達量在1 d、2 d、4 d和8 d時均顯著降低,說明CPPU處理抑制了VlCKX5基因的表達,有研究發現CPPU可以抑制CKX的活性,是提高作物生產力的農用制劑[37]。CKX基因對調控植物產量發揮重要功能[38-39]。在大麥中沉默HvCKX1基因可降低細胞分裂素氧化酶/脫氫酶水平,提高植株產量[40]。在水稻中,降低OsCKX2的表達量會提高細胞分裂素在花序分生組織中積累從而提高籽粒產量[41]。在筆者課題組前期的研究中發現CPPU處理降低了葡萄內源細胞分裂素含量并促進葡萄坐果[30],在擬南芥中過表達葡萄VlCKX4基因可促進擬南芥坐果使角果數增多[42]。因此推測VlCKX5基因可能通過提高細胞分裂素水平促進葡萄坐果,從而提高葡萄產量。
在基因表達的過程中,啟動子是決定基因是否被轉錄以及轉錄效率的關鍵因素之一。本研究中對VlCKX5啟動子序列的順式作用元件進行預測,結果顯示VlCKX5啟動子區域的順式作用元件包含多種與生長素、水楊酸和脫落酸相關的響應元件。GUS染色試驗結果顯示,IBA、SA、ABA和CPPU激素的處理都增強了VlCKX5啟動子的活性,IBA、SA、ABA和CPPU激素可能串擾調控VlCKX5的表達,影響葡萄的生長發育。CKX可能是赤霉素/細胞分裂素串擾的一個重要環節[43]。BjuCKX基因具有生長素、脫落酸、茉莉酸甲酯、乙烯和水楊酸的激素響應元件,可以不同程度應答激素[44]。細胞分裂素與脫落酸密切串擾,細胞分裂素水平及其信號轉導的調節影響脫落酸依賴和脫落酸不依賴的途徑,使植物適應不利條件[45]。綜上所述,VlCKX5可能受IBA、SA、ABA和CPPU激素的串擾影響參與到多種激素通路中發揮作用。
MADS-box基因編碼高度保守的DNA結合轉錄因子,可調控花和果實發育過程[46]。有研究發現MADS-box基因的表達模式與指定功能之間存在密切的相關性[47],而在本研究中VlAGL6a在葡萄花序中的相對表達量最高,其次是在果實和卷須中,在根、莖和葉組織中表達量極低,VlAGL6a可能在葡萄的生殖生長中發揮重要作用。與此一致的是,AcMADS1啟動子在番茄和擬南芥的花器官和果實發育中的高水平表達可能促進花發育和果實成熟[48],PeMADS5基因在花中高水平表達促進了竹子開花[49],TaAGL6在花器官開始分化時的穗中高表達,過表達TaAGL6會導致小麥小穗數和穗粒數增多[50]。酵母單雜交和雙熒光素酶分析結果顯示MADS-box轉錄因子VlAGL6a可以靶向VlCKX5促進其表達。有研究表明MADS家族的FUL2和MBP20可以抑制CKX5/6/8基因的表達,從而促進花分生組織中的細胞分裂素信號轉導,促進植物向生殖生長過渡,進而抑制番茄花序分枝[51]。TaMADS-GS蛋白會抑制TaCKX基因的表達使小麥籽粒發育早期的細胞分裂素含量維持在正常水平,促使籽粒正常發育[52]。因此推測VlAGL6a可以靶向VlCKX5通過調控細胞分裂素水平影響葡萄坐果。
4 結 論
VlCKX5基因編碼546個氨基酸,具有FAD和CK-bind的典型CKXs家族保守結構域。VlCKX5具有組織表達特異性,在根和葉中高表達,其次是花序中。CPPU處理后VlCKX5的表達量顯著降低。外源CPPU處理可以激活VlCKX5啟動子活性。轉錄因子VlAGL6a定位在細胞核中,在花序中高表達。CPPU處理后VlAGL6a的相對表達量顯著下調,與VlCKX5的表達趨勢一致。進一步研究證明,VlAGL6a可以與VlCKX5相互作用并促進VlCKX5的表達,因此,VlAGL6a通過靶向VlCKX5調控細胞分裂素水平而影響葡萄坐果,為CPPU激素調控葡萄坐果提供了理論依據。
參考文獻 References:
[1] SABRA A,NETTICADAN T,WIJEKOON C. Grape bioactive molecules,and the potential health benefits in reducing the risk of heart diseases[J]. Food Chemistry:X,2021,12:100149.
