Bi3.25Nd0.75Ti3O12納米結構:可控合成及其可見光催化活性
林 雪 關慶豐 李海波 李洪吉 巴春華 鄧海德
(1江蘇大學材料科學與工程學院,江蘇鎮江212013;2吉林師范大學化學學院,環境友好材料制備與應用教育部重點實驗室,吉林四平136000;3吉林師范大學物理學院,吉林四平136000)
Bi3.25Nd0.75Ti3O12納米結構:可控合成及其可見光催化活性
林 雪1,2關慶豐1,*李海波3李洪吉2巴春華2鄧海德2
(1江蘇大學材料科學與工程學院,江蘇鎮江212013;2吉林師范大學化學學院,環境友好材料制備與應用教育部重點實驗室,吉林四平136000;3吉林師范大學物理學院,吉林四平136000)
用水熱法制備不同形貌的摻釹鈦酸鉍(Bi3.25Nd0.75Ti3O12,BNdT)納米粉體.透射電子顯微鏡(TEM)結果表明,通過控制OH-濃度可以得到不同形貌的納米粉體.基于不同條件下制備的樣品微結構分析,提出了這些不同形貌的形成機制.紫外-可見漫反射光譜(UV-Vis DRS)表明BNdT樣品的帶隙能(Eg)約為1.984 eV.利用可見光照射下甲基橙降解實驗評價了BNdT樣品的光催化性能.結果表明,BNdT的光催化活性比商用TiO2催化劑P25和摻氮TiO2高得多.OH-濃度為10 mol·L-1時制備的BNdT納米線光催化效率最高,經可見光照射360 min,濃度為0.01 mmol·L-1甲基橙溶液的降解率可達到93.0%,且循環使用4次后,其光催化活性并沒有明顯降低,表明BNdT是一種穩定有效的可見光催化劑.
鈦酸鉍;摻釹;納米結構;水熱合成;光催化降解;可見光照射
1 Introduction
Environmental and energy issues are very important topics on a global scale.Natural energy,such as sunlight,can be employed to help human being to curb the damage that polluted wastewater has on the environment.Semiconductor photocatalysis offers the potential technology for complete elimination of toxic chemicals through its efficiency and potentially broad applicability.1Various new compounds and materials for photocatalysis have been synthesized in the past few decades.2-6A successful example is TiO2,a metal oxide often used as a catalyst in photochemistry.7-12However,the band gap energy of the TiO2is 3.2 eV.It absorbs only the ultraviolet light(λ≤386.5 nm)which only accounts for about 4.0%of the sunlight.Therefore,studies on attempting to eliminate these drawbacks on photocatalysts have been performed.13-16
In recent years,bismuth titanate photocatalysts,such as Bi12TiO20,17-19Bi2Ti2O7,20Bi20TiO32,21,22and Bi4Ti3O12,23,24have been widely studied as a class of promising photocatalysts which can respond under visible light.In heterogeneous photocatalysis,the morphology of the catalyst plays an important role in catalytic activity.25,26Nanowire photocatalyst has attracted extensive attention in environmental remediation due to its great specific surface area.27On the one hand,conventional film photocatalysts can be fixed and reclaimed easily,but their low surface areas decrease the photocatalytic activities.On the other hand,the application of particulate photocatalysts is limited owing to the difficulties in separation,which may repollute the treated water.Compared to film and particulate counterparts,nanowire photocatalysts not only possess large specific surface area,which allows for their surface active sites to be accessible for reactants more efficiently,but also owe high lengthto-diameter aspect ratio,which makes the separation of photocatalysts more easily.Furthermore,Metal element doping is one of the typical approaches to extend the spectral response of bismuth titanate photocatalysts by providing defect states in the band gap.28
Methyl orange(MO)is an azo dye and also has a variety of uses in texitiles,paper,pulp and environment thus causing toxicity problems.Many efforts have been made to study the photodegradation of MO.Bi4Ti3O12is generally considered to be one of the promising photocatalysts and has the ability to detox water from MO.29To our knowledge,the morphological control of Bi4Ti3O12-related layered-perovskites nanostructures is relatively unexplored due to the lack of synthetic capability. On the basis of our previous work30on the preparation of bismuth titanate,we have introduced a low-temperature solution-phase route without the use of any surfactant and template to synthesize Bi3.25Nd0.75Ti3O12(BNdT)nanostructures with well controlled shapes.As stimulated by the promising applications, the synthesis of BNdT nanowires is a subject of considerable research interest.In this work,BNdT nanostructures with high photocatalytic activity have been successfully synthesized by means of a facile template-free hydrothermal method process. There are two significant aspects of the work described in this paper.Firstly,the synthesis of shape-controlled BNdT nanostructures has been found to be extremely evasive to date. Hence,the facile and template-free hydrothermal synthesis of BNdT nanostructures with well controlled shapes should be an important progress that may inspire subsequent catalytic materials synthesis.Secondly,catalysis by BNdT crystals has been studied recently,28but the test of BNdT nanowires associated optical properties and photocatalytic activities in the degradation of MO under visible-light irradiation has been rarely reported.Hence,this work may be of interest to both materials scientists and those working in the area of catalyst design.
