李PsNAC基因家族鑒定及其在空腔褐變果實中的表達模式分析
摘 " "要:【目的】NAC轉錄因子廣泛參與植物生長發育和應對逆境脅迫過程,被認為是植物次生細胞壁生物合成轉錄調控的一級開關。鑒定李PsNAC轉錄因子家族,探索其與皇冠李果實空腔褐變的關系。【方法】采用生物信息學方法,分析李PsNAC家族成員、理化性質、系統發育和基因結構等,并通過qRT-PCR技術,分析PsNACs在空腔褐變皇冠李果實中的表達模式。【結果】李PsNACs包含115個成員,不均勻地分布在8條染色體上,可分為17個亞族,與植物次生細胞壁合成相關的OsNAC003和OsNAC7亞族分別含6和10個PsNACs。PsNACs啟動子區域含有豐富的激素響應元件和MYB結合位點。10個PsNACs在皇冠李空腔褐變果實中的表達量均高于非空腔褐變果實。【結論】本結果為研究PsNAC家族成員與皇冠李果實空腔褐變的具體關聯性奠定了重要基礎。
關鍵詞:皇冠李;NAC轉錄因子;基因家族;空腔褐變;基因表達量
中圖分類號:S662.3 文獻標志碼:A 文章編號:1009-9980(2025)01-0048-15
Identification of plum PsNAC gene family and its expression patterns in development of fruit hollowness and browning
DENG Honghong, PENG Chao#, LIANG Xi, ZHANG Ziyang, LIU Junwei, LI Binqi, WEI Mingkang, WANG Xueying, LI Liumin, CHEN Faxing*
(College of Horticulture, Fujian Agriculture and Forestry University, Fuzhou 350002, Fujian, China)
Abstract: 【Objective】 The NAC transcription factor family is one of the plant-specific transcription factor families and plays a pivotal role in plant growth and development and responses to biotic and abiotic stresses. Huangguan plum is a newly developed high-quality plum variety bred by our team specifically suited for cultivation in Fujian Province, China. Its fruit has the advantages of excellent taste, pleasant flavor, and rich nutrient profile. However, in our previous long-term observations, Huangguan plum has been found to be prone to fruit hollowness and browning (HB), characterized by rough and crystalline fruit pulp surfaces undergoing lignification and browning. We found that lignin biosynthesis and accumulation is one of the predominant biochemical responses to HB. The NAC transcription factor is recognized as the primary regulator in the transcriptional control of plant secondary wall synthesis. This study aims to characterize the PsNAC gene family members in plum and investigate their association with fruit HB in Huangguan plum. 【Methods】 The molecular weight, theoretical isoelectric point, and other physicochemical properties were predicted by the online tool ExPASy. The subcellular localization of PsNACs was predicted by the online software WoLF PSORT. The MEGA 11 software was used to construct a phylogenetic tree. The online tool Simple MEME Wrapper was used to analyze the motifs. The conserved motifs and gene structure maps were drawn by Tbtools. To analyze the cis-acting elements in the promoter region of PsNACs, the upstream 2000 bp promoter sequences were extracted from the genomic sequences and submitted to the Plant TBDB website for the identification of cis-elements in the promoter region. The analysis results were organized and displayed using Simple BioSequence Viewer. The expression of ten PsNACs in Huangguan fruit with and without HB was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). 【Results】 There are 115 PsNAC members identified in Prunus salicina Lindl., with protein sequences ranging from 182 to 861 amino acids, molecular weights from 20.98 to 95.97 ku, theoretical isoelectric points from 4.43 to 9.55, and the instability index from 27.84 (PsNAC60) to 61.36 (PsNAC087). The grand average of hydropathy values of PsNAC gene family members were negative, indicating that these proteins are hydrophilic in nature. Transmembrane structure analysis revealed that 94% of PsNAC gene family members do not possess a transmembrane domain. The subcellular localization prediction results showed that 91 PsNAC gene family members were located in the nucleus, and the rest were distributed in structures such as the cytoplasm, Golgi apparatus, peroxisome, cytoskeleton, mitochondria, chloroplasts, plasma membrane, and vacuole. Chromosomal localization analysis revealed uneven distribution across the plum’s eight chromosomes, with chromosomes 2 and 3 harboring the highest number counts (17.4%), followed by chromosome 5 (15.2%), and the fewest on chromosomes 6 and 7 (10 PsNACs each). Phylogenetic tree analysis between Arabidopsis thaliana and P. salicina Lindl. classified PsNAC genes into 17 subfamilies, with 6 and 10 members clustered in OsNAC003, and OsNAC7, respectively, which are associated with plant secondary wall biosynthesis. The number of coding sequence segments in PsNACs ranged from 1 to 8, with most containing 3 to 6 segments. Analysis of gene annotation files identified a total of 10 conserved motifs among PsNACs, with varying positions and frequencies. Motif 1, motif 2, motif 3, and motif 6 were found in the majority of PsNACs, typically located towards the N-terminus of the sequences. PsNAC members within the same subfamily exhibited similar motif distributions and gene structure characteristics including the CDS and UTR regions, suggesting potential functional similarity. Analysis of the 2000 bp upstream sequences from the transcription start site of PsNACs identified a total of 3069 cis-elements. The most significant core elements included phytohormone-responsive elements, MYB binding sites, low temperature responsiveness, drought-inducible elements, and light-responsive elements. Intraspecific synteny analysis revealed that the PsNAC gene family contained 13 pairs of duplicated genes within the plum genome. The relative expression levels of PsNAC26, PsNAC57, PsNAC77, and PsNAC95 were highest at the fruit expansion period and gradually decreased as fruit development progressed. Conversely, PsNAC54 and PsNAC74 exhibited their lowest expression levels at the fruit expansion period, and increased gradually with fruit development and ripening. The expression levels of PsNAC26, PsNAC57, PsNAC77, PsNAC95, PsNAC54, and PsNAC74 were higher in Huangguan fruit showing hollowness and browning compared to those without. 【Conclusion】 This study represents the first comprehensive analysis of the PsNAC gene family in plum, identifying and characterizing 115 members of the PsNAC gene family. We explored their physical and chemical properties, gene structures, chromosome locations, phylogenetic relationships, and subcellular localization characteristics. Furthermore, using qRT-PCR technology, we investigated the gene expression patterns of PsNAC gene family members in Huangguan fruit exhibiting HB and non-HB across various developmental stages. The findings of this study will serve as a crucial foundation for further exploration into the biological functions of PsNAC gene family and the molecular mechanism by which PsNAC gene family members regulate fruit HB in Huangguan plum.