[2] ZHOU D D,LI J H,XIONG R G,SAIMAITI A,HUANG S Y,WU S X,YANG Z J,SHANG A,ZHAO C N,GAN R Y,LI H B. Bioactive compounds,health benefits and food applications of grape[J]. Foods,2022,11(18):2755.
[3] B?TTCHER C,DAVIES C. Hormonal control of grape berry development and ripening[M]. Sharjah:The Bentham Science Publishers Ltd.,2012:194-217.
[4] RIMPIKA,JAIN S,RATHOD M,BANJARE R,NIDHI N,SOOD A,SHILPA,SHARMA R. Physiological aspects of flowering,fruit setting,fruit development and fruit drop,regulation and their manipulation:A review[J]. International Journal of Environment and Climate Change,2023,13(12):205-224.
[5] CROSBY K E,AUNG L H,BUSS G R. Influence of 6-benzylaminopurine on fruit-set and seed development in two soybean,Glycine max (L.) Merr. genotypes[J]. Plant Physiology,1981,68(5):985-988.
[6] CLIFFORD P E. Control of reproductive sink yield in mung beans[J]. Zeitschrift Für Pflanzenphysiologie,1981,102(2):173-181.
[7] ZU?IGA-MAYO V M,BA?OS-BAYARDO C R,DíAZ-RAMíREZ D,MARSCH-MARTíNEZ N,DE FOLTER S. Conserved and novel responses to cytokinin treatments during flower and fruit development in Brassica napus and Arabidopsis thaliana[J]. Scientific Reports,2018,8(1):6836.
[8] MOSTAFA L Y,MOSTAFA Y S,EL-BERRY I M.Effect of NAA and CPPU on fruit drop,yield and quality of avocado trees[J]. Egyptian Journal of Horticulture,2020,47(2):137-147.
[9] YU Y H,LI X F,YANG S D,BIAN L,YU K K,MENG X X,LIU H N,PEI M S,WEI T L,GUO D L. CPPU-induced changes in energy status and respiration metabolism of grape young berry development in relation to berry setting[J]. Scientia Horticulturae,2021,283:110084.
[10] LIU Y,LI Y,GUO H X,LV B S,FENG J,WANG H H,ZHANG Z H,CHAI S. Gibberellin biosynthesis is required for CPPU-induced parthenocarpy in melon[J]. Horticulture Research,2023,10(6):uhad084.
[11] WANG C N,WANG H,ZHU H,JI W K,HOU Y L,MENG Y Y,WEN J Q,MYSORE K S,LI X S,LIN H. Genome-wide identification and characterization of cytokinin oxidase/dehydrogenase family genes in Medicago truncatula[J]. Journal of Plant Physiology,2021,256:153308.
[12] 王澤琛,肖榮,歐陽樂軍,李莉梅,梁楚炎,潘璟茵,劉智超. 基于CRISPR/Cas9的擬南芥CKX3基因編輯載體構建及轉化研究[J]. 植物研究,2021,41(6):1015-1022.
WANG Zechen,XIAO Rong,OUYANG Lejun,LI Limei,LIANG Chuyan,PAN Jingyin,LIU Zhichao. Construction and transformation of Arabidopsis CKX3 gene editing vector based on CRISPR/Cas9[J]. Bulletin of Botanical Research,2021,41(6):1015-1022.
[13] LIU Y,WANG X,WANG X F,GAO W S,YOU C X. Identification and functional characterization of apple MdCKX5.2 in root development and abiotic stress tolerance[J]. Horticulturae,2022,8(1):62.
[14] LI S X,AN Y R,HAILATI S,ZHANG J,CAO Y M,LIU Y S,GENG J C,HU T M,YANG P Z. Overexpression of the cytokinin oxidase/dehydrogenase (CKX) from Medicago sativa enhanced salt stress tolerance of Arabidopsis[J]. Journal of Plant Biology,2019,62(5):374-386.