2 Experimental
2.1 Preparation of BNdT photocatalysts
All the chemicals were analytically graded(purchased from Shanghai Chemical Industrial Company)and used without further purification.A surfactant-free and template-free solutionphase synthesized route to BNdT samples is described below. Bismuth nitrate(Bi(NO3)3·5H2O),neodymium nitrate(Nd(NO3)3· 6H2O),and titanium tetrachloride(TiCl4)were chosen as starting materials with the bismuth:neodymium:titanium ions molar ratios of 3.25:0.75:3.00.TiCl4(10 mL)was dissolved in 50 mL cold H2O under vigorous stirring,then mixed with Bi(NO3)3· 5H2O and Nd(NO3)3·6H2O.
The concentration of the alkali solution was adjusted using KOH,which in effect served as a capping agent.Before being transferred to a 20 mL stainless steel autoclave,the solution mixture was prepared under an ultrasonic water bath for 30 min in order to avoid the premature formation of bismuth titanate nuclei induced by the concentration of KOH and kept at a filling ratio of 70%(volume ratio).The autoclave was kept at 180°C for 24 h,and then cooled to room temperature.The precipitates were washed with deionized water and ethanol three times,separately.The final products were dried at 100°C for 2 h in a vacuum box.The sample prepared for comparison are(i) bismuth titanate(BIT)obtained according to our previous work30and(ii)N-doped TiO2(N-TiO2)synthesized according to literature.31
2.2 Characterization of BNdT photocatalysts
The crystal structures of the samples were characterized by X-ray diffraction(XRD,America PE,D/max 2500)with Cu Kαradiation.The chemical composition of the compound was determined by scanning electron microscope-X-ray energy dispersion spectrum(SEM-EDX,Japan JEOL,JSM-7001F).Trans-mission electron microscope(TEM)analysis of the samples was done using a JEM-2100F(Japan JEOL)instrument and the electron beam accelerating voltage was 200 kV.The surface areas of BNdT nanowires and nanosheets were measured by Tri-Star 3000-BET/BJH Surface Area.The infrared(IR)spectrum was measured by infrared spectrometer(America Perkin Elmer,Spectrum One).The UV-Vis diffuse reflectance spectra were recorded for the dry-pressed disk sample using a scan UV-Vis spectrophotometer(UV-Vis DRS,Japan SHIMADZU, UV-2550)equipped with an integrating sphere assembly.
2.3 Photocatalytic activity test
The photocatalytic degradation of MO was employed to evaluate the photocatalytic activities of the samples.A 300 W Xe lamp(λ>420 nm)was employed to provide visible light irradiation.About 0.1 g of photocatalyst was added to 100 mL of MO solution(0.01 mmol·L-1).Before irradiation,the suspensions were magnetically stirred in the dark for 30 min to ensure the adsorption-desorption equilibrium between the photocatalyst and MO.Then the solution was exposed to visible light irradiation under magnetic stirring.At given time intervals,4 mL of suspension was sampled and centrifuged to remove the photocatalyst particles.Then,the catalyst-free dye solution was analyzed by a UV-2550 spectrometer to record intensity of the maximum band at 462 nm in the UV-Vis absorption spectrum.
3 Results and discussion
3.1 XRD analysis
XRD patterns of the as-prepared BNdT products synthesized at different OH-concentrations of 3,5,8,10 mol·L-1are shown in Fig.1.All the reflection peaks can be indexed according to the JCPDS card No.36-1486,suggesting that the as-prepared products are of layered-perovskite structure(Bi4Ti3O12). No peaks of impurities were detected from the patterns.The strong and sharp peaks indicate high crystallinities of BNdT samples.However,the intensities of the peaks of BNdT samples are different which proves that there are some differences among the samples prepared with different OH-concentrations.BNdT sample prepared at OH-concentration of 10 mol· L-1has stronger peak intensity,which means that the crystallinity of the sample was developing with the increase of OH-concentration.