Key words: Huangguan plum; NAC transcription factor; Gene family; Fruit hollowness and browning; Gene expression
轉錄因子是植物生長發育和外部(非)生物脅迫響應的主要調控因子[1]。目前已知植物中存在的轉錄因子有58種,其中,NAC轉錄因子是超大轉錄因子家族之一,是植物特有的基因家族[2]。NAC這一命名源自矮牽牛(Petunia hybrida)的NAM(no apical meristem)基因、擬南芥(Arabipopsis thaliana)的ATAF1/ATAF2(actiation factor 1/2)基因和CUC2(cup-shaped cotyledon 2)基因的首字母[3]。NAC蛋白的N末端區域含有約150個高度保守氨基酸的NAC結構域,負責DNA結合和二聚體形成,可分為A~E 5個亞結構,其中A、C和D高度保守,B和E較為多變。NAC蛋白的C端由一些簡單氨基酸重復序列構成高度可變的調控區域(transcription regulation region,TRR),具有蛋白結合活性,能夠轉錄激活或者抑制[4]。
植物基因組中NAC轉錄因子成員眾多,目前已在多種植物鑒定出這一家族成員,包括模式植物擬南芥(Arabidopsis thaliana,117個)[5]和毛果楊(Populus trichocarpa,170個)[6]、作物水稻(Oryza sativa,151)[5]、玉米(Zea mays,148個)[7]和大豆(Glycine max,152個)[8]、蔬菜作物番茄(Solanum lycopersicum,93個)[9]、辣椒(Capsicum annuum,104個)[10]、大白菜(Brassica rapa L. ssp. pekinensis,188個)[11]和果樹作物蘋果(Malus domestica,180個)[12]、梨(Pyrus bretschneideri,185)[13]、火龍果(Hylocereus undatus,64個)[14]、菠蘿(Ananas comosus,73個)[15]、歐李(Cerasus humilis,76個)[16]。
研究表明,NAC轉錄因子廣泛參與植物多種生命代謝活動,包括在植物調控生長發育、響應逆境脅迫[17-20]、花器官形成[21]、器官邊界和植物形態建成[22-23]、次生細胞壁形成與增厚[24-25]、芽和根尖分生組織形成[1,26]、側根發育[20,27]、纖維發育[24]、植株衰老調節[28]、果實生長發育[29]、果實風味形成[30-31]、果實成熟[1,32]等方面發揮著重要作用。
皇冠李(Prunus salicina Lindl. var. cordata ‘Huangguan’)是筆者團隊選育的一種適宜福建地區栽培的優質柰李新品種。該品種于2018年12月通過福建省林木良種審定,編號為閩S-SV-PS-2018。皇冠李的果實呈鮮亮的黃色,口味鮮甜,風味濃郁,成熟期在5月底至6月初,是早熟柰李的優新品種。然而,皇冠李是屬于典型的柰李類,存在的突出問題是果實內部的核頂端常與果肉分離,形成蛀孔狀(似蟲蛀而非蟲蛀)的空腔(hollowness,or cavity)。筆者課題組前期的長期觀察發現,這種現象使得空腔周圍的果肉表面變得粗糙呈結晶狀,同時出現木質化(lignification)褐變(化)(browning),降低了果實內在品質與商品價值。這種空腔褐變特征,可能會讓消費者誤認為是爛果,從而對產業造成嚴重的經濟損失。
果肉組織的木質化過程是木質素積累的結果,受到木質素生物合成和轉錄調控的相關基因調控[33-34]。木質素生物合成是一個復雜的過程,其中轉錄因子如NAC、MYB、WRKY和bHLH等在調控木質素生物合成中發揮著關鍵作用。在這些轉錄因子中,NAC和MYB被認為是木質素生物合成調控網絡的關鍵上游轉錄因子,擔任主調節開關角色[37]。NAC轉錄因子特別被視為植物次生壁生物合成轉錄調控的一級開關[35-37]。
本研究利用生物信息學手段,鑒定李PsNAC基因家族成員,并系統分析PsNAC家族基因的理化性質、基因結構、染色體定位、系統進化、亞細胞定位等特征,采用qRT-PCR技術分析PsNAC家族基因在皇冠李不同發育時期的空腔褐變果和非空腔褐變果的基因表達模式。研究結果將為進一步研究PsNAC家族基因的生物學功能、尋找可能參與木質素合成調控柰李果實空腔褐變的PsNAC候選基因提供重要參考,并有望為未來制定柰李果實空腔褐變防控技術方案、品種改良和種質創新提供重要依據。
1 材料和方法
1.1 基因家族成員鑒定、染色體定位和理化性質分析