[15] TANG D,LI Y J,ZHAI L M,LI W,KUMAR R,YER H,DUAN H,CHENG B P,DENG Z N,LI Y. Root predominant overexpression of iaaM and CKX genes promotes root initiation and biomass production in citrus[J]. Plant Cell,Tissue and Organ Culture,2023,155(1):103-115.
[16] ZHAO X,ZHAO Z X,CHENG S S,WANG L H,LUO Z,AI C F,LIU Z G,LIU P,WANG L L,WANG J R,LIU M Z,LI Y,LIU M J. ZjWRKY23 and ZjWRKY40 promote fruit size enlargement by targeting and downregulating Cytokinin oxidase/dehydrogenase 5 expression in Chinese jujube[J]. Journal of Agricultural and Food Chemistry,2023,71(46):18046-18058.
[17] ZENG J Y,YAN X Y,BAI W Q,ZHANG M,CHEN Y,LI X B,HOU L,ZHAO J,DING X Y,LIU R C,WANG F L,REN H,ZHANG J Y,DING B,LIU H R,XIAO Y H,PEI Y. Carpel-specific down-regulation of GhCKXs in cotton significantly enhances seed and fiber yield[J]. Journal of Experimental Botany,2022,73(19):6758-6772.
[18] ZHANG W,PENG K X,CUI F B,WANG D L,ZHAO J Z,ZHANG Y J,YU N N,WANG Y Y,ZENG D L,WANG Y H,CHENG Z K,ZHANG K W. Cytokinin oxidase/dehydrogenase OsCKX11 coordinates source and sink relationship in rice by simultaneous regulation of leaf senescence and grain number[J]. Plant Biotechnology Journal,2021,19(2):335-350.
[19] JOSHI R,SAHOO K K,TRIPATHI A K,KUMAR R,GUPTA B K,PAREEK A,SINGLA-PAREEK S L. Knockdown of an inflorescence meristem-specific cytokinin oxidase - OsCKX2 in rice reduces yield penalty under salinity stress condition[J]. Plant,Cell amp; Environment,2018,41(5):936-946.
[20] SCHWARZ I,SCHEIRLINCK M T,OTTO E,BARTRINA I,SCHMIDT R C,SCHMüLLING T. Cytokinin regulates the activity of the inflorescence meristem and components of seed yield in oilseed rape[J]. Journal of Experimental Botany,2020,71(22):7146-7159.
[21] CHENG Y Z,SUN Y D,PEI M S,LIU H N,WEI T L,GUO D L. Transcription factor VviAGL6a regulates fruit ripening by directly activating grape VviJMJ21[J]. Scientia Horticulturae,2024,336:113396.
[22] ZHANG L M,ZHAO J,FENG C F,LIU M J,WANG J R,HU Y F. Genome-wide identification,characterization of the MADS-box gene family in Chinese jujube and their involvement in flower development[J]. Scientific Reports,2017,7(1):1025.
[23] PARENICOVá L,DE FOLTER S,KIEFFER M,HORNER D S,FAVALLI C,BUSSCHER J,COOK H E,INGRAM R M,KATER M M,DAVIES B,ANGENENT G C,COLOMBO L. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis:New openings to the MADS world[J]. The Plant Cell,2003,15(7):1538-1551.
[24] 付學森,劉紫璇,王玲,龍雨青,曾娟,周日寶,劉湘丹. 灰氈毛忍冬MADS-box基因家族鑒定與CMB1基因克隆[J]. 湖南中醫藥大學學報,2024,44(3):383-394.
FU Xuesen,LIU Zixuan,WANG Ling,LONG Yuqing,ZENG Juan,ZHOU Ribao,LIU Xiangdan. Identification of the MADS-box gene family and cloning of the CMB1 gene in Lonicera macranthoides[J]. Journal of Hunan University of Chinese Medicine,2024,44(3):383-394.
[25] SCHILLING S,KENNEDY A,PAN S R,JERMIIN L S,MELZER R. Genome-wide analysis of MIKC-type MADS-box genes in wheat:pervasive duplications,functional conservation and putative neofunctionalization[J]. New Phytologist,2020,225(1):511-529.