Fig.1 XRD patterns of samples BNdT and BIT(a)BIT,(b)BNdT(3 mol·L-1OH-),(c)BNdT(5 mol·L-1OH-), (d)BNdT(8 mol·L-1OH-),(e)BNdT(10 mol·L-1OH-).T=180°C,t=24 h
3.2 TEM analysis
The morphologies and structure details of BNdT products were studied by TEM,as shown in Fig.2.The influence of OH-concentration on the morphologies of BNdT crystals was presented using KOH with concentrations in the range from 3 to 10 mol·L-1.Fig.2a gives the TEM image of a typical example of nanosheets.In addition to nanosheets,nano-sized particles were also observed in the sample prepared at OH-concentration of 3 mol·L-1(Fig.2a).However,when the OH-concentration is increased to 5 mol·L-1,a large number of nanosheets of BNdT were formed,accompanied by the disappearance of the spherical particles(as illustrated in Fig.2b).When the OH-concentration is further increased to 8 mol·L-1,as shown in Fig.2c,BNdT nanowires as well as nanosheets were formed. Fig.2d shows BNdT sample obtained at OH-concentration of 10 mol·L-1.It shows that the as-prepared product consists of a large amount of nanowires with width of approximate 100 nm and lengths up to several micrometers.These results indicate that OH-concentration seems to play an important role in determining final morphologies of BNdT.Namely,morphology of BNdT powders can be controlled through varying the concentration of OH-.The relationship between the OH-concentration and morphology of BNdT crystals will be discussed later.
Further structure details of BNdT nanostructures were obtained by TEM,as shown in Fig.3.It can be seen that BNdT products prepared at OH-concentration of 3 mol·L-1were composed of nanosheets as well as spherical nano-sized particles with average size of about 8 nm(as shown in Fig.3a). Fig.3b shows TEM image of BNdT nanosheets obtained at OH-concentrations of 5 mol·L-1.It reveals that BNdT nanos-heets are made up of nanoparticles with average size of about 5 nm.Fig.3(c,d)gives the TEM images of a typical example of nanowires.It also can be observed that BNdT nanowires are made up of nanoparticles with average size of 10 nm,approximately.

Fig.2 TEM images of BNdT samples obtained at different concentrations of OH-c(OH-)/(mol·L-1):(a)3,(b)5,(c)8,(d)10
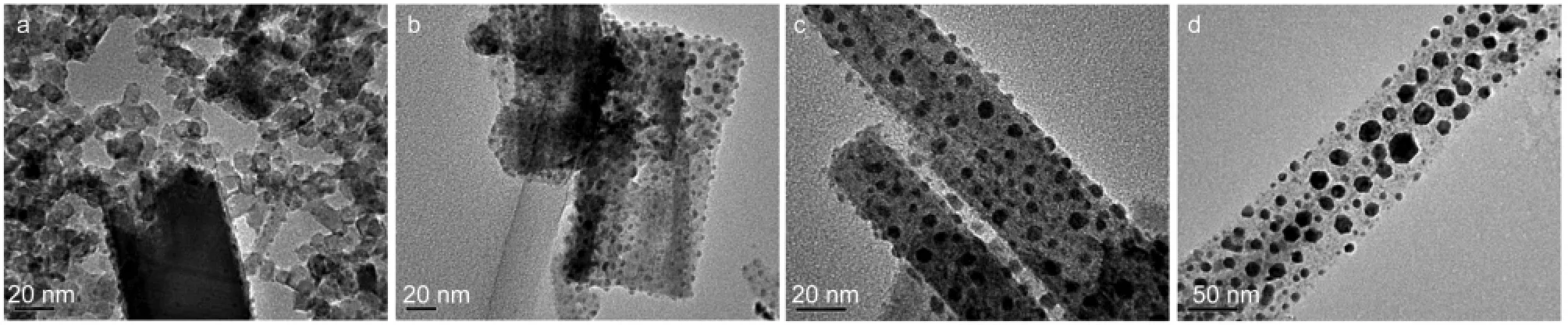
Fig.3 TEM images of BNdT samples prepared at different concentrations of OH-c(OH-)/(mol·L-1):(a)3,(b)5,(c)8,(d)10

Fig.4 High-resolution TEM image of BNdT nanowires
In order to investigate the detailed crystal structure of as-prepared samples,HRTEM images for BNdT nanowires were measured as shown in Fig.4.It reveals the fringes with an interval of 0.3843 nm,which is in good agreement with the(111) lattice planes of the layered-perovskite Bi4Ti3O12.This result might indicate that the low concentration doping of Nd3+ions did not induce the formation of separate purity phases(neodymium metal).
