Synthesis and Crystal Structure of a Two-Dimensional Hofmann-Type Bimetallic Complex Cu(DMF)2[Pt(CN)4]
LU Run-Qin ZHOU Hu YUAN Ai-Hua
(School of Material Science and Engineering,Jiangsu University of Science and Technology,Zhenjiang,Jiangsu 212003)
研究簡報
Synthesis and Crystal Structure of a Two-Dimensional Hofmann-Type Bimetallic Complex Cu(DMF)2[Pt(CN)4]
LU Run-Qin ZHOU Hu YUAN Ai-Hua*
(School of Material Science and Engineering,Jiangsu University of Science and Technology,Zhenjiang,Jiangsu212003)
A two-dimensional cyanide-bridged Cu(Ⅱ)-Pt(Ⅱ) bimetallic complex has been synthesized by solution diffusion method using [Pt(CN)4]2-and [Cu(L)]2+(L=3,10-diethanol-1,3,5,8,10,12-hexaazacyclotetradecane)as buildingblocks.Unexpectedly,the obtained complex Cu(DMF)2[Pt(CN)4](1)is an analogue of the well-known Hofmann -type clathrate without macrocyclic ligand.Single-crystal X-ray diffraction reveals that 1 crystallizes in monoclinic, space group C2/m,a=1.6248(6)nm,b=0.7393(3)nm,c=0.6955(3)nm,β=108.969(4)°.The crystal structure of 1 consists of two-dimensional corrugated metal cyanide sheets without interpenetration stacking along the a axis in an ABAB packing mode.CCDC:756100.
cyanide-bridged;macrocyclic ligand;Hofmann-type;crystal structure
0 Introduction
Coordination polymers have attracted much interest because of their multi-functional properties[1-4]. Cyano-bridged metal complexes are among the first coordination complexes but are still extensively investigated in recent years for their unique magnetic[5-6],spincrossover[7-9],catalytic[10],host-guest[11],gas-storage[12-13]and negative thermal expansion properties[14-15].One strategy to assemble 1D,2D and 3D coordination polymers is to combine cyanometalates building blocks such as[M(CN)4]2-(M=Ni,Pd,and Pt),[M(CN)6]3-(M= Fe,Mn,Cr and Co)and[M(CN)8]3-/4-(M=Mo,W and Nb) with mononuclear complexes containing macrocyclic ligands[16-18].Cyanometalates exhibit bridging character involving either one or several of the cyano groups and can constructvariouscoordination polymerswith different structural topologies.Macro-cyclic ligands usually occupy several coordination sites of the metal ions and leave axial sites available for cyano groups.
Herein,we chose[Pt(CN)4]2-and[Cu(L)]2+(L=3,10-diethanol-1,3,5,8,10,12-hexaazacyclotetradecane) asbuilding blocks and attempt to construct a new cyanobridged coordination polymer containing macrocyclic ligand.However,an unexpected two-dimensional Hofmann-DMF-type complex Cu(DMF)2[Pt(CN)4](1) without macrocyclic ligand was obtained instead.
1 Experimental
1.1 Materials and physical measurements
All chemicals and solvents in the synthesis were of reagent grade and used as received.[Cu(L)](ClO4)2were synthesized as described in the literature[19].Caution! Perchlorate salts of metal complexes with organic ligands are potentially explosive and should be handled in small quantities with great care.Elemental analyses for C,H,and N were carried out with a Perkin-Elmer 240C analyzer.
1.2 Synthesis
Well-shaped brightgreen-blue crystalsof1 suitable for X-ray single-crystal structure determination were grown at room temperature by slow diffusion of a DMF solution(2 mL)of[Cu(L)](ClO4)2(0.05 mmol)and an aqueous solution(20 mL)of K2[Pt(CN)4]·3H2O(0.05 mmol)for about 3 weeks.The resulting crystals were collected,washed with H2O and dried in air.Yield 30%.Anal.Calcd.for C10H14N6PtO2Cu(%):C 23.60,H 2.77,N 16.51;Found(%):C 23.52,H 2.78,N 16.48.
1.3 X-ray crystallography
Diffraction data for 1 was collected at 173(2)K on a Bruker Smart ApexⅡ CCD diffractometer with graphite monochromatic Mo Kα radiation(λ=0.710 73 nm)using the φ-ω scan mode.Diffraction data analysis and reduction were performed within SMART and SAINT[20].Correction for Lorentz,polarization,and absorption effects were performed within SADABS[21].Structures were solved using Patterson method within SHELXS-97 and refined using SHELXL-97[22].All nonhydrogen atoms were refined anisotropically.The sites of hydrogen atoms were placed by the geometry.The C (H)atoms of the DMF ligand were placed in calculated position and refined using a riding model,with Uiso(H)= 1.5Ueq(C).Selectedcrystaldatacollectionandrefinement parameters are given in Table 1.
CCDC:756100.

