含吡嗪甲酸配體的雙核鉻配合物的水熱合成、晶體結構及熒光性質
郭玉華 郁有祝*,, 呂 壘 王軍月 黃 勇 李大成
(1安陽工學院化學與環境工程學院,安陽 455000)
(2聊城大學化學化工學院,聊城 252059)
含吡嗪甲酸配體的雙核鉻配合物的水熱合成、晶體結構及熒光性質
郭玉華1郁有祝*,1,2呂 壘2王軍月1黃 勇1李大成2
(1安陽工學院化學與環境工程學院,安陽 455000)
(2聊城大學化學化工學院,聊城 252059)
通過水熱法合成了1個雙核鉻配合物[Cr2(OH)2(2-pca)4]·3H2O(2-Hpca=2-吡嗪甲酸),并對其進行了X-射線單晶衍射、紅外光譜、元素分析、熒光和熱的表征及研究。該配合物屬于單斜晶系,P21/c空間群。晶體學數據:a=1.56635(15),b=0.84770(6),c=1.97460(18)nm,β=90.6450(10)°,C20H20Cr2N8O13,Mr=684.44,V=2.6217(4)nm3,Dc=1.734 g·cm-3,F(000)=1392,μ=0.913 cm-1,Z=4,最終R=0.0447,wR=0.1120用于3251個可觀測點。在晶體結構中,每個鉻原子均為六配位,其中來自兩分子2-吡嗪甲酸的2個氮原子、2個羧酸基氧原子和2個橋連羥基上的氧原子,呈現畸變的八面體幾何構型。
2-吡嗪甲酸;晶體結構;鉻配合物
In the field of inorganic-organic framework materials,the pyrazinecarboxylate ligand(pca)and its substituted derivatives have proven to be extremely versatile for the synthesis of novel coordination framework structures due to their ability to engage simultaneously in several different coordination modes[1-6].The vast majority of pca containing mixed-metal coordination polymers reported to date have utilized pca as a linear component of the metal-containing building blocks(MCBB)[7].Chromium-containing compounds have been the focus of research interest because of their potential applications in chemistry,biology,medicine andindustrial process[8].However,chromium complexes of pyrazine-2-carboxylic acid have been infrequently described in the literature[7,9].Joseph synthesized and characterized Cr(pca)3isolated from the solution reaction between pcaH and chromium metal salt where the pca complex was used not as linear MCBBs,but as pyramidal shaped MCBBs[7].[{M2(2-pca)2(H2O)4}{CrMo6(OH)7O17}]·17H2O(M=Co and Cu)were the first examples where the coordination polymer is coordinated to an Anderson-Evans cluster[9].In this paper we report the hydrothermal synthesis and crystal structure of a new chromium(Ⅲ)complex,dihydroxytetra[pyrazine-2-carboxylate]dichromium(Ⅲ)trihydrate.
1 Experimental
1.1 Materials and methods
All starting reagents were of A.R.grade and used as purchased without further purification.Analyses of C,H and N were determined on a Perkin-Elmer 240 Elemental analyzer.IR spectrum was recorded as KBr discs on a Perkin-Elmer SP-RX1 infrared spectrophotometer in the 4 000~400 cm-1range.The crystal data were collected on a Bruker SMART1000-CCD diffractometer.Thermogravimetric analysis(TG)data were collected on a DTG-60(H)instrument under N2atmosphere with a heating rate of 10℃·min-1.
1.2 Synthesis of the title complex
CrCl3(1 mmol,0.266 5 g),Hpca(3 mmol,0.372 3 g)and NaOH(3 mmol,0.120 g)were mixed in H2O(15 mL)and heated at 443 K for 4 d in a sealed 25 mL Teflon-lined stainless steel vessel under autogenous pressure.After cooling to room temperature at a rate of 10℃·h-1,red block crystals were isolated,washed with methanol and then dried in air,obtaining crystals in 30%yield.Anal.Calcd.(%):C,35.10;H,2.95;N,16.37.Found(%):C,35.07;H,2.99;N,16.33.