[26] SUN X M,ZHANG S L,LI X M,ZHANG X M,WANG X H,WANG L,LI Z,WANG X P. A MADS-box transcription factor from grapevine,VvMADS45,influences seed development[J]. Plant Cell,Tissue and Organ Culture,2020,141(1):105-118.
[27] YOO S K,WU X L,LEE J S,AHN J H. AGAMOUS-LIKE 6 is a floral promoter that negatively regulates the FLC/MAF clade genes and positively regulates FT in Arabidopsis[J]. The Plant Journal,2011,65(1):62-76.
[28] PI M T,HU S Q,CHENG L C,ZHONG R H,CAI Z Y,LIU Z C,YAO J L,KANG C Y. The MADS-box gene FveSEP3 plays essential roles in flower organogenesis and fruit development in woodland strawberry[J]. Horticulture Research,2021,8(1):247.
[29] LI G,KUIJER H N J,YANG X J,LIU H R,SHEN C Q,SHI J,BETTS N,TUCKER M R,LIANG W Q,WAUGH R,BURTON R A,ZHANG D B. MADS1 maintains barley spike morphology at high ambient temperatures[J]. Nature Plants,2021,7(8):1093-1107.
[30] WANG L L,SHI Q F,JING P W,WANG R X,ZHANG H M,LIU Y T,LI C Y,SHI T Z,ZHANG L X,YU Y H. VlMYB4 and VlCDF3 co-targeted the VlLOG11 promoter to regulate fruit setting in grape (Vitis vinifera L.)[J]. Plant Cell Reports,2024,43(8):194.
[31] SUN Y D,YUE Y H,LI X F,LI S Q,SHI Q F,YU Y H. Transcription factor VviWOX13C regulates fruit set by directly activating VviEXPA37/38/39 in grape (Vitis vinifera L.)[J]. Plant Cell Reports,2023,43(1):19.
[32] LI S M,ZHENG H X,ZHANG X S,SUI N. Cytokinins as central regulators during plant growth and stress response[J]. Plant Cell Reports,2021,40(2):271-282.
[33] WANG Y G,LIU H H,XIN Q G. Genome-wide analysis and identification of cytokinin oxidase/dehydrogenase (CKX) gene family in foxtail millet (Setaria italica)[J]. The Crop Journal,2014,2(4):244-254.
[34] LIU Z N,LV Y X,ZHANG M,LIU Y P,KONG L J,ZOU M H,LU G,CAO J S,YU X L. Identification,expression,and comparative genomic analysis of the IPT and CKX gene families in Chinese cabbage (Brassica rapa ssp. pekinensis)[J]. BMC Genomics,2013,14:594.
[35] SHARMA S,ARPITA K,NIRGUDE M,SRIVASTAVA H,KUMAR K,SREEVATHSA R,BHATTACHARYA R,GAIKWAD K. Genomic insights into cytokinin oxidase/dehydrogenase (CKX) gene family,identification,phylogeny and synteny analysis for its possible role in regulating seed number in Pigeonpea [Cajanus cajan (L.) Millsp.][J]. International Journal of Biological Macromolecules,2024,277:134194.
[36] RONG C Y,LIU Y X,CHANG Z Y,LIU Z Y,DING Y F,DING C Q. Cytokinin oxidase/dehydrogenase family genes exhibit functional divergence and overlap in rice growth and development,especially in control of tillering[J]. Journal of Experimental Botany,2022,73(11):3552-3568.
[37] KHABLAK S H,SPIVAK S I,PASTUKHOVA N L,YEMETS A I,BLUME Y B. Cytokinin oxidase/dehydrogenase as an important target for increasing plant productivity[J]. Cytology and Genetics,2024,58(2):115-125.
[38] CHEN L,ZHAO J Q,SONG J C,JAMESON P E. Cytokinin dehydrogenase:A genetic target for yield improvement in wheat[J]. Plant Biotechnology Journal,2020,18(3):614-630.
[39] SHARMA A,PRAKASH S,CHATTOPADHYAY D. Killing two birds with a single stone-genetic manipulation of cytokinin oxidase/dehydrogenase (CKX) genes for enhancing crop productivity and amelioration of drought stress response[J]. Frontiers in Genetics,2022,13:941595.
[40] ZALEWSKI W,GALUSZKA P,GASPARIS S,ORCZYK W,NADOLSKA-ORCZYK A. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity[J]. Journal of Experimental Botany,2010,61(6):1839-1851.