Table 1 Data collection and structure refinement for 1
2 Results and discussion
2.1 Crystal structure
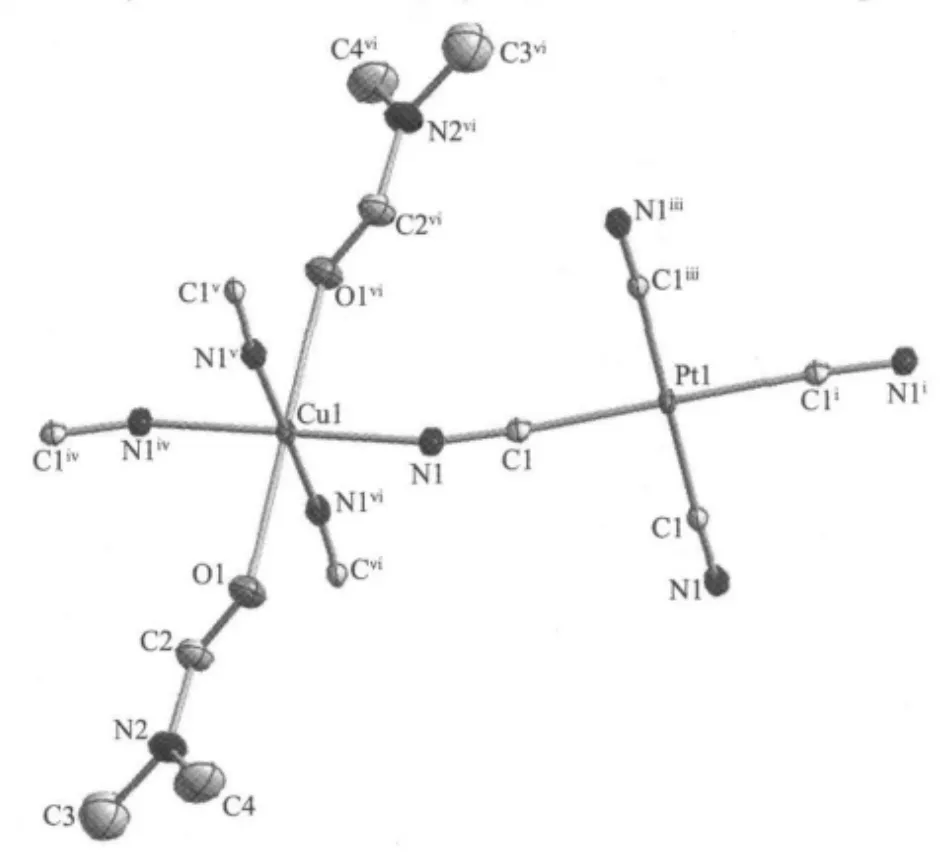
Fig.1 ORTEP diagram of 1,Displacement ellipsoids are drawn at the 30%probability level and H atoms are omitted for clarity
ORTEP and packing diagrams of 1 are shown in Fig.1 and Fig.2,respectively.Selected bond lengths and bond angles are given in Table 2.The crystal structure of 1 consists of two-dimensional corrugated metal cyanide networks(Fig.2a).The similar corrugated polymeric sheets are also reported in some related complexes with H2O molecules as the axial ligands[23-25].The network is formed with linkages of cyanide between Cu and Pt centers.In the structure,the[Pt (CN)4]moiety has a square planar geometry with four bridging cyano ligands.The coordination sphere of Cu center is six-coordinated by four cyano nitrogens and two oxygen atoms from two DMF ligands,describing a slightly distorted octahedral geometry.The equatorial coordination sites of Cu atom are occupied by four N atoms from the bridging cyano groups,whereas two O atoms from two DMF ligands are in the axial positions. The DMF molecules,bound to the Cu atom in trans position,are located on both sides of the network planes (Fig.1).The average Cu-N and Cu-O bond lengths are ca.0.199 4(3)nm and 0.236 4(4)nm,respectively.The Pt-C-N bond is nearly linear with the bond angle of 176.2(3)°.
As displayed in Fig.2b,the two-dimensional sheets of 1 are square grid-like networks and the length of side is ca.0.51 nm (Cu…Pt distance).In addition,these sheets stack together in an ABAB packing mode along the a axis without interpenetration.The distance between the adjacent A and B sheets is ca.0.772 8 nm (Cu…Pt distance),while the offset distance between the adjacent sheets is ca.0.25 nm (about the half distance of Cu…Pt)in the bc plane.

