異煙酰胺基間苯二甲酸根構筑的混核鎳-鋱配合物的合成、晶體結構及熒光性質
譚雄文 鄧奕芳 張春華 鄺代治 陳滿生
(功能金屬有機材料湖南省普通高等學校重點實驗室,衡陽師范學院化學與材料科學系,衡陽 421008)
異煙酰胺基間苯二甲酸根構筑的混核鎳-鋱配合物的合成、晶體結構及熒光性質
譚雄文 鄧奕芳 張春華*鄺代治 陳滿生*
(功能金屬有機材料湖南省普通高等學校重點實驗室,衡陽師范學院化學與材料科學系,衡陽 421008)
水熱合成了一個的雜金屬配合物{[NiTb2(INAIP)4(H2O)6]·8H2O}n(1)(INAIP2-=異煙酰胺吡啶基異酞酸根),并對其進行了元素分析、IR及X-射線衍射法表征。晶體結構研究表明:配合物1屬于三斜晶系,P1空間群。配合物1是由配體異煙酰胺吡啶基異酞酸連接而成的二維雙層狀結構,該二維層通過氫鍵延伸為三維超分子結構。熒光測試研究表明配合物1具有典型的稀土鋱離子綠色熒光。
3d-4f配合物;晶體結構;熒光性質;氫鍵
In the last decade,the rational design and synthesis of higher-dimensional transition-lanthanide metal(d-f)heterometallic networks have attracted increasing attention,which is justified not only by the fascinating structural diversity of the architectures but also by the potential applications of these complexes as important functional solid materials[1-8].On the other hand,the useful way to synthesis of the heterometallic coordination polymers is the assembly from the mixed metal ions and logic multidentate organic ligands.Furthermore,the lanthanide ions usually prefer O-to N-donors,while transition metal ions have a strongtendency to coordinate to both N-and O-donors[9-11].Thus,ligands containing O-and N-donors can elaborately be selected and bond relatively easily to both Ln ions and d-block metal ions to form heterometallic MOFs.5-(isonicotinamido)isophthalic acid(H2INAIP)can also show richer coordination modes due to its two carboxylate groups and one pyridyl group,accordingly,it is an excellent candidate for the construction of metal organic frameworks.Herein we report the synthesis,crystal structure and photoluminescence property of a new mixed metal coordination polymers,namely{[NiTb2(INAIP)4(H2O)6]·8H2O}n(1).
1 Experimental
1.1 Materials and instruments
The regents were used from commercial sources without further purification.Elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer.The IR spectra were recorded on Bruker Vector22 FTIR spectrophotometer using KBr discs.Thermogravimetric analyses were performed on a simultaneous SDT 2960 thermal analyzer under nitrogen with a heating rate of 10℃·min-1.The luminescent spectra for the solid powdered samples were recorded at room temperature on an Aminco Bowman Series 2 spectrophotometer with xenon arc lamp as the light source.In the measurements of the emission and excitation spectra,the pass width was 4.0 nm.All the measurements were carried out under the same conditions.
1.2 Synthesis of the complex 1
Complex 1 was synthesized by hydrothermal method in a 16 mL Teflon-lined autoclave by heating a mixture containing NiSO4·6H2O(26.2 mg,0.1 mmol),Tb(NO3)3·6H2O(46.4 mg,0.1 mmol),H2INAIP(28.7 mg,0.1 mmol),and NaOH(6.0 mg,0.15 mmol)dissolved in 10 mL H2O and heated at 160℃for 5 d.Block pale green single crystals of 1 were collected by filtration and washed with water and ethanol for several times with a yield of 31%(based on H2INAIP).Anal.Calcd.for C56H60NiTb2N8O34(%):C 38.06;H 3.40;N 6.34;found(%):C 38.14;H 3.46;N 6.28.IR(KBr pellet,cm-1):3 422(s),1 665(m),1 619(m),1 542(s),1 429(m),1 378(s),1 287(m),889(w),785(w),726(m),616(m),598(w).
1.3 X-ray crystallography
The X-ray diffraction measurement for 1 was performed on the Bruker Smart ApexⅡCCD diffractometer with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at room temperature.The data were integrated by using the SAINT program[12],and the intensity was corrected for Lorentz and polarization effect.An empirical absorption correction was applied using the SADABS program[13].The structures were solved by direct methods using the program SHELXS-97 and all the non-hydrogen atoms were refined anisotropically on F2by the full-matrix least-squares technique using the SHELXL-97crystallo-graphic software package[14-15].Crystal data and structure refinement parameters are listed in Table 1.The selected bond lengths are given in Table 2.
CCDC:853173.