從Pfam數據庫(http://pfam.xfam.org/)下載NAC.hmm(PF01849)和NAM.hmm(PF02365)隱馬爾結構域模型,利用TBtools軟件的Simple HMM search功能鑒定李NAC家族蛋白序列(E-value設定為1e-5)。利用NCBI在線CD-search工具,檢測候選蛋白序列的保守結構域,剔除冗余和無效基因模型,最終獲得PsNAC基因家族成員及其序列信息。
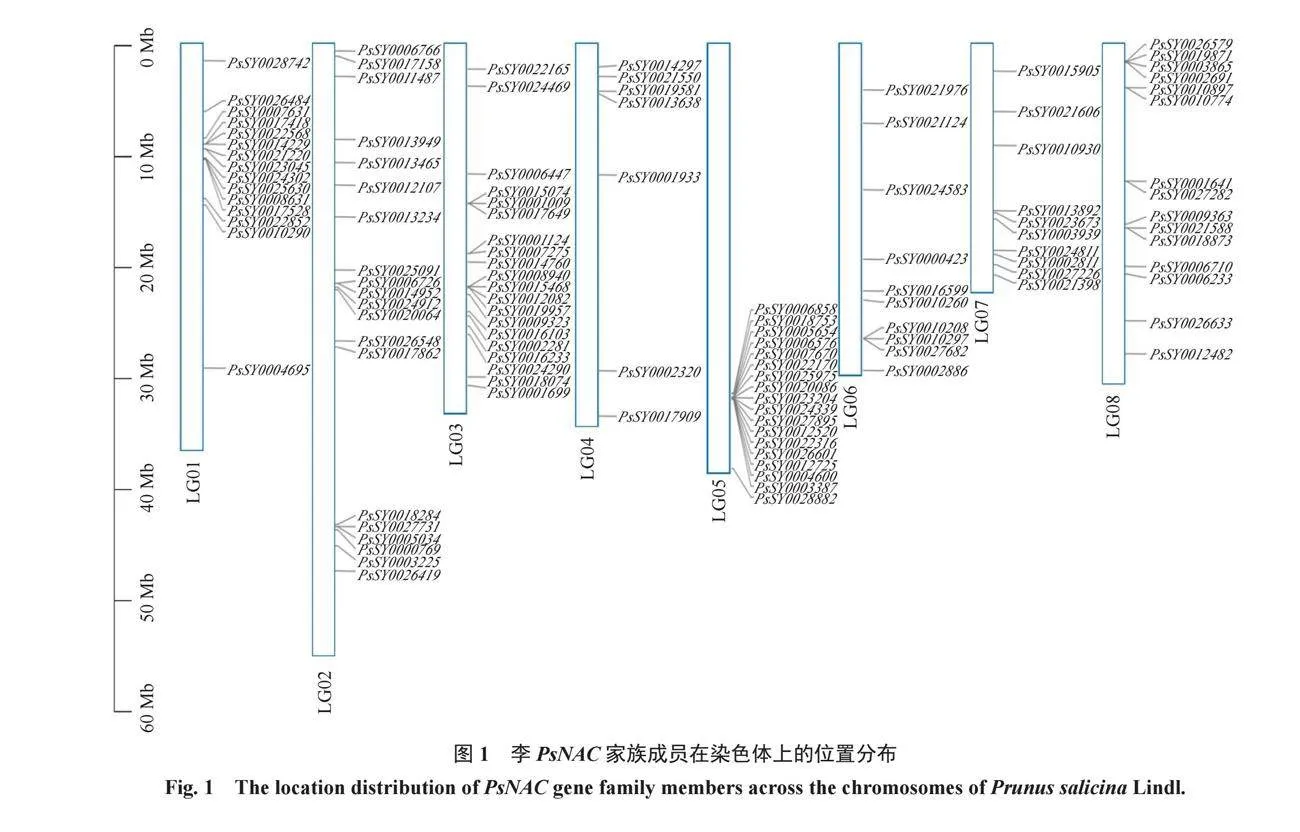
基于三月李(P. salicina Lindl.)基因組注釋的GFF文件,采用TBtools對PsNAC基因家族成員進行染色體定位分析,并根據它們在染色體上的位置順序進行重命名。
利用ExPASy網站(https://web.expasy.org/protparam/)分析PsNAC家族成員的理化性質,包括等電點、分子質量、不穩定系數、氨基酸數目等。使用SignaIP4.1(https://services.healthtech.dtu.dk/services/SignalP-4.1/)預測這些PsNAC家族成員是否存在信號肽。采用TMHMM 2.0(https://dtu.biolib.com/DeepTMHMM)預測PsNAC家族成員的跨膜結構、利用WoLF PSORT網站(https://www.genscript.com/wolf-psort.html)預測PsNAC家族成員的亞細胞定位。
1.2 基因家族成員多序列比對與系統進化樹的構建
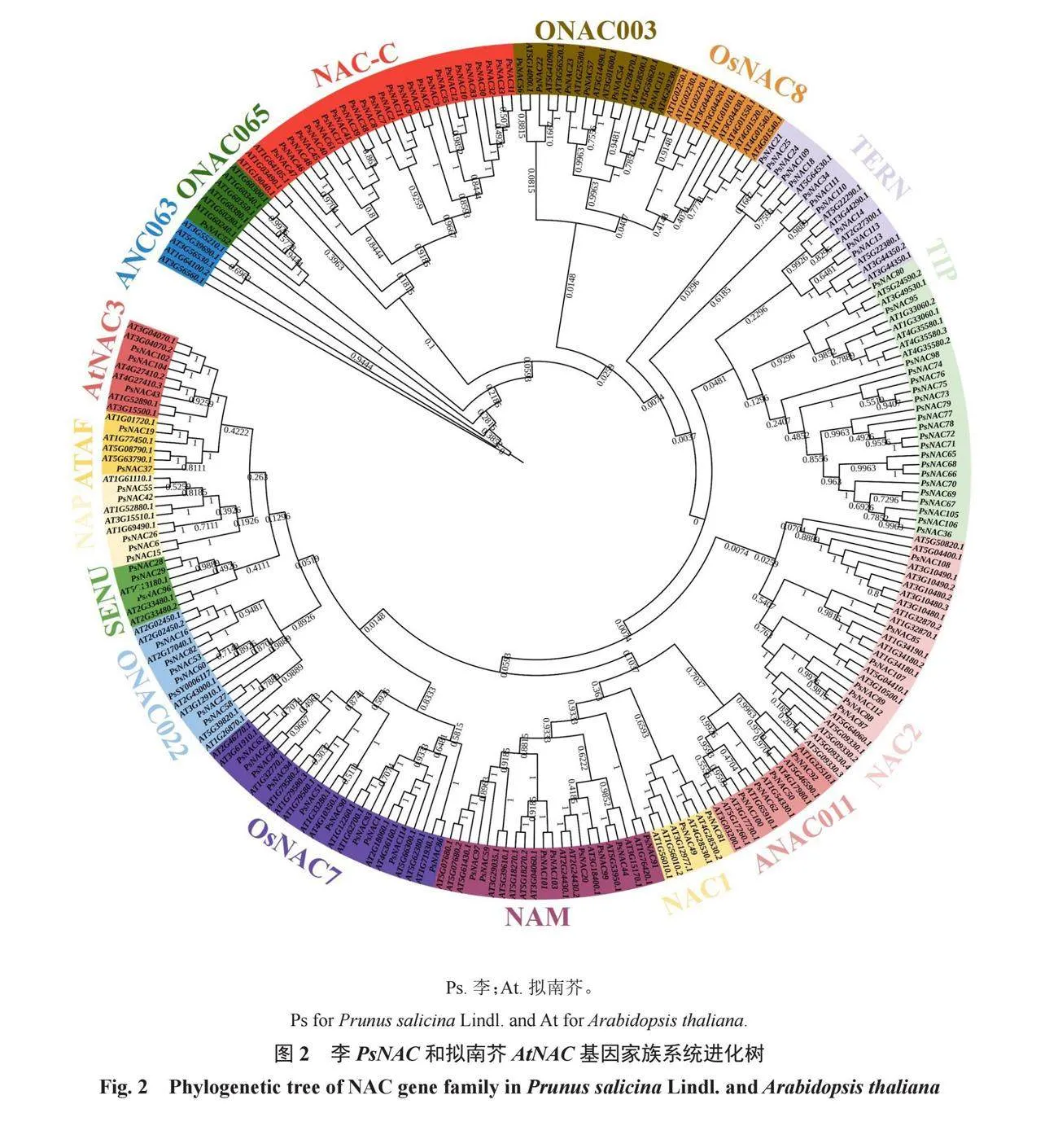
從PlantTFDB(http://planttfdb.gao-lab.org/)獲取擬南芥NAC基因(125個)的蛋白序列,采用MEGA 11軟件的ClustalW進行李和擬南芥NAC蛋白序列比對,去除差異較大的氨基酸序列。采用Neihgbor-Joining方法構建系統進化樹(Bootstrap設置為1000次)。利用iTOL網站(https://itol.embl.de/tree/)美化系統進化樹。最后參考已知的擬南芥NAC基因家族分類信息,對構建好的系統發育樹進行亞族分類。
1.3 基因家族成員保守基序和基因結構分析
根據亞族分類結果,利用TBtools的Simple MEME Wrapper功能對PsNAC家族的保守基序進行分析,最大motif數設置為20,其他參數為默認值。根據生成的XML文件和家族成員ID,使用TBtools將PsNAC亞族成員的保守基序進行可視化繪制。根據三月李基因注釋文件,使用TBtools將PsNAC亞族成員的編碼區和非編碼區的基因結構進行可視化繪制。
1.4 基因家族成員的啟動子順式作用元件預測
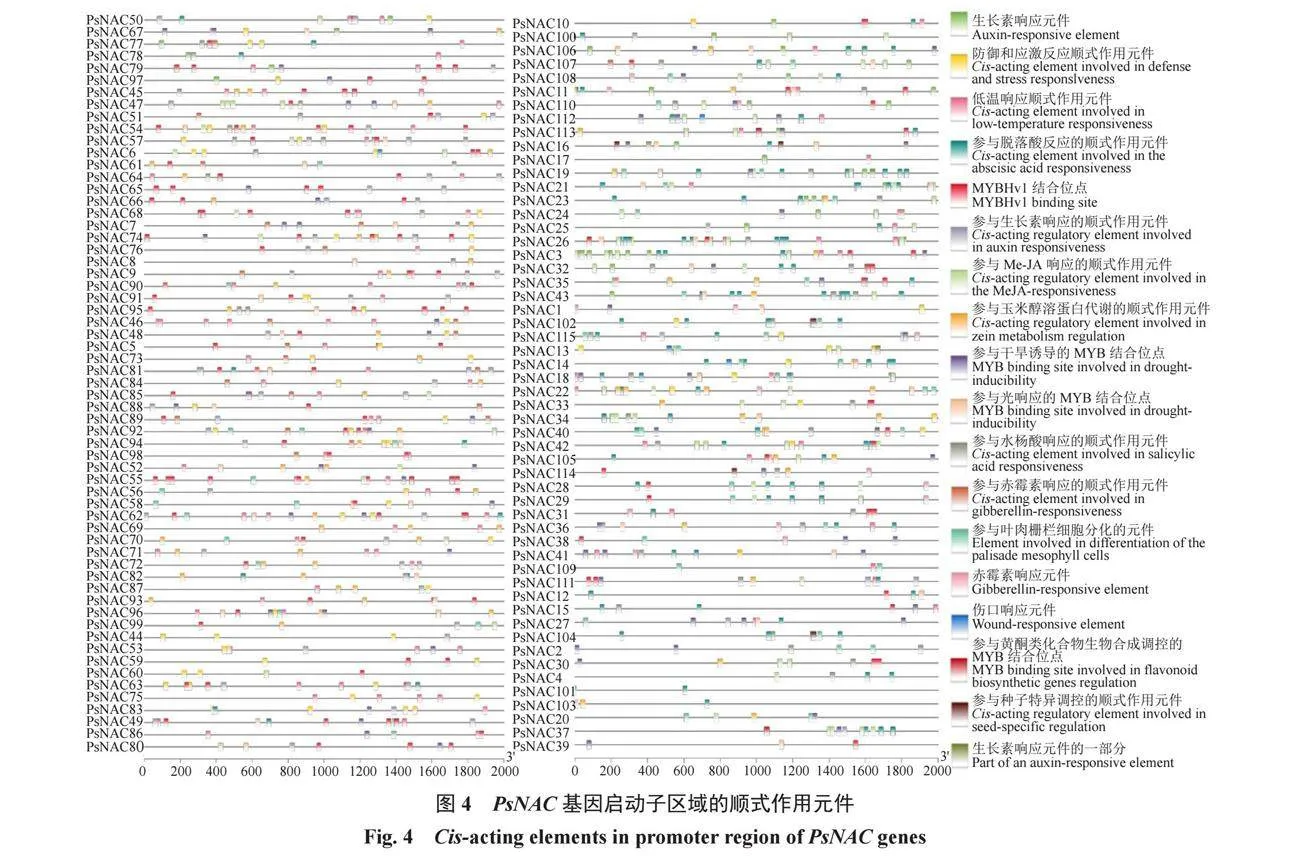
根據三月李基因組文件和基因結構注釋信息文件,使用TBtools篩選PsNAC家族成員基因轉錄起始位點上游2000 bp的序列,并將這些序列上傳到Plant TFDB網站,進行啟動子區域順式作用元件的預測與分析。最后,通過TBtools的Simple BioSequence Viewer功能,將篩選出的啟動子順式作用元件數據進行可視化展示。
1.5 基因家族成員共線性分析
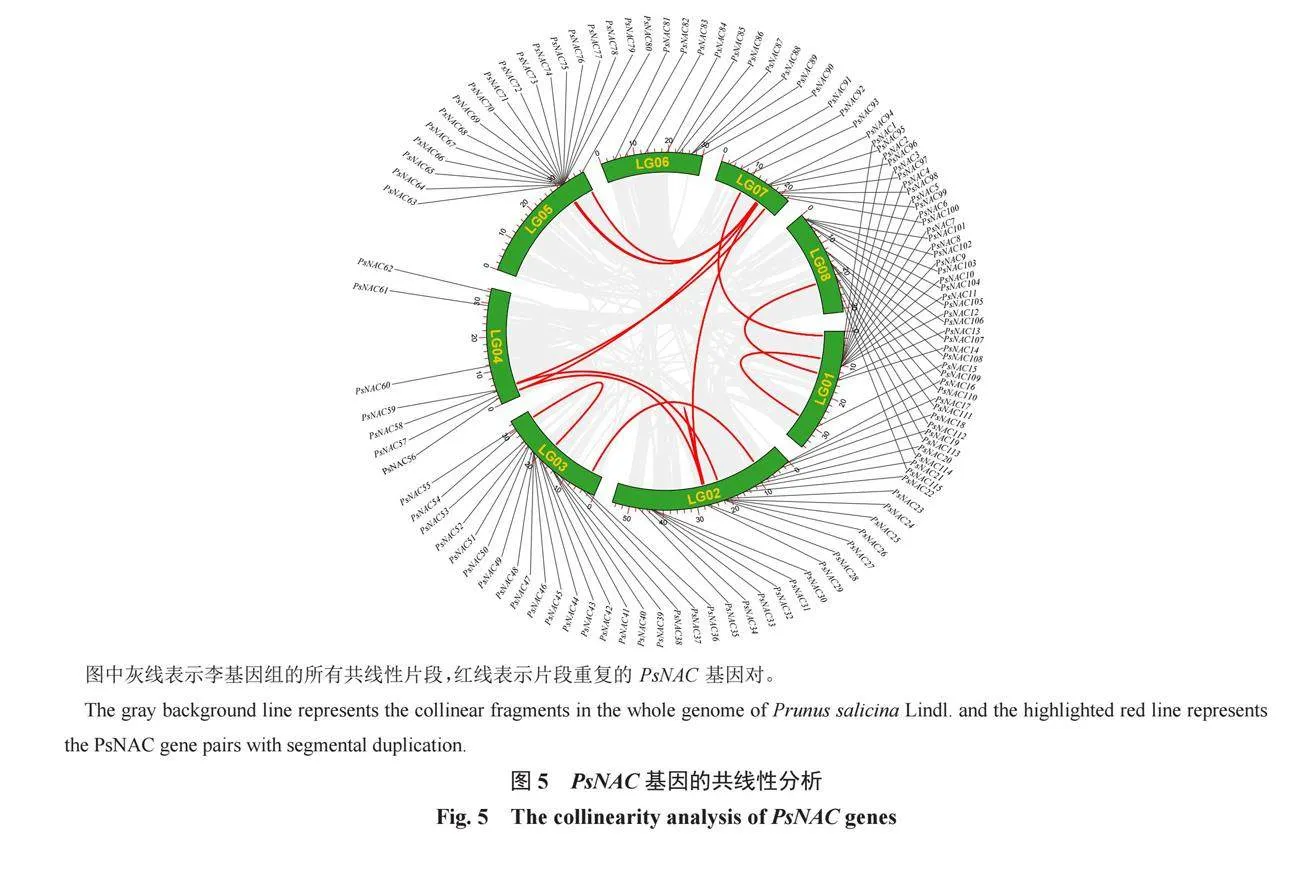
從擬南芥基因組數據庫(http: //www. arabidopsis.org/)下載擬南芥基因組和基因注釋文件,從三月李基因組(https://www.rosaceae.org/Analysis/9450778)下載全基因組文件和注釋文件,利用MCScanX軟件對這2個物種進行共線性比較。
1.6 PsNAC基因家族成員的表達模式分析
以皇冠李不同發育時期,包括膨大期、轉色期、成熟期和后熟期的果實作為試驗材料,采自福建省寧德市古田縣旸谷村李園(E 118°49′4″,N 26°39′3″,海拔280 m)。選取樹齡為5年,株行距3.5 m′4.0 m,生長一致、長勢良好,水肥管理水平一致的植株為采樣樹。采樣時,選擇樹體東、南、西、北四個方向高度一致、外圍花束狀果枝上果形均勻一致的果實,每3株樹的樣本作為1個生物學重復,用冰盒放置帶回實驗室。用手術刀將果實切開,判斷是否為空腔褐變果實。
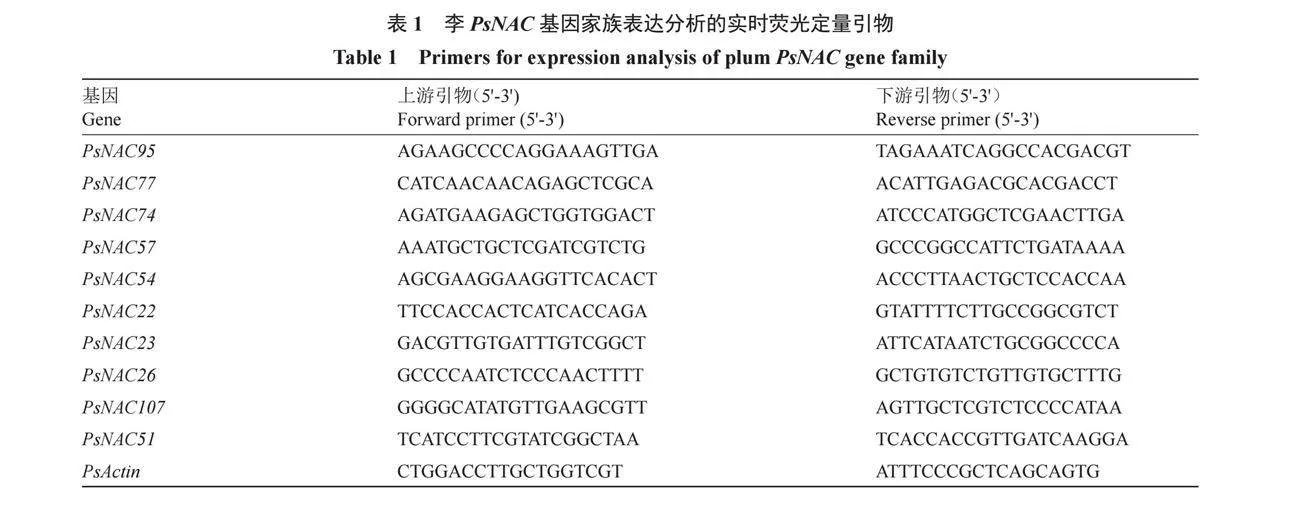
采用qRT-PCR技術分析PsNAC家族基因的相對表達量。采用RNAprep Pure多糖多酚植物總RNA提取試劑盒(DP441,天根生化科技北京有限公司,北京)提取RNA,M5 Super plus qPCR RT kit with gDNA remover試劑盒(北京聚合美生物科技有限公司,北京)合成cDNA第一鏈。使用Primer Premier 5設計實時熒光定量引物,選用Actin為內參基因(表1),引物由擎科偉業生物技術有限公司進行合成。qRT-PCR反應使用LightCycler 480 SYBR Green I Master試劑盒(Roche),通過2-??CT法計算基因的相對表達量。