Fig.2 View of the stacking without interpenetration of sheets along the a and b axis of 1
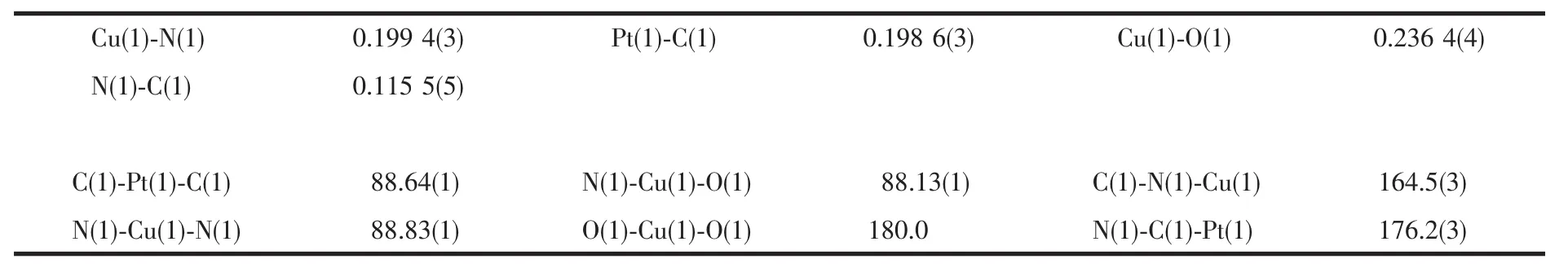
Table 2 Selected bond lengths(nm)and angles(°)
[1]Kitagawa S,Kitaura R,Noro S.Angew.Chem.Int.Ed.,2004, 43:2334-2375
[2]Cheetham A K,Rao C N R,Feller R K.Chem.Commun., 2006:4780-4795
[3]Robin A Y,Fromm K M.Coord.Chem.Rev.,2006,250:2127-2157
[4]Férey G.Chem.Soc.Rev.,2008,37:191-214
[5]Kahn O.Nature,1995,378:667-667
[6]Sato O,Iyoda T,Fujishima A,et al.Science,1996,272:704-705
[7]Kitazawa T,Gomi Y,Takahashi M,et al.J.Mater.Chem., 1996,6(1):119-121
[8]Niel V,Martinez-Agudo J M,Mu?oz M C,et al.Inorg.Chem., 2001,40(16):3838-3839
[9]Agustí G,Cobo S,Gaspar A B,et al.Chem.Mater.,2008,20 (21):6721-6732
[10]Robertson N J,Qin Z,Dallinger G C,et al.Dalton Trans., 2006:5390-5395
[11]Yuge H,Kim C H,Iwamoto T,et al.Inorg.Chim.Acta,1997, 257:217-224
[12]Li Y,Liu Y,Wang Y T,et al.Int.J.Hydrogen Energy,2007, 32:3411-3415
[13]Culp J T,Natesakhawat S,Smith M R,et al.J.Phys.Chem.C, 2008,112(17):7079-7083
[14]Goodwin A L,Calleja M,Conterio M J,et al.Science,2008, 319:794-797
[15]Phillips A E,Goodwin A L,Halder G J,et al.Angew.Chem. Int.Ed.,2008,47:1396-1399
[16]Yuan A H,Liu W Y,Zhou H,et al.J.Mol.Struct.,2009,919: 356-360
[17]Lim J H,You Y S,Yoo H S,et al.Inorg.Chem.,2007,46(25): 10578-10586
[18]Jiang L,Feng X L,Su C Y,et al.Inorg.Chem.,2007,46(7): 2637-2644
[19]Suh M P,Kang S G.Inorg.Chem.,1988,27(14):2544-2546
[20]SMART,SAINT and XPREP,Area Detector and Data Integration and Reduction Software,BrukerAnalytical Instruments Inc.,Madison,WI,1995.
[21]Sheldrick G M.SADABS,Empirical Adsorption Correction Program for Area Detector Data,University of G?ttingen, G?ttingen,Germany,1996.
[22]Sheldrick G M.SHELXS-97 and SHELXL-97,Program for Crystal Structural Solution and Refinement,Bruker Analytical Instruments Inc.,Madison,WI,1997.
[23]Ham W K,Weakley T J R,Page C J.J.Solid State Chem., 1993,107:101-107
[24]Yuge H,Kim C,Iwamoto T,et al.Inorg.Chim.Acta,1997, 257:217-224
[25]Niu T Y,Crisci G,Lu J,et al.Acta Cryst.,1998,C54:565-567
二維Hofmann類雙金屬配合物Cu(DMF)2[Pt(CN)4]的合成及晶體結構
陸潤芹 周 虎 袁愛華*
(江蘇科技大學材料科學與工程學院,鎮(zhèn)江 212003)
氰基橋聯(lián);大環(huán)配體;Hofmann類;晶體結構
O614.121;O614.82+6
A
1001-4861(2010)02-0347-04
2009-07-12。收修改稿日期:2009-11-16。
陸潤芹,女,25歲,碩士研究生;研究方向:材料化學。
江蘇省高校自然科學基金資助項目(No.07KJB150030)。
*通訊聯(lián)系人。E-mail:aihuayuan@163.com,Tel:0511-84448290