1.3 X-ray structure determination
A dark-red crystal with dimensions of 0.49 mm×0.31 mm×0.18 mm was mounted on a glass fiber.All diffraction data were collected on a Bruker SMART 1000 CCD diffractometer equipped with a graphitemonochromatized Mo Kα radiation(λ=0.071 073 nm)at 293(2)K by using an ω-2θ scan mode(1.3°≤θ≤25.02°).A total of 13 161 reflections were collected with 4 619 unique ones(Rint=0.037 9).Corrections for Lp factors and empirical absorption were applied.The structure was solved by directmethods with SHELXS-97 program[10]and refined by full-matrix least-squares techniques on F2with SHELXL-97[11].All non-hydrogen atoms were refined anisotropically and hydrogen atoms isotropically.The final R=0.044 7 and wR=0.1120(w=1/[σ2(Fo2)+(0.0698P)2+3.51P],where P=(Fo2+2Fc2)/3)for 3251 observed reflections(I>2σ(I)).(Δ/σ)max=0.000,S=1.007,(Δρ)max=878 e·nm-3and(Δρ)min=-387 e·nm-3.
CCDC:752196.
2 Rsults and discussion
2.1 Infrared spectrum
The FTIR spectra of the title compound(in KBr)show the bands as follows:3 452(s),3 103(w),1 678(s),1 595(w),1 473(w),1 356(w),1 339(m),1 060(m),1 024(w),864(m),791(m)and 556(m)cm-1.IR spectrum of the compound shows the typical(1 678 cm-1)stretching bands of carboxylate groups.Peak at 1595.6 cm-1could be attributed to the stretching vibration of C=N bond.The absence of strong peaks around 1700 cm-1suggests that all carboxylic groups are deprotonated[12].In addition,the strong and broad band centered at 3 452 cm-1for the title compound is attributable to the H-O-H stretching vibration of water molecule and OH group on the basis of known structure.
2.2 Structure analysis
The crystal structure of the title compound is revealed in Fig.1.The selected bond lengths and bond angles are listed in Table 1.The complex,crystallized as[Cr2(OH)2(2-pca)4]·3H2O,belongs to the monoclinic system.The Cr1(Ⅲ)ion displays distorted octahedral coordination environment,with the O(1),O(2),N(1),N(3)formingthe basal plane and the O(3),O(5)occupying the axial position.The deviation of the chromium atom is 0.0011 nm from the basal plane.Two[Cr(pca)]units are bridged by two hydroxo ligands to give a chromium to chromium separation of 0.297 5 nm in the structure.The Cr(1)-Cr(2)-O(1)-O(2)bridging unit is nearly planar.The deviation of O(1)and O(2)is 0.004 5 nm from the basal plane.The Cr(1)-O(1)-Cr(2)and Cr(1)-O(2)-Cr(2)angle in the bridge is respectively 100.25(12)°and 99.84(12)°,while the O(1)-Cr(1)-O(2)and O(1)-Cr(2)-O(2)angle is 79.96(11)°and 79.45(11)°.These parameters are within the ranges of the core distances and angles for the 12 previously characterized bis(μ-hydroxo)-bridged dimers[13-16],which are as follows:Cr…Cr,0.294 9~0.308 6 nm;Cr-OH,0.191 1~0.198 8 nm;Cr-O-Cr,97.57°~104.08°;O-Cr-O,75.92°~82.40°.There is no crystallographically imposed symmetry in the dimer;On one side of the dimer(Cr1)the pyridine rings are cis to one another and trans to the bridging hydroxide groups;on the other side(Cr2)they are trans to one another and cis to the bridging hydroxide groups.

Table 1 Selected bond lengths(nm)and bond angles(°)

Fig.1 Molecular structure of the chromium complex at 30%probability thermal ellipsoids
Hydrogen bonding interactions are ususllyimportant in the synthesis of supramoleculararchitecture [17]. The molecular conformation isstabilized by intramolecular O-H…O and O-H…Nbonds. In the crystal structure, complex molecules andhydrate solvent molecules are linked into a threedimensionalnetwork by O -H … O and O -H … Nhydrogen-bonding interactions. The hydrogen bonds ofthe title compound are listed in Table 2 and theselected hydrogen bonding interactions among thecomplexes are revealed in Fig.2.