2 結果與分析
2.1 PsNAC基因家族成員的鑒定及理化性質特征
通過三月李NAC轉錄因子特異隱馬可夫模型文件的構建及對三月李蛋白數據庫的二次搜索,共提取到117個PsNAC轉錄因子家族成員。經過NCBI CD-search功能驗證蛋白序列的保守結構域,剔除冗余和無效基因模型,最終獲得了115個PsNAC轉錄因子基因。根據其在染色體位置的排列順序,依次編號為PsNAC01~PsNAC115。
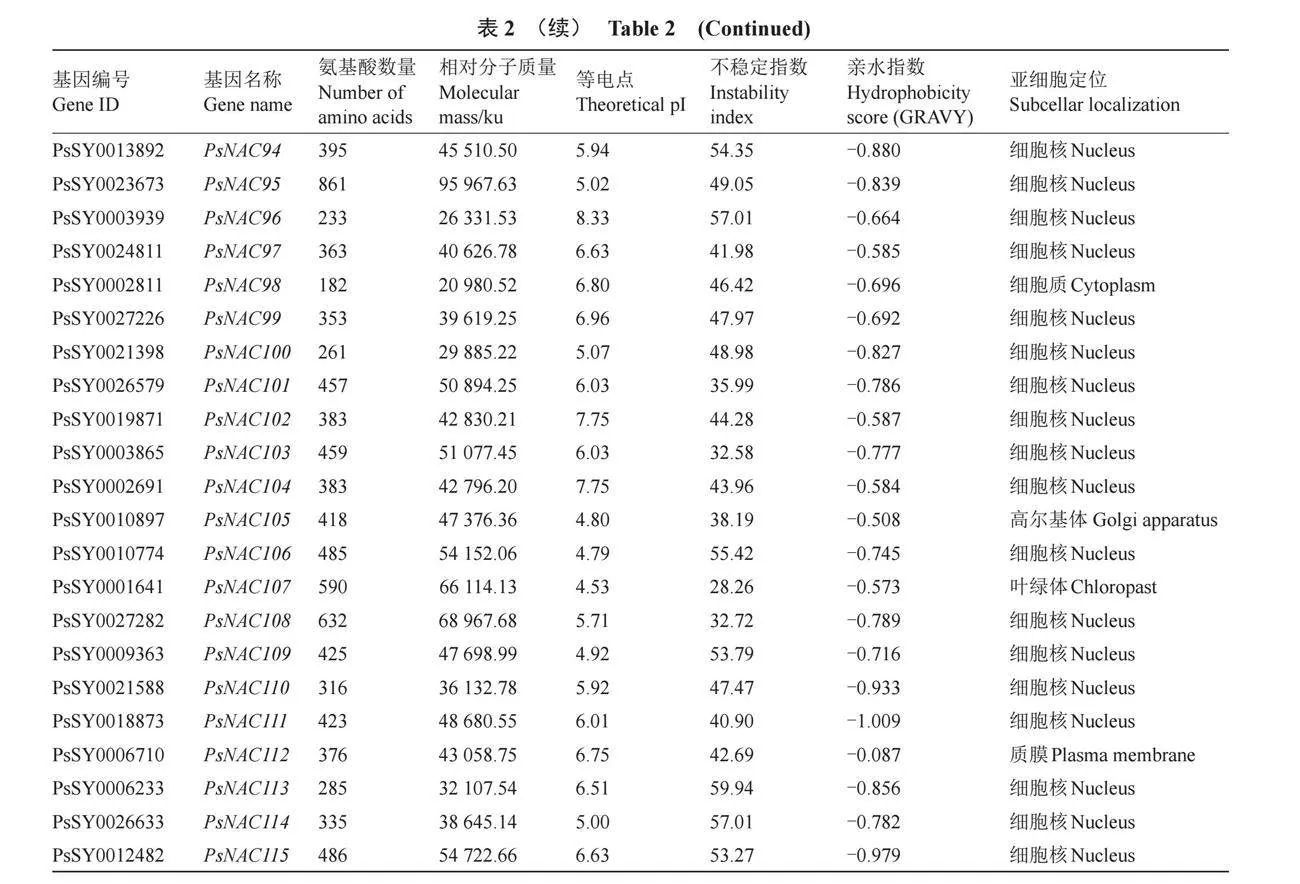
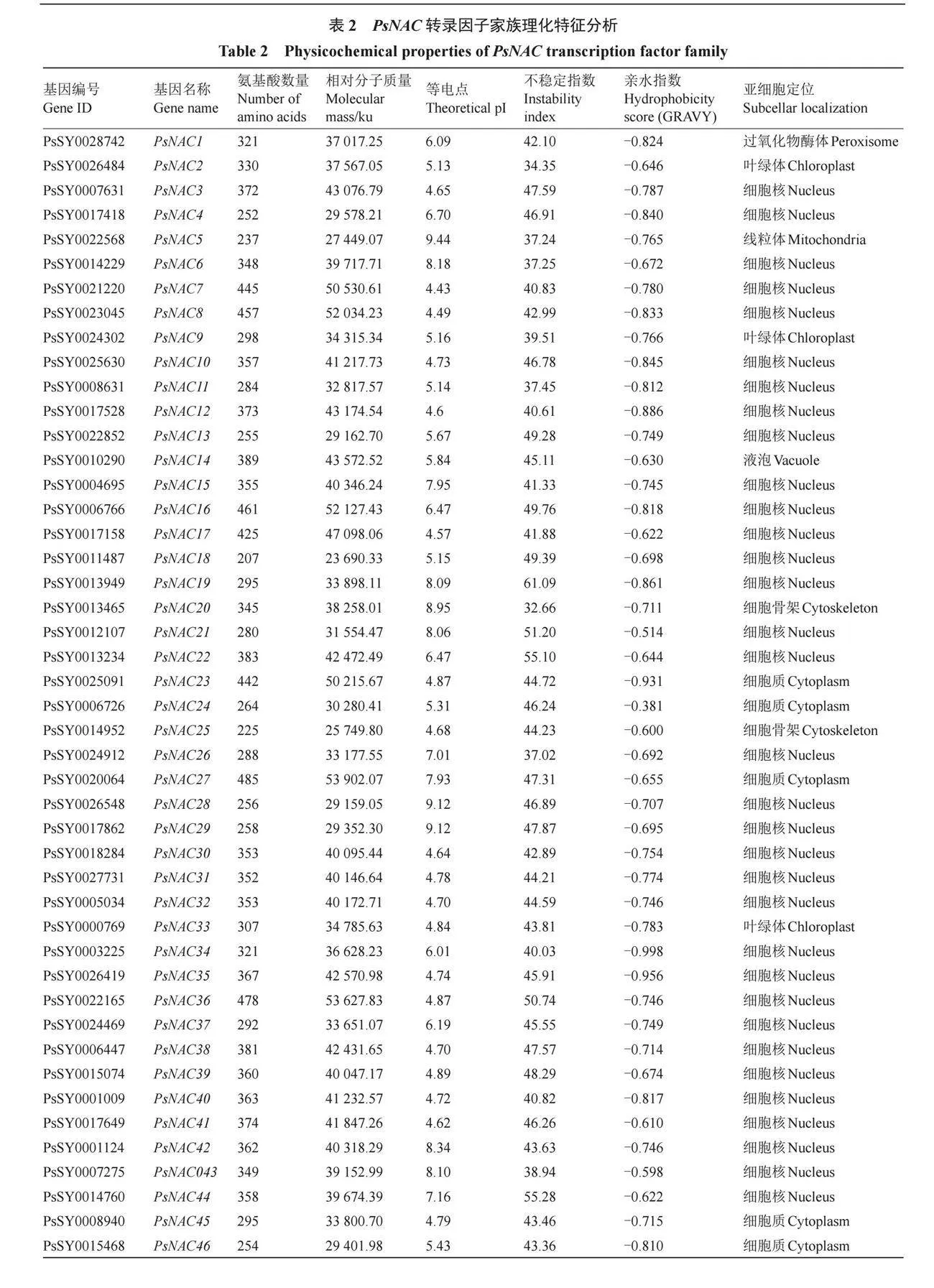
對PsNAC家族基因蛋白序列進行基本理化性質分析,如表2所示,PsNACs編碼蛋白質的氨基酸序列長度范圍是182(PsNAC98)至861(PsNAC95)個,平均氨基酸數量366個。蛋白理論等電點值(pI)分布在4.43~9.55之間,其中86個為酸性蛋白,29個為堿性蛋白。相對分子質量在20 980.52(PsNAC98)~95 967.63(PsNAC95)之間。不穩定指數范圍在27.64(PsNAC60)~61.36(PsNAC093),其中,108個PsNAC家族成員不穩定指數大于35,7個PsNAC家族成員不穩定指數則小于35。PsNAC家族成員的GRAVY值均為負數,說明這些PsNAC家族成員蛋白均為親水性蛋白。
跨膜結構分析顯示,94%的PsNAC家族成員沒有跨膜結構域,而PsNAC14、PsNAC80、PsNAC85、PsNAC95、PsNAC105和PsNAC107則各含有1個跨膜結構域,僅PsNAC112具有3個跨膜結構域。亞細胞定位的預測結果顯示,91個PsNAC家族成員位于細胞核,其余分布于細胞質、高爾基體、過氧化物酶體、細胞骨架、線粒體、葉綠體、質膜和液泡等結構中。
2.2 PsNAC基因染色體定位
染色體定位結果(圖1)顯示,115個PsNAC基因不均勻地定位到8對染色體上,其中,第2號和3號染色體上分布的PsNAC家族成員最多(占17.4%),其次是第5號染色體上(占15.7%),第6號和7號染色體上分布的基因數量最少,僅含有10個PsNACs。
2.3 PsNAC多序列比對與系統發育樹構建
利用擬南芥NAC基因序列作為對照,可將PsNACs分為17個亞族,不同亞族在功能和進化關系上存在差異。OsNAC8和ANC063亞族不含PsNAC家族成員,其余亞族均含有數量不等的PsNAC家族成員。NAC-C和TIP亞族的PsNAC家族成員數量超過20個,其中NAC-C亞族包含PsNAC家族成員數量最多,為26個。OsNAC003亞族含6個PsNAC家族成員,分別是PsNAC22、PsNAC23、PsNAC54、PsNAC56、PsNAC57和PsNAC115;OsNAC7亞族含10個PsNAC家族成員,分別是PsNAC1、PsNAC51、PsNAC63、PsNAC64、PsNAC84、PsNAC86、PsNAC90、PsNAC93、PsNAC94和PsNAC114(圖2)。
2.4 PsNAC家族成員保守基序和基因結構
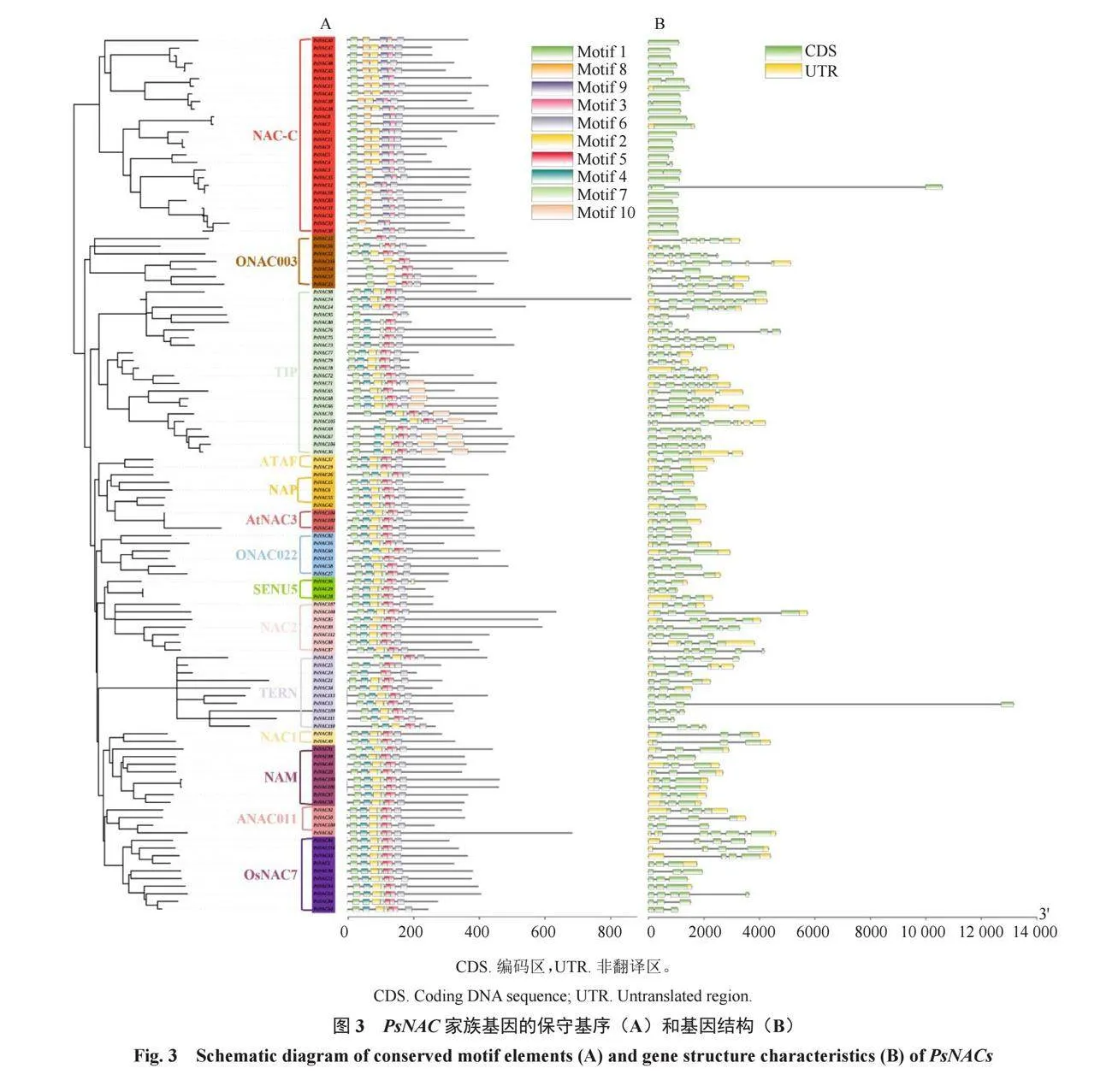
PsNAC基因的CDS片段數量在1~8個不等,多數PsNAC基因具有3~6個CDS片段。基于基因注釋文件的分析,共識別出10個保守motifs,它們的分布位置和數量在PsNAC基因中并不完全一致。其中,motif 1、motif 2、motif 3和motif 6出現的次數最多,且通常是依次分布在N端。同一個亞族的PsNAC成員具有相似的motif分布和基因結構(包括CDS和UTR區域),這說明每個亞族的PsNAC成員可能具有類似的功能。PsNAC33和PsNAC95所具有的motif數量最少,僅有3個(圖3)。
2.5 PsNAC家族成員啟動子順式作用元件
為便于預測PsNAC基因所具有的潛在功能,對PsNAC基因起始密碼子ATG上游2000 bp的序列進行分析,共預測到3069個功能不同的順式作用元件(圖4)。其中,最主要的響應元件是植物激素響應元件,包括茉莉酸甲酯響應元件(MeJA-responsive element,320個)、脫落酸響應元件(abscisic acid-responsive element,296個)、赤霉素響應元件(gibberellin-responsive element,127個)和生長素響應元件(auxin-responsive element,78個)。此外,在非生物脅迫中,PsNAC家族還含有87、64和49個響應元件,分別參與干旱誘導反應的MYB結合位點(MYB binding site involved in drought-inducibility)、參與低溫(Cis-acting element involved in low-temperature responsiveness)和光(MYB binding site involved in light responsiveness)響應。