The SEM-EDX analysis reveals that BNdT has a homogenous atomic distribution with no other impure elements,as shown in Fig.5.An average atomic ratio of Bi:Nd:Ti(3.25:0.75: 3.00)for Bi3.25Nd0.75TiO12was obtained from measurements at different points.Based on the above results,we can conclude that the resulting materials are of high purity under our preparation conditions.

Fig.5 EDX spectrum of the as-prepared BNdT sample
Fig.6 schematically outlines the possible mechanism involved in the hydrothermal synthesis.Although the crystal growth habit is mainly determined by the intrinsic structure,it is also affected by the external conditions such as pH value of the solution,saturation,temperature,etc.As we all know,OH-concentration in the precursor solution has been found to be very important for the microstructure.It has been reported that the morphologies of Bi2Ti2O7crystals can be controlled by adjusting the OH-concentrations suggesting that OH-ionscan behave as a surfactant,20obtaining a better understanding of the role of OH-ions in the hydrothermal process.On the basis of previous report about Na0.5Bi0.5TiO3nanostructure and Na0.5Bi0.5TiO3(NBT)nanoparticles and nanowires,32at lower concentration of OH-and for shorter reaction time,only nanoparticles were obtained.When the OH-concentrations increase,primary nanoparticles grow and aggregate,picking up freshly formed nanosheets.When the OH-concentration is further increased,nanowires were obtained.Our experimental results are in accordance with the above mechanism.Thus,the pH value plays an important role in controlling the formation of seeds and the growth rates to shape the BNdT particles.

Fig.6 Schematic illustration of plausible mechanism involved in the synthesis
In this work,the condition of the alkaline medium as a factor is considered to play a key part in the formation of BNdT nanowires.At lower OH-concentration(OH-concentration:3 mol·L-1),BNdT nuclei produced in solution can aggregate to form small particles.These particles may serve as crystal seeds to grow the nanosheet and nanowire structures.With the alkalinity increases continuing(OH-concentrations:8 and 10 mol· L-1),a large amount of BNdT nuclei produced in the solution lead to forming the very high supersaturation solution,which favors the formation of wire structure.When OH-concentration is relatively lower(OH-concentration:5 mol·L-1),only BNdT nanosheets are obtained because of lower driving force, which comes from the lower chemical potential.Hence,the special growth behavior formation of BNdT nanosheets and nanowires in the present route is attributed to the highly alkaline medium.33
3.3 IR spectral analysis
It has been reported that the type and number of OH groups revealed by infrared(IR)spectra depend on the sample origin as well as on the preparation conditions(temperature,water pressure,and OH-concentration)leading to different surface species or possibly to different morphologies.34In order to explore the further impact of OH-ions on the reactivity and interaction with the active phase,IR spectrum has been recorded. At the same time,many relevant IR spectroscopy studies show that some OH-stretching frequencies were observed between 3350 and 3500 cm-1,34which is in agreement with our experimental results.The absorption band of 3444 cm-1belongs to OH-stretching frequencies(as shown in Fig.7).35
3.4 UV-Vis diffuse reflectance spectral analysis
Fig.8 shows the UV-Vis diffuse reflectance spectrum of the prepared BNdT photocatalyst.As a comparison,the spectra of P25 TiO2,N-TiO2,and BIT were also measured.The absorption onset wavelength(λg)of BNdT sample is around 625 nm, which is shifted 125,175,and 200 nm to visible region compared to BIT,N-TiO2and P25 TiO2.It shows that the performance of BNdT is better.
The absorption coefficient(α)as a function of photon energy can be expressed by the Tauc relation:28

Fig.7 IR spectrum of the as-prepared BNdT sampleT=180°C,t=24 h

Fig.8 UV-Vis diffuse reflectance spectra of different samples(a)P25 TiO2,(b)N-TiO2,(c)BIT,(d)BNdT.A:absorbance.The inset shows the plot of(Ahv)2as a function of photon energy(hv).