[41] ASHIKARI M,SAKAKIBARA H,LIN S Y,YAMAMOTO T,TAKASHI T,NISHIMURA A,ANGELES E R,QIAN Q,KITANO H,MATSUOKA M. Cytokinin oxidase regulates rice grain production[J]. Science,2005,309(5735):741-745.
[42] SHI Q F,LI X F,YANG S D,ZHAO X C,YUE Y H,YANG Y J,YU Y H. Dynamic temporal transcriptome analysis reveals grape VlMYB59- VlCKX4 regulatory module controls fruit set[J]. Horticulture Research,2024,11(9):uhae183.
[43] TODOROVA D,VASEVA I,MALBECK J,TRáVNí?KOVá A,MACHá?KOVá I,KARANOV E. Cytokinin oxidase/dehydrogenase activity as a tool in gibberellic acid/cytokinin cross talk[J]. Biologia Plantarum,2007,51(3):579-583.
[44] LI M Y,ZHOU J,GONG L,ZHANG R,WANG Y,WANG C,DU X M,LUO Y,ZHANG Y,WANG X R,TANG H R. Identification and expression analysis of CKX gene family in Brassica juncea var. tumida and their functional analysis in stem development[J]. Horticulturae,2022,8(8):705.
[45] HA S,VANKOVA R,YAMAGUCHI-SHINOZAKI K,SHINOZAKI K,TRAN L S P. Cytokinins:Metabolism and function in plant adaptation to environmental stresses[J]. Trends in Plant Science,2012,17(3):172-179.
[46] GRIMPLET J,MARTíNEZ-ZAPATER J M,CARMONA M J. Structural and functional annotation of the MADS-box transcription factor family in grapevine[J]. BMC Genomics,2016,17:80.
[47] ALVAREZ-BUYLLA E R,LILJEGREN S J,PELAZ S,GOLD S E,BURGEFF C,DITTA G S,VERGARA-SILVA F,YANOFSKY M F. MADS-box gene evolution beyond flowers:Expression in pollen,endosperm,guard cells,roots and trichomes[J]. Plant Journal,2000,24(4):457-466.
[48] MOYLE R L,KOIA J H,VREBALOV J,GIOVANNONI J,BOTELLA J R. The pineapple AcMADS1 promoter confers high level expression in tomato and Arabidopsis flowering and fruiting tissues,but AcMADS1 does not complement the tomato LeMADS-RIN (rin) mutant[J]. Plant Molecular Biology,2014,86(4/5):395-407.
[49] ZHANG Y T,TANG D Q,LIN X C,DING M Q,TONG Z K. Genome-wide identification of MADS-box family genes in moso bamboo (Phyllostachys edulis) and a functional analysis of PeMADS5 in flowering[J]. BMC Plant Biology,2018,18(1):176.
[50] KONG X C,WANG F,GENG S F,GUAN J T,TAO S,JIA M L,SUN G L,WANG Z Y,WANG K,YE X G,MA J,LIU D C,WEI Y M,ZHENG Y L,FU X D,MAO L,LAN X J,LI A L. The wheat AGL6-like MADS-box gene is a master regulator for floral organ identity and a target for spikelet meristem development manipulation[J]. Plant Biotechnology Journal,2022,20(1):75-88.
[51] JIANG X B,LUBINI G,HERNANDES-LOPES J,RIJNSBURGER K,VELTKAMP V,DE MAAGD R A,ANGENENT G C,BEMER M. FRUITFULL-like genes regulate flowering time and inflorescence architecture in tomato[J]. The Plant Cell,2022,34(3):1002-1019.
[52] ZHANG J N,ZHANG Z H,ZHANG R J,YANG C F,ZHANG X B,CHANG S Y,CHEN Q,ROSSI V,ZHAO L,XIAO J,XIN M M,DU J K,GUO W L,HU Z R,LIU J,PENG H R,NI Z F,SUN Q X,YAO Y Y. Type I MADS-box transcription factor TaMADS-GS regulates grain size by stabilizing cytokinin signalling during endosperm cellularization in wheat[J]. Plant Biotechnology Journal,2024,22(1):200-215.