2.6 PsNAC基因家族種內共線性
為了鑒定PsNAC基因家族內是否存在基因復制現象,通過MCScanX共線性分析,總共鑒定出16對片段重復基因復制事件(圖5,紅線相連的是基因復制事件的基因對),片段重復是PsNAC基因家族進化重要事件。其中,PsNAC37與PsNAC19、PsNAC29與PsNAC56、PsNAC28與PsNAC96、PsNAC26與PsNAC58、PsNAC96與PsNAC56、PsNAC80與PsNAC94、PsNAC93與PsNAC1、PsNAC94與PsNAC71發生的是兩兩基因片段復制,PsNAC97、PsNAC59與PsNAC58,PsNAC79、PsNAC94與PsNAC95,PsNAC29、PsNAC28與PsNAC96發生的是3個基因之間的基因片段復制。PsNAC13、PsNAC14、PsNAC113與PsNAC114發生的是4個基因之間的基因片段復制。根據片段重復的基因結構分析和系統發育樹構建,推測這些片段重復基因的功能可能存在相似性。
2.7 PsNAC基因家族在空腔褐變皇冠李果實中的表達模式分析
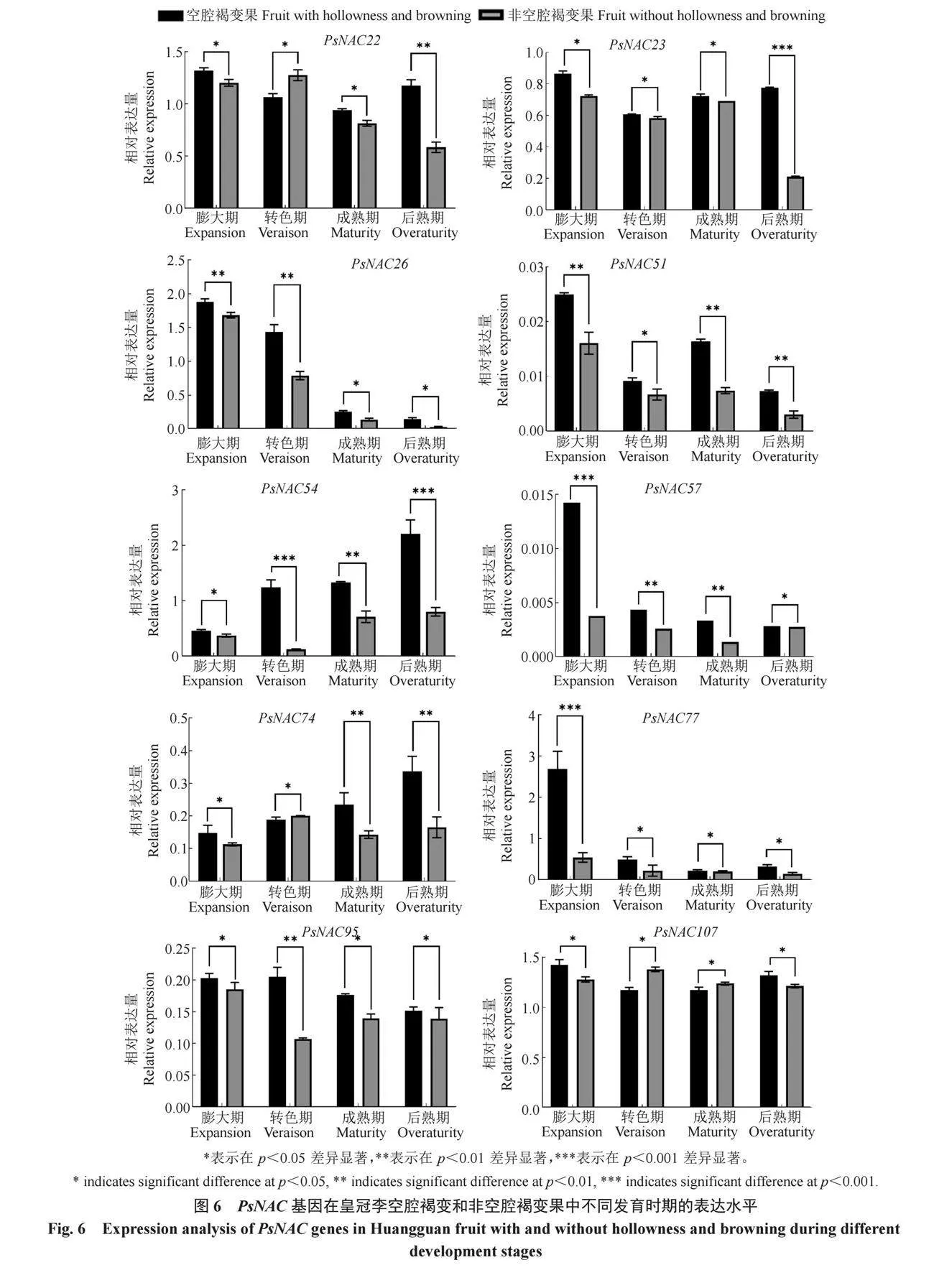
植物次生細胞壁加厚過程包含纖維素、半纖維素和木質素的合成,同時結合PsNAC基因家族成員的順式作用元件預測結果,對10個PsNAC基因家族成員的基因表達量進行了分析(圖6)。其中,PsNAC57、PsNAC54和PsNAC51既是OsNAC7和OsNAC003的亞族成員,又同時具有生長素和赤霉素響應元件,在激素參與木質素生物合成的轉錄調控網絡中具有重要作用。PsNAC26、PsNAC57、PsNAC77和PsNAC95在果實膨大期的基因相對表達量最高,隨果實發育逐漸降低,在空腔褐變果實中的表達量高于非空腔褐變果。PsNAC54和PsNAC74在果實膨大期的表達量最低,隨果實發育逐漸上升,在空腔褐變果實中的表達量高于非空腔褐變果。PsNAC22和PsNAC23在果實膨大期基因相對表達量最高,隨果實發育呈現先下降后上升的趨勢。PsNAC51基因相對表達量隨果實發育呈現先下降后上升的波動變化,后熟期的相對表達量最低,而PsNAC107在各時期的相對表達量基本保持不變。
3 討 論
NAC轉錄因子家族是植物中最大的轉錄因子家族之一,在調節植物的生長發育、抵御病原菌、響應非生物脅迫(如干旱、高鹽和低溫等)以及調控果實品質等方面發揮著重要作用。盡管對NAC基因家族的鑒定和功能分類在多種植物中已有廣泛的研究,但NAC轉錄因子在李的系統性研究方面尚不充分。木質素與果實品質和風味密切相關,其過度積累可能導致園藝植物的果實口感變差、果肉變硬、風味變淡、顏色變褐加深等,致使感官特性劣變、商品價值降低[38-39]。筆者課題組前期觀察發現,皇冠李果實空腔褐變這一產業問題與木質素密切相關。作為木質素生物合成途徑的上游調控因子[35-37],NAC轉錄因子在木質素生物合成的轉錄調控方面發揮關鍵作用。因此,對PsNAC轉錄因子進行全面生物信息學鑒定和分析,有望為未來制定防控柰李果實空腔褐變的技術方案、品種改良和種質創新提供重要參考。
筆者在本研究中鑒定到115個PsNAC轉錄因子成員,該數量多于番茄(93個)[9]、辣椒(104個)[10]、火龍果(64個)[14]、菠蘿(73個)[15]和歐李(76個)[16],但低于擬南芥(117個)[5]、毛果楊(170個)[6]、水稻(151個)[5]、玉米(148個)[7]、大豆(152個)[8]、大白菜(188個)[11]、蘋果(180個)[12]和梨(185個)[13]。與其他物種相比,李PsNAC基因家族成員數量存在一定的差異,顯示出明顯的物種間的差異性。
筆者在本研究中的蛋白理化性質分析結果顯示,李PsNAC基因家族成員在氨基酸序列長度、相對分子質量、等電點、CDS長度等存在明顯差異,表明了PsNAC基因家族成員在結構和功能上的多樣性。盡管PsNAC蛋白呈現親水性特征,但其穩定性存在較大變異,反映了它們在不同生物學環境中的適應性和功能多樣性。亞細胞定位預測結果顯示,PsNAC家族成員主要定位于細胞核中,也有少數位于細胞質、葉綠體、線粒體等中,說明李PsNAC轉錄因子可能在不同細胞部位發揮著多樣性的功能,從而為植物體的正常生命活動提供保障。
以擬南芥NAC基因序列作為對照,將PsNACs基因分為17個亞族。其中,6個PsNAC基因聚集在OsNAC003亞族,10個PsNACs聚集在OsNAC7亞族。前人研究表明,OsNAC003和OsNAC7亞族的大部分基因參與植物的次生細胞壁合成[40]。因此,推測聚類在這2個亞族的PsNACs可能具有相似的功能。OsNAC7亞族在NAC家族中被廣泛研究,包括SND1、NST1、URP7、BRN1/2、VND1-7基因,其主要功能集中在調控莖、根和花藥次生壁的形成,在木質素調控植物生長發育和逆境脅迫中起重要作用[41-42]。