where hv,C,and Egare the photon energy,a constant,and the band gap energy,respectively.And n is an index determined by the nature of the electron transition during the absorption process.It is well known that there are two types of fundamental optical transitions,namely direct(n=1/2)and indirect(n=2). For BNdT,it is a direct band gap semiconductor,so here n= 1/2.Since absorbance(A)is proportional to absorption coefficient α,we use absorbance A to substitute absorption coefficient α.The plot of(Ahv)2versus hv is presented in the inset of Fig.8.Thus,The band gap energy of BNdT is calculated to be 1.984 eV,which displays a marked red shift in the absorbance compared to P25 TiO2and N-TiO2due to the contribution of 6s electrons from Bi3+.28It indicates that BNdT photocatalyst has a suitable band gap for photocatalytic decomposition of organic contaminants under visible light irradiation.The DRS spectrum of BNdT photocatalyst has steep shape which shows that the absorption relevant to the band gap is due to the intrinsic transition of the nanomaterials.36
3.5 Degradation of MO using BNdT photocatalysts
Photodegradation experiments of MO were carried out under visible light irradiation in order to test the photocatalytic performance of BNdT photocatalysts.For comparison,the photodegradation of MO by N-doped TiO2,P25 TiO2,BIT,and that without any catalyst were also carried out.UV-Vis spectral changes of MO solution by BNdT are displayed in Fig.9A while the temporal courses of the photodegradation of MO in different catalyst aqueous dispersions are shown in Fig.9B.It shows that the peaks at 462 and 271 nm were reduced with the increase of irradiation time(as illustrated in Fig.9A).The results show that MO solution is stable under visible light irradiation in the absence of any catalyst(as illustrated in Fig.9B). When BNdT nanowires have been added to MO solution,the total degradation rate is over 93.0%within 360 min irradiation, much higher than those of P25 TiO2and N-TiO2,which only reach 18.0%and 50.0%,respectively.Thus,the addition of BNdT photocatalyst leads to the obvious degradation of MO. In comparison,the photocatalytic degradation rate of MO over BIT is about 80.0%.Thus,Nd doping is one of the typical approaches to improve the performance of BIT photocatalysts. Besides,as shown in Fig.9B,the degradation rate of MO by BNdT nanowires is higher than that of BNdT nanosheets, which reaches 85.0%of the total degradation,approximately. However,as the BET surface area of the samples increases, there is a resulting increase in adsorption percentages of MO molecules.The BET surface area of BNdT nanosheets is 11.2 m2·g-1,which is relatively lower than that of nanowires.The larger BET surface area(15.0 m2·g-1)of BNdT nanowires provides more active sites for photocatalytic reaction,resulting in the superior photocatalytic activity.Besides,crystallinity also has important influence on the activity of degrading MO.36Based on the above analysis,it can be deduced that the better photocatalytic performance of BNdT nanowires is due to the higher crystallinity and larger BET surface area.
3.6 Stability of BNdT as the photocatalyst
Fig.10 indicates the XRD patterns of the BNdT sample after 360 min of visible light irradiation.Both the position and the intensity of the peaks in the XRD patterns are almost the same to those of BNdT before irradiation.As shown in this result, BNdT photocatalyst is considered to be relatively stable to visible light irradiation under the present experimental conditions. This result indicates a possibility for application of BNdT photocatalyst in the waste water treatment.
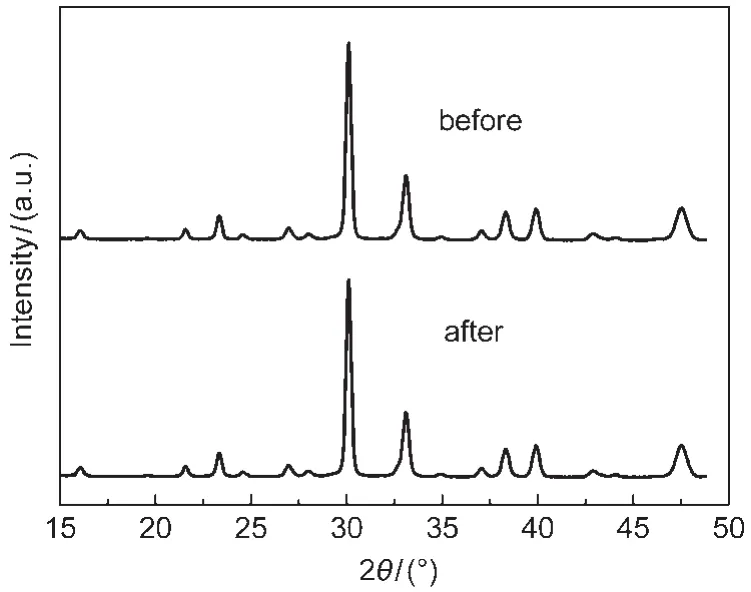
Fig.10 XRD patterns of BNdT before and after visible light irradiation
The stability tests were also investigated by carrying out recycling reactions four times for the photodegradation of MO over BNdT photocatalyst under visible light irradiation,and the results are shown in Fig.11.No significant decrease in catalytic activity was observed in the recycling reactions.Combined with the XRD patterns,all evidences demonstrate that the BNdT photocatalyst has good stability.