Table 2 Hydrogen bonds of the title compound

Fig.2 Selected hydrogen bonding interactions among the complexes
2.3 Thermal analysis
The thermal analysis experiment was performed on samples consisting of numerous single crystals of the title complex under N2 atmosphere.The sample of the complex was heated to 800℃(Fig.3).The first weight loss of 8.67%(calcd.7.89%)between 60.9 and 177.8℃corresponds to the release of uncoordinated water molecules in the complex.An obvious weight loss occurred in the range of 298.1~470.8℃,which may be attributed to the elimination of ligands.The complex continued to decompose from 470.8℃.
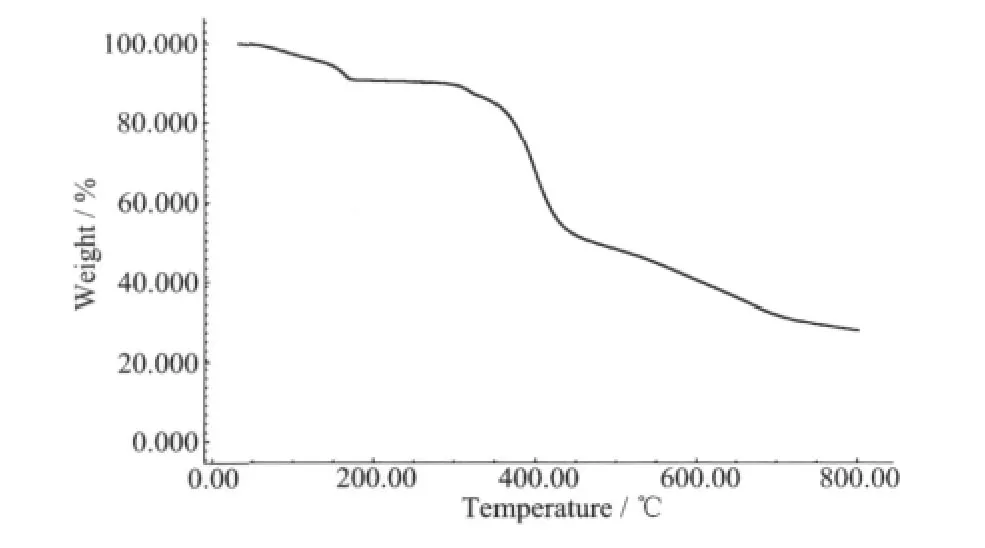
Fig.3 TG curve of the title complex
2.4 Luminescent properties
Luminescent compounds are currently of great interest because of their various applications inchemical sensors, photochemistry, and electroluminescent(EL) displays[18].To examine the luminescent properties of 2-pca ligand and its complex,they were investigated at room temperature.As shown in Fig.4,while 2-pca ligand displays purple luminescence in the solid state at λmax=421 nm upon excitation at λ=262 nm,under the same experimental conditions,its complex exhibits the weak luminescent emissions at λmax=423 nm upon excitations at 260 nm in the purple fluorescent regions.We believe that the complex in this work might absorb the luminous energy and then emit fluorescence mainly through their fluorene ring of ligand resulting from the ligand-to-ligand charge transfer(LLCT),similar to the reported results on coordinationpolymers[19-22].In addition,the changes of luminescences of 2-pca ligand and its complex may be attributed to the chelating and/or bridging effect of the relevant ligands to the metal centres,which may change the rigidity of the ligand and the loss of energy via a radiationless pathway[19,21-24].