啟動子順式作用元件的預測可以為進一步研究基因的轉錄調控機制和潛在功能提供理論依據[43]。本研究中啟動子順式作用元件分析結果表明,PsNACs含大量與激素相關元件的成員,推測這些成員在激素調控木質素生物合成的轉錄調控網絡中發揮重要作用。研究表明,NAC轉錄因子在植物次生細胞壁合成的轉錄調控網絡起關鍵作用,是該復雜調控網絡的主要開關。枇杷EjNAC1通過反式激活木質素合成相關基因EjPAL1和Ej4CL1的啟動子,使其表達上調,從而導致果實木質素積累,呈現出明顯的木質化特征[33]。Ge等[34]發現,EjNAC3蛋白能直接結合并激活木質素合成結構基因EjCAD-like的啟動子,參與調控枇杷果實木質化過程。三紅蜜柚CgNAC043能激活下游轉錄因子CgMYB46和木質素合成基因CgC3H和CgCCoAOMT1的啟動子,共同參與蜜柚汁胞木質素合成的轉錄調控[44]。冬棗MYB激活子(LOC107425254)和抑制子(LOC107415078)通過調節F5H和CCR參木質素生物合成,而NAC轉錄因子(LOC107435239)則促進F5H的表達,從而正向調控木質素的積累[45]。石榴NAC類蛋白PgSND1-like能結合木質素合成酶基因啟動子特異元件,提高PAL、4CL、F4H、CCR和CAD表達水平,從而調控石榴籽粒的硬度[46]。
筆者在本研究中結合PsNAC基因家族成員順式作用元件預測,共鑒定到10個PsNAC基因家族成員,其中PsNAC57、PsNAC54和PsNAC51既是OsNAC7和OsNAC003的亞族成員,又同時具有生長素和赤霉素響應元件。因此,這3個基因在激素參與木質素生物合成的轉錄調控網絡中至關重要。PsNAC26、PsNAC57、PsNAC77和PsNAC95在果實膨大期表達量最高,隨果實發育逐漸降低,在空腔褐變果中的表達量高于非空腔褐變果,推測這些家族成員可能負向調控木質素生物合成。PsNAC54和PsNAC74在果實膨大期的表達量最低,隨果實發育逐漸上升,在空腔褐變果中的表達量高于非空腔褐變果,PsNAC54和PsNAC74可能正向調控木質素生物合成。本研究為后續研究皇冠李空腔褐變的分子機制提供了重要靶標。
4 結 論
首次鑒定并分析了115個李PsNAC基因家族成員,分析了PsNAC家族基因的理化性質、基因結構、染色體定位、系統進化、亞細胞定位等特征,采用qRT-PCR技術分析PsNAC家族基因在皇冠李不同發育時期的空腔褐變果和非空腔褐變果的基因表達模式。研究結果將為進一步研究PsNAC家族基因的生物學功能、PsNAC家族成員與皇冠李果實空腔褐變的具體關聯性奠定了重要基礎。
參考文獻References:
[1] LIU G S,LI H L,GRIERSON D,FU D Q. NAC transcription factor family regulation of fruit ripening and quality:A review[J]. Cells,2022,11(3):525.
[2] SINGH S,KOYAMA H,BHATI K K,ALOK A. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement[J]. Journal of Plant Research,2021,134(3):475-495.
[3] AIDA M,ISHIDA T,FUKAKI H,FUJISAWA H,TASAKA M. Genes involved in organ separation in Arabidopsis:An analysis of the cup-shaped Cotyledon mutant[J]. The Plant Cell,1997,9(6):841-857.
[4] PURANIK S,SAHU P P,SRIVASTAVA P S,PRASAD M. NAC proteins:Regulation and role in stress tolerance[J]. Trends in Plant Science,2012,17(6):369-381.
[5] NURUZZAMAN M,MANIMEKALAI R,SHARONI A M,SATOH K,KONDOH H,OOKA H,KIKUCHI S. Genome-wide analysis of NAC transcription factor family in rice[J]. Gene,2010,465(1/2):30-44.