3.7 Photocatalytic activity mechanism
In a typical photodegradation of organic pollutants process, when the semiconductor is irradiated by light,the photoexcited electrons can be transferred to the conduction band(CB)from the valence band(VB)and whilst the holes form in the VB. Then the photoexcited holes in the VB can form·OH(hydroxyl radical)that can oxidize the organic pollutants and the electrons in the CB participate in the reduction process.Thus,the photocatalytic activity of the semiconductor is very closely related to its corresponding band structure.The band gap of oxides is generally defined by the O 2p level and transition metal d level.22

Fig.11 Stability evaluation for BNdTfour reaction cycles for photodegradation of MO under visible light irradiation
As calculated by Goto37and Cai38et al.the CB and VB of BNdT consist mostly of empty Ti 3d and occupied O 2p orbitals,respectively,and the latter is hybridized with Bi 6s and Nd 5d.These bands meet the potential requirements of organic oxidation.Therefore,in the present hybridized valence band com-posed of O 2p and Bi 6s,the photogenerated carriers may own a high mobility.Then it will reduce the recombination opportunities of the photogenerated electron-hole pairs that could effectively move to the crystal surface to degrade the absorbed MO molecules.Based on the above consideration,the possible photocatalytic mechanism of BNdT is established and a schematic diagram is shown in Fig.12.The higher photocatalytic activity of BNdT over TiO2and BIT is attributed to the suitable band gap and stable electron-hole pair formation in the VB formed by the hybrid orbitals of Bi 6s,Nd 5d,and O 2p and the CB of Ti 3d.
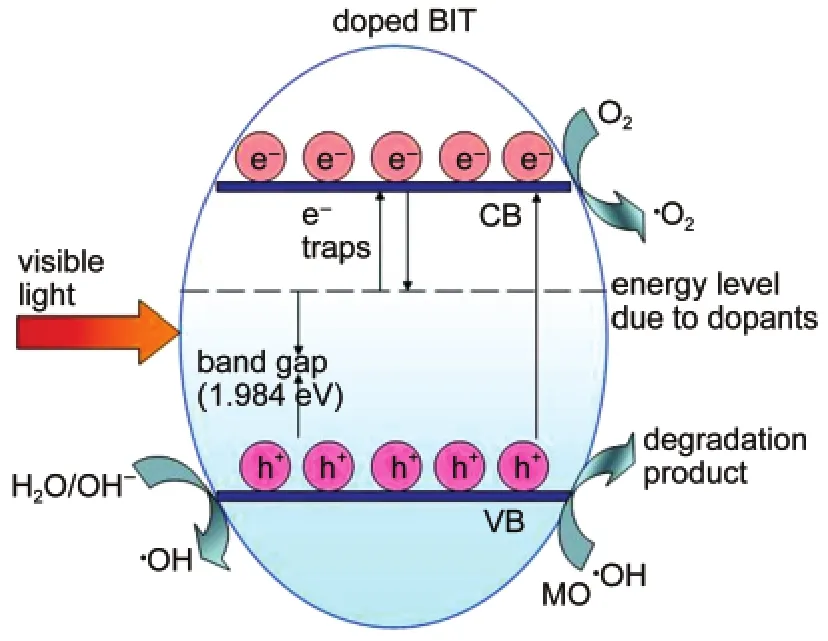
Fig.12 Schematic diagram of photocatalytic mechanism for BNdT sample
4 Conclusions
In summary,Bi3.25Nd0.75Ti3O12nanostructures were synthesized by a facile hydrothermal process without the use of any surfactant or template.The optical band gap energy of BNdT nanowires was estimated to be about 1.984 eV,which proved that BNdT photocatalyst can respond to the visible light.Besides,based on the structural analysis of samples obtained at different conditions,we also proposed a possible mechanism for the formation of these distinctive morphologies.Most importantly,BNdT photocatalysts with good stability exhibited higher photocatalytic performance in the degradation of methyl orange under visible light irradiation than traditional N-doped TiO2and commercial P25 TiO2.
(1) Uyguner-Demirel,C.S.;Bekbolet,M.Chemosphere 2011,84, 1009.
(2)Yu,J.G.;Xiang,Q.J.;Zhou,M.H.Appl.Catal.B:Environ. 2009,90,595.
(3)Xu,D.;Gao,A.M.;Deng,W.L.Acta Phys.-Chim.Sin.2008, 24(7),1219. [許 迪,高愛梅,鄧文禮.物理化學學報,2008, 24(7),1219.]
(4) Xie,J.;Wang,H.;Duan,M.Acta Phys.-Chim.Sin.2011,27(1), 193. [謝 娟,王 虎,段 明.物理化學學報,2011,27(1), 193.]