Fig.4 Emission spectra of 2-pca ligand and its complex in the solid state at room temperature
[1]Ciurtin D M,Smith M D,zur Loye H C.Chem.Commun.,2002:74-75
[2]Dong Y B,Smith M D,zur Loye H C.Angew Chem.Int Ed.,2000,39:4271-4273
[3]Ciurtin D M,Smith M D,zur Loye H C.J.Chem.SOC.,Dalton Trans.,2003:1245-1250
[4]Ellsworth J M,Su C Y,Khaliq Z,et al.J.Mol.Struct.,2006,796:86-94
[5]Zheng L M,Whitefield T,Wang X.Q,et al.Angew.Chem.Int.Ed.,2000,112:4702-4705
[6]Maggard P A,Yan B B,Luo J H.Angew.Chem.Int.Ed.,2005,44:2553-2556
[7]Ellsworth J M,Khaliq Z M,Seward K L,et al.J.Chem.Cryst.,2007,37:749-754
[8]Ramos R L,Martinez A J,Coronado G R M.Water Sci.Technol.,1994,30:191-197
[9]Singh M,Ramanan A.Cryst.Growth Des.,2011,11:3381-3394
[10]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Sturcture,University of G?ttingen,Germany,1997.
[11]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Sturcture,University of G?ttingen,Germany,1997.
[12]Dai Y M,Tang E,Li Z J,et al.Chin.J.Struct.Chem.,2006,25:971-974
[13]Diane M S,William H A.Inorg.Chem.,1992,31:5178-5184
[14]Gafford B G,Holwerda R A.Inorg.Chem.,1989,28:60-66
[15]Lethbridge J W.Chem.Soc.,Dalton Trans.,1980:2039-2041
[16]Heinrichs M A,Hodgson D J,Michelsen K,et al.Inorg.Chem.,1984,23:3174-3180
[17]LI Xiu-Mei(李秀梅),WANG Qing-Wei(王慶偉),LIU Bo(劉博),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(6):1207-1211
[18]Liu C S,Chen P Q,Chang Z,et al.Inorg.Chem.Commun.,2008,11:159-163
[19]Liu C S,Wang J J,Yan L F,et al.Inorg.Chem.,2007,46:6299-6310
[20]Chang Z,Zhang A S,Hu T L,et al.Cryst.Growth Des.,2009,9:4840-4846
[21]Liu C S,Wang J J,Chang Z,et al.CrystEngComm.,2010,12:1833-1841
[22]Wang J J,Zhu H M,Wang S M.Transition Met.Chem.,2011,36:579-584
[23]Wang J J,Shi Y F,Li S J.Z.Anorg.Allg.Chem.,2011,637:1590-1596
[24]Wang J J,Hu T L,Bu X H.CrystEngComm.,2011,13:5152-5161
Hydrothermal Synthesis,Crystal Structure and Luminescence of a Binuclear Chromium Complex with Pyrazinecarboxylate Ligand
GUO Yu-Hua1YU You-Zhu*,1,2LüLei1WANG Jun-Yue1HUANG Yong1LI Da-Cheng2
(1College of Chemistry and Environmental Engineering,Anyang Institute of Technology,Anyang,Henan 455000,China)
(2College of Chemistry and Chemical Engineering,Liaocheng University,Liaocheng,Shandong 252059,China)
Abinuclear chromium complex[Cr2(OH)2(2-pca)4]·3H2O(2-pca=pyrazine-2-carboxylate anion)was hydrothermally prepared and characterized by single crystal X-ray diffraction,FTIR spectra,elemental analysis,luminescence and thermal analysis.It crystallizes in the monoclinic system,space group P21/c,with a=1.56635(15),b=0.847 70(6),c=1.974 60(18)nm,β=90.645 0(10)°,C20H20Cr2N8O13,Mr=684.44,V=2.621 7(4)nm3,Dc=1.734 g·cm-3,F(000)=1392,μ=0.913 cm-1,Z=4,the final R=0.0447 and wR=0.1120 for 3251 reflections with I>2σ(I).X-ray diffraction analysis reveals that each Cr(Ⅲ)center is octahedrally coordinated by two nitrogen atoms and two oxygen atoms from two pyrazine-2-carboxylate anion ligands,and another two oxygen atoms from two hydroxyl groups.The two Cr(Ⅲ)centers are bridged by two μ2-O of OH group.CCDC:752196.
pyrazine-2-carboxylic acid;crystal structure;chromium(Ⅲ)complex
O614.61+1
A
1001-4861(2012)04-0846-05
2011-06-21。收修改稿日期:2011-12-23。
國家自然科學基金(No.20671048,21041002)資助項目。
*通訊聯系人。E-mail:119yyz@163.com