[6] MENG L,CHEN S Y,LI D W,HUANG M R,ZHU S. Genome-wide characterization and evolutionary expansion of poplar NAC transcription factors and their tissue-specific expression profiles under drought[J]. International Journal of Molecular Sciences,2022,24(1):253.
[7] PENG X J,ZHAO Y,LI X M,WU M,CHAI W B,SHENG L,WANG Y,DONG Q,JIANG H Y,CHENG B J. Genomewide identification,classification and analysis of NAC type gene family in maize[J]. Journal of Genetics,2015,94(3):377-390.
[8] LE D T,NISHIYAMA R,WATANABE Y,MOCHIDA K,YAMAGUCHI-SHINOZAKI K,SHINOZAKI K,TRAN L S P. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress[J]. DNA Research,2011,18(4):263-276.
[9] JIN J F,WANG Z Q,HE Q Y,WANG J Y,LI P F,XU J M,ZHENG S J,FAN W,YANG J L. Genome-wide identification and expression analysis of the NAC transcription factor family in tomato (Solanum lycopersicum) during aluminum stress[J]. BMC Genomics,2020,21(1):288.
[10] DIAO W P,SNYDER J C,WANG S B,LIU J B,PAN B G,GUO G J,GE W,DAWOOD M H S A. Genome-wide analyses of the NAC transcription factor gene family in pepper (Capsicum annuum L.):Chromosome location,phylogeny,structure,expression patterns,Cis-elements in the promoter,and interaction network[J]. International Journal of Molecular Sciences,2018,19(4):1028.
[11] MA J,WANG F,LI M Y,JIANG Q,TAN G F,XIONG A S. Genome wide analysis of the NAC transcription factor family in Chinese cabbage to elucidate responses to temperature stress[J]. Scientia Horticulturae,2014,165:82-90.
[12] SU H Y,ZHANG S Z,YUAN X W,CHEN C T,WANG X F,HAO Y J. Genome-wide analysis and identification of stress-responsive genes of the NAM–ATAF1 2–CUC2 transcription factor family in apple[J]. Plant Physiology and Biochemistry,2013,71:11-21.
[13] AHMAD M,YAN X H,LI J Z,YANG Q S,JAMIL W,TENG Y W,BAI S L. Genome wide identification and predicted functional analyses of NAC transcription factors in Asian pears[J]. BMC Plant Biology,2018,18(1):214.
[14] HU X L,XIE F F,LIANG W W,LIANG Y H,ZHANG Z K,ZHAO J T,HU G B,QIN Y H. HuNAC20 and HuNAC25,two novel NAC genes from pitaya,confer cold tolerance in transgenic Arabidopsis[J]. International Journal of Molecular Sciences,2022,23(4):2189.