(5) Yang,X.H.;Liu,C.;Liu,J.K.;Zhu,Z.C.Acta Phys.-Chim. Sin.2011,27(12),2939.[楊小紅,劉 暢,劉金庫,朱子春.物理化學學報,2011,27(12),2939.]
(6) Hu,Y.F.;Li,Y.X.;Peng,S.Q.;Lü,G.X.;Li,S.B.Acta Phys.-Chim.Sin.2008,24(11),2071.[胡元方,李越湘,彭紹琴,呂功煊,李樹本.物理化學學報,2008,24(11),2071.]
(7) Li,A.C.;Li,G.H.;Zheng,Y.;Feng,L.L.;Zheng,Y.J.Acta Phys.-Chim.Sin.2012,28(2),457.[李愛昌,李桂花,鄭 琰,馮玲玲,鄭彥俊.物理化學學報,2012,28(2),457.]
(8)Zhang,Q.;He,Y.Q.;Chen,X.G.;Hu,D.H.;Li,L.J.;Yin,T.; Ji,L.L.Acta Phys.-Chim.Sin.2010,26(3),654.[張 瓊,賀蘊秋,陳小剛,胡棟虎,李林江,尹 婷,季伶俐.物理化學學報,2010,26(3),654.]
(9) Shen,J.J.;Liu,C.;Zhu,Y.D.;Li,W.;Feng,X.;Lu,X.H.Acta Phys.-Chim.Sin.2009,25(5),1013.[沈晶晶,劉 暢,朱育丹,李 偉,馮 新,陸小華.物理化學學報,2009,25(5), 1013.]
(10) Ghorai,T.K.;Biswas,S.K.;Pramanik,P.Appl.Surf.Sci.2008, 254,7498.
(11)Wang,H.Q.;Wu,Z.B.;Liu,Y.;Wang,Y.J.Chemosphere 2008, 74,773.
(12) Zhang,J.W.;Jin,Z.S.;Feng,C.X.;Yu,L.G.;Zhang,J.W.; Zhang,Z.J.J.Solid State Chem.2011,184,3066.
(13) Liu,D.R.;Jiang,Y.S.;Gao,G.M.Chemosphere 2011,83, 1546.
(14) Yu,J.Q.;Zhang,Y.;Kudo,A.J.Solid State Chem.2009,182, 223.
(15) Zhang,L.;Cao,X.F.;Chen,X.T.;Xue,Z.L.J.Colloid Interface Sci.2011,354,630.
(16) Zhang,L.S.;Wang,H.L.;Chen,Z.G.;Wong,P.K.;Liu,J.S. Appl.Catal.B:Environ.2011,106,1.
(17) Hou,J.G.;Wang,Z.;Jiao,S.Q.;Zhu,H.M.J.Hazard.Mater. 2011,192,1772.
(18)Hou,J.G.;Cao,R.;Jiao,S.Q.;Zhu,H.M.;Kumar,R.V.Appl. Catal.B:Environ.2011,104,399.
(19)Thanabodeekij,N.;Gulari,E.;Wongkasemjit,S.Powder Technol.2005,160,203.
(20) Hou,J.G.;Jiao,S.Q.;Zhu,H.M.;Kumar,R.V.J.Solid State Chem.2011,184,154.
(21) Zhou,T.F.;Hu,J.C.Environ.Sci.Technol.2010,44,8698.
(22)Cheng,H.F.;Huang,B.B.;Dai,Y.;Qin,X.Y.;Zhang,X.Y.; Wang,Z.Y.;Jiang,M.H.J.Solid State Chem.2009,182,2274.
(23) Lin,X.;Guan,Q.F.;Liu,Y.;Li,H.B.Chin.Phys.B 2010,19, 107701.
(24) Lin,X.;Guan,Q.F.;Li,H.B.;Liu,Y.;Zou,G.T.Sci. China-Phys.Mech.Astron.2012,55,33.
(25) Xu,J.J.;Chen,M.D.;Fu,D.G.Appl.Surf.Sci.2011,257, 7381.
(26) Xu,J.;Wang,W.Z.;Shang,M.;Gao,E.P.;Zhang,Z.J.;Ren,J. J.Hazard.Mater.2011,196,426.
(27)Yu,H.G.;Yu,J.G.;Cheng,B.Chemosphere 2007,66,2050.
(28)Wang,Z.Z.;Qi,Y.J.;Qi,H.Y.;Lu,C.J.;Wang,S.M.J.Mater. Sci.:Mater.Electron.2010,21,523.
(29)Yao,W.F.;Xu,X.H.;Wang,H.;Zhou,J.T.;Yang,X.N.; Zhang,Y.;Shang,S.X.;Huang,B.B.Appl.Catal.B:Environ. 2004,52,109.
(30) Xu,G.C.;Pan,L.;Guan,Q.F.;Zou,G.T.Acta Physica Sinica 2006,55,3080. [徐國成,潘 玲,關慶豐,鄒廣田.物理學報,2006,55,3080.]
(31)Hou,Y.D.;Wang,X.C.;Wu,L.;Chen,X.F.;Ding,Z.X.; Wang,X.X.;Fu,X.Z.Chemosphere 2008,72,414.
(32) Jiang,X.P.;Lin,M.;Tu,N.;Chen,C.;Zhou,S.L.;Zhan,H.Q. J.Alloy.Compd.2011,509,9346.
(33) Yang,J.H.;Zheng,J.H.;Zhai,H.J.;Yang,L.L.;Lang,J.H.; Gao,M.J.Alloy.Compd.2009,481,628.
(34)Arrouvel,C.;Digne,M.;Breysse,M.;Toulhoat,H.;Raybaud,P. J.Catal.2004,222,152.
(35)Hou,L.;Hou,Y.D.;Song,X.M.;Zhu,M.K.;Wang,H.;Yan, H.Mater.Res.Bull.2006,41,1330.
(36) Zhu,X.Q.;Zhang,J.L.;Chen,F.Chemosphere 2010,78,1350.
(37)Goto,T.;Noguchi,Y.;Soga,M.;Miyayama,M.Mater.Res. Bull.2005,40,1044.
(38)Cai,M.Q.;Yin,Z.;Zhang,M.S.;Li,Y.Z.Chem.Phys.Lett. 2004,399,89.
February 13,2012;Revised:March 29,2012;Published on Web:March 31,2012.
Bi3.25Nd0.75Ti3O12Nanostructures:Controllable Synthesis and Visible-Light Photocatalytic Activities
LIN Xue1,2GUAN Qing-Feng1,*LI Hai-Bo3LI Hong-Ji2BA Chun-Hua2DENG Hai-De2
(1School of Materials Science and Engineering,Jiangsu University,Zhenjiang 212013,Jiangsu Province,P.R.China;2College of Chemistry,Key Laboratory of Preparation and Application Environmentally Friendly Materials of the Ministry of Education,Jilin Normal University,Siping 136000,Jilin Province,P.R.China;3College of Physics,Jilin Normal University, Siping 136000,Jilin Province,P.R.China)
Neodymium-doped bismuth titanate(Bi3.25Nd0.75Ti3O12,BNdT)nanostructures with different morphologies were synthesized hydrothermally without using surfactant or template.Transmission electron microscopy(TEM)results showed that different morphologies could be fabricated simply by manipulating the concentration of OH-ions during hydrothermal synthesis.Hydroxide ions played an important role in controlling the formation of seeds and the growth rate of BNdT particles.On the basis of structural analysis of samples obtained under different conditions,a possible mechanism for the formation of these distinctive morphologies was proposed.A UV-visible diffuse reflectance spectrum(UV-Vis DRS)of an as-prepared BNdT sample revealed that its band gap energy(Eg)was about 1.984 eV.BNdT photocatalysts exhibited higher photocatalytic activities for the degradation of methyl orange(MO)under visible light irradiation than those for traditional commercial P25 TiO2and N-doped TiO2(N-TiO2).BNdT nanowires prepared using a hydroxide concentration of 10 mol·L-1showed the highest photocatalytic activity among the samples.Over this catalyst,93.0%degradation of MO(0.01 mmol·L-1)was obtained after irradiation with visible light for 360 min.In addition,there was no significant decrease in photocatalytic activity after the catalyst was used 4 times,indicating that BNdT is a stable photocatalyst for degradation of MO under visible light irradiation.
Bismuth titanate;Neodymium doping;Nanostructure;Hydrothermal synthesis; Photocatalytic degradation; Visible light irradiation
10.3866/PKU.WHXB201203313
?Corresponding author.Email:guanqf@ujs.edu.cn;Tel:+86-13852904936;Fax:+86-434-3290363.
The project was supported by the Key Laboratory of Preparation andApplication Environmentally Friendly Materials of the Ministry of Education of China,Scientific Research Innovation Plan for Young Talented Person and Plans of Scientific Research Innovation for Postgraduates of Jilin Normal University,China.
環境友好材料制備與應用教育部重點實驗室項目和吉林師范大學青年科研創新計劃創新人才項目、研究生科研創新項目資助
O643