[15] HE Q,LIU Y H,ZHANG M,BAI M Y,PRIYADARSHANI S V G N,CHAI M N,CHEN F Q,HUANG Y M,LIU L P,CAI H Y,QIN Y. Genome-wide identification and expression analysis of the NAC transcription factor family in pineapple[J]. Tropical Plant Biology,2019,12(4):255-267.
[16] 張忠鑫,郭夕雯,汪澤文,王鵬飛,張建成,杜俊杰,穆霄鵬. 歐李NAC基因家族的鑒定及表達分析[J]. 果樹學報,2023,40(2):206-222.
ZHANG Zhongxin,GUO Xiwen,WANG Zewen,WANG Pengfei,ZHANG Jiancheng,DU Junjie,MU Xiaopeng. Identification and expression analysis of NAC gene family in Cerasus humilis[J]. Journal of Fruit Science,2023,40(2):206-222.
[17] NURUZZAMAN M,SHARONI A M,KIKUCHI S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants[J]. Frontiers in Microbiology,2013,4:248.
[18] HOU X M,ZHANG H F,LIU S Y,WANG X K,ZHANG Y M,MENG Y C,LUO D,CHEN R G. The NAC transcription factor CaNAC064 is a regulator of cold stress tolerance in peppers[J]. Plant Science,2020,291:110346.
[19] XU Y,LI P F,MA F N,HUANG D M,XING W T,WU B,SUN P G,XU B Q,SONG S. Characterization of the NAC transcription factor in passion fruit (Passiflora edulis) and functional identification of PeNAC-19 in cold stress[J]. Plants,2023,12(6):1393.
[20] HE X J,MU R L,CAO W H,ZHANG Z G,ZHANG J S,CHEN S Y. AtNAC2,a transcription factor downstream of ethylene and auxin signaling pathways,is involved in salt stress response and lateral root development[J]. Plant Journal,2005,44(6):903-916.
[21] SABLOWSKI R W M,MEYEROWITZ E M. A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA[J]. Cell,1998,92(1):93-103.
[22] BERGER Y,HARPAZ-SAAD S,BRAND A,MELNIK H,SIRDING N,ALVAREZ J P,ZINDER M,SAMACH A,ESHED Y,ORI N. The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves[J]. Development,2009,136(5):823-832.
[23] KATO H,MOTOMURA T,KOMEDA Y,SAITO T,KATO A. Overexpression of the NAC transcription factor family gene ANAC036 results in a dwarf phenotype in Arabidopsis thaliana[J]. Journal of Plant Physiology,2010,167(7):571-577.
[24] KO J H,YANG S H,PARK A H,LEROUXEL O,HAN K H. ANAC012,a member of the plant-specific NAC transcription factor family,negatively regulates xylary fiber development in Arabidopsis thaliana[J]. Plant Journal,2007,50(6):1035-1048.
[25] NAKANO Y,YAMAGUCHI M,ENDO H,REJAB N A,OHTANI M. NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants[J]. Frontiers in Plant Science,2015,6:288.
[26] SOUER E,VAN HOUWELINGEN A,KLOOS D,MOL J,KOES R. The No apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries[J]. Cell,1996,85(2):159-170.
[27] XIE Q,FRUGIS G,COLGAN D,CHUA N H. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development[J]. Genes amp; Development,2000,14(23):3024-3036.
[28] PODZIMSKA-SROKA D,O’SHEA C,GREGERSEN P L,SKRIVER K. NAC transcription factors in senescence:From molecular structure to function in crops[J]. Plants,2015,4(3):412-448.
[29] FORLANI S,MIZZOTTI C,MASIERO S. The NAC side of the fruit:Tuning of fruit development and maturation[J]. BMC Plant Biology,2021,21(1):238.
[30] CAO X M,WEI C Y,DUAN W Y,GAO Y,KUANG J F,LIU M C,CHEN K S,KLEE H,ZHANG B. Transcriptional and epigenetic analysis reveals that NAC transcription factors regulate fruit flavor ester biosynthesis[J]. Plant Journal,2021,106(3):785-800.
[31] FU B L,WANG W Q,LI X,QI T H,SHEN Q F,LI K F,LIU X F,LI S J,ALLAN A C,YIN X R. A dramatic decline in fruit citrate induced by mutagenesis of a NAC transcription factor,AcNAC1[J]. Plant Biotechnology Journal,2023,21(8):1695-1706.
[32] 卓茂根,王惠聰. NAC轉錄因子在果實成熟中的調控作用[J]. 果樹學報,2023,40(7):1455-1470.
ZHUO Maogen,WANG Huicong. The roles of NAC transcription factors in regulating fruit ripening[J]. Journal of Fruit Science,2023,40(7):1455-1470.
[33] XU Q,WANG W Q,ZENG J K,ZHANG J,GRIERSON D,LI X,YIN X R,CHEN K S. A NAC transcription factor,EjNAC1,affects lignification of loquat fruit by regulating lignin[J]. Postharvest Biology and Technology,2015,102:25-31.
[34] GE H,ZHANG J,ZHANG Y J,LI X,YIN X R,GRIERSON D,CHEN K S. EjNAC3 transcriptionally regulates chilling-induced lignification of loquat fruit via physical interaction with an atypical CAD-like gene[J]. Journal of Experimental Botany,2017,68(18):5129-5136.
[35] 郭光艷,柏峰,劉偉,秘彩莉. 轉錄因子對木質素生物合成調控的研究進展[J]. 中國農業科學,2015,48(7):1277-1287.
GUO Guangyan,BAI Feng,LIU Wei,BI Caili. Advances in research of the regulation of transcription factors of lignin biosynthesis[J]. Scientia Agricultura Sinica,2015,48(7):1277-1287.
[36] 張雨,趙明潔,張蔚. 植物次生細胞壁生物合成的轉錄調控網絡[J]. 植物學報,2020,55(3):351-368.
ZHANG Yu,ZHAO Mingjie,ZHANG Wei. Transcriptional regulatory network of secondary cell wall biosynthesis in plants[J]. Chinese Bulletin of Botany,2020,55(3):351-368.
[37] 陳倩,游雙梅,邢樂華,徐凡,羅明,郭啟高. 果樹NAC轉錄因子的研究進展[J]. 分子植物育種,2021,19(19):6396-6405.
CHEN Qian,YOU Shuangmei,XING Lehua,XU Fan,LUO Ming,GUO Qigao. Research progress of NAC transcription factors in fruit trees[J]. Molecular Plant Breeding,2021,19(19):6396-6405.
[38] 薛維文,周顯芳,張昭其,方方. 果蔬采后木質素積累及其調控對品質的影響研究進展[J]. 園藝學報,2022,49(9):2023-2036.
XUE Weiwen,ZHOU Xianfang,ZHANG Zhaoqi,FANG Fang. Advances in lignin accumulation and its regulation on the quality of postharvest fruit and vegetables[J]. Acta Horticulturae Sinica,2022,49(9):2023-2036.
[39] SHI Y N,LI B J,SU G Q,ZHANG M X,GRIERSON D,CHEN K S. Transcriptional regulation of fleshy fruit texture[J]. Journal of Integrative Plant Biology,2022,64(9):1649-1672.
[40] HUSSEY S G,MIZRACHI E,SPOKEVICIUS A V,BOSSINGER G,BERGER D K,MYBURG A A. SND2 a NAC transcription factor gene,regulates genes involved in secondary cell wall development in Arabidopsis fibres and increases fibre cell area in Eucalyptus[J]. BMC Plant Biology,2011,11:173.
[41] MITSUDA N,SEKI M,SHINOZAKI K,OHME-TAKAGI M. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence[J]. The Plant Cell,2005,17(11):2993-3006.
[42] ZHONG R Q,DEMURA T,YE Z H. SND1,a NAC domain transcription factor,is a key regulator of secondary wall synthesis in fibers of Arabidopsis[J]. The Plant Cell,2006,18(11):3158-3170.
[43] HERNANDEZ-GARCIA C M,FINER J J. Identification and validation of promoters and Cis-acting regulatory elements[J]. Plant Science,2014,217:109-119.
[44] LI X T,WANG N Y,SHE W Q,GUO Z X,PAN H L,YU Y,YE J W,PAN D M,PAN T F. Identification and functional analysis of the CgNAC043 gene involved in lignin synthesis from Citrus grandis ‘San Hong’[J]. Plants,2022,11(3):403.
[45] ZHANG Q,WANG L H,WANG Z T,ZHANG R T,LIU P,LIU M J,LIU Z G,ZHAO Z H,WANG L L,CHEN X,XU H F. The regulation of cell wall lignification and lignin biosynthesis during pigmentation of winter jujube[J]. Horticulture Research,2021,8(1):238.
[46] XIA X C,LI H X,CAO D,LUO X,YANG X W,CHEN L N,LIU B B,WANG Q,JING D,CAO S Y. Characterization of a NAC transcription factor involved in the regulation of pomegranate seed hardness (Punica granatum L.)[J]. Plant Physiology and Biochemistry,2019,139:379-388.