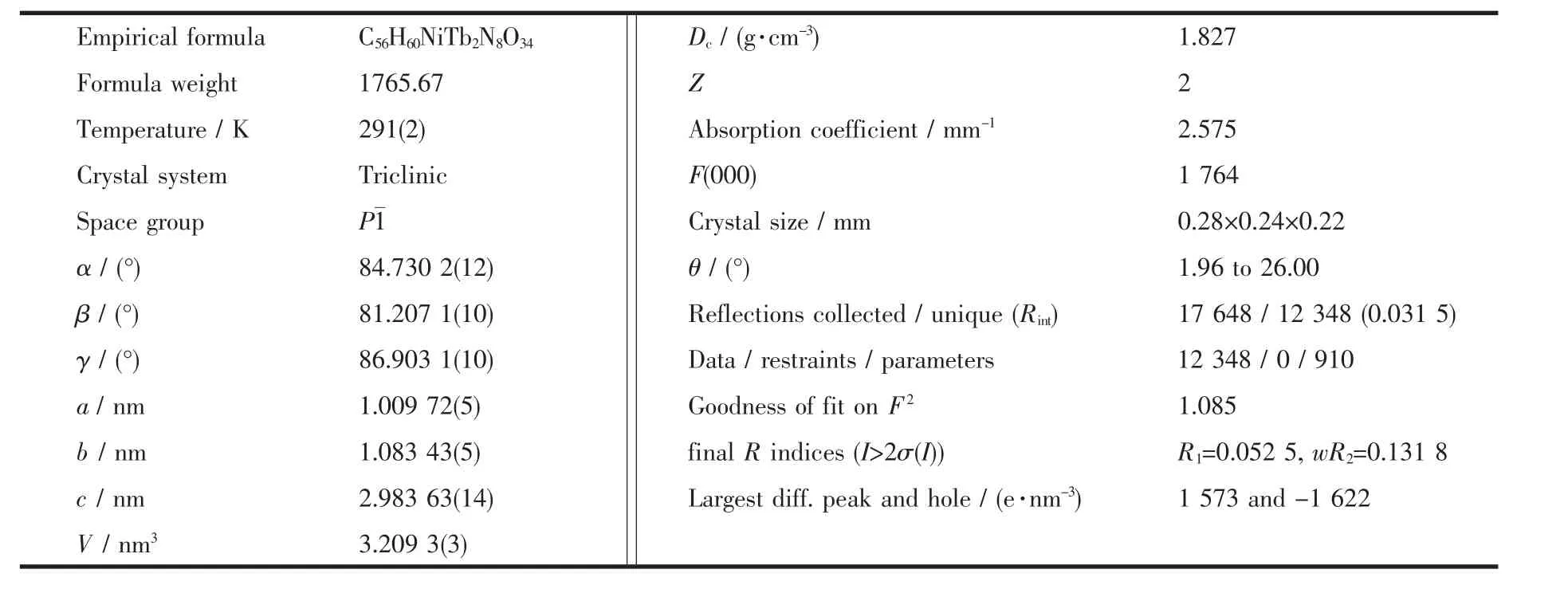
Table 1 Crystal data and structure parameters for complex 1
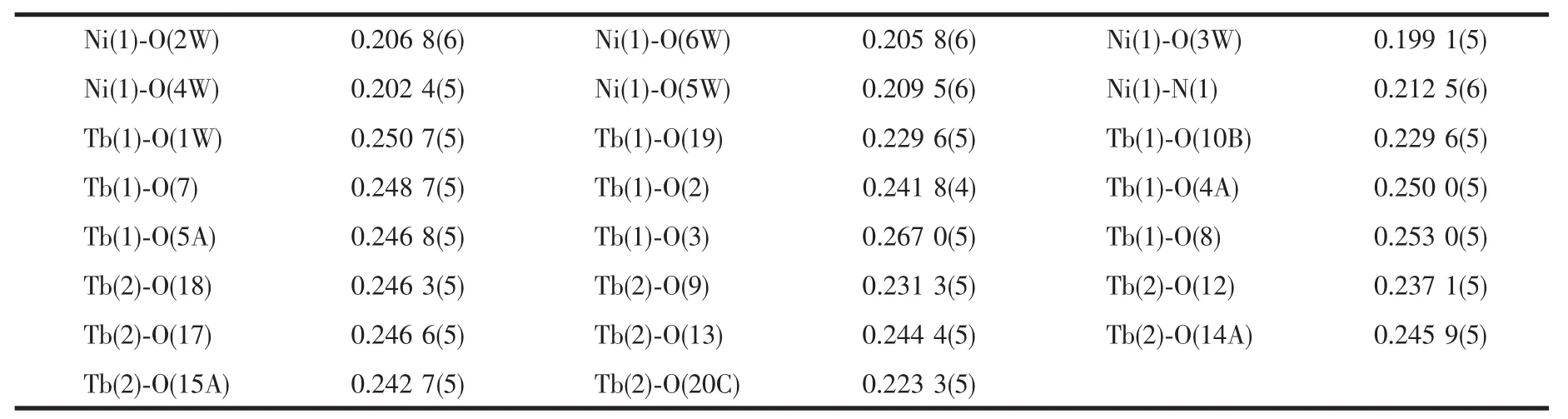
Table 2 Selected bond lengths(nm)for the title complex
2 Results and discussion
2.1 Structure description
X-ray diffraction analysis reveals that a 2D bilayer of 1 crystallizes in space group P1.As illustrated in Fig.1,the asymmetric unit of 1 contains one Ni(Ⅱ)atom,two Tb(Ⅲ)atoms,four INAIP2-ligands,six coordinated and eight non-coordinated water molecules.Each Ni(Ⅱ)atom of1 with six-coordinated octahedral coordination geometry is coordinated by one pyridyl nitrogen(N1)and five oxygen(O2W,O3W,O4W,O5W,O6W)atoms from five water molecules with Ni1-N bond length of 0.2125(6)nm,the Ni1-O ones in the range of 0.199 1(5)~0.209 5(6)nm and bond angles around the Ni(Ⅱ)in the range of 83.5(3)°to 176.0(2)°(Table 2).The Tb1 in 1 is nine-coordinated with the distorted tricapped trigonal prism coordination geometry by eight carboxylate oxygen atoms from five INAIP2-ligands and another oxygen atom from one coordinated water molecule(Fig.1).However,Tb2 is eight coordinated with eight carboxylate groups O atoms from five INAIP2-ligands,forming a bicapped trigonal prism,rather than that of Tb1.It is noteworthy that three unique INAIP2-ligands in 1 exhibit three different kinds of coordination modes:one coordinates to two Tb(Ⅲ))atoms using its two carboxylate groups with μ1-η1∶η1-chelate coordina-tion modes and the pyridyl group is bonded to one Ni(Ⅱ)atom,another one coordinates to two Tb(Ⅲ)through the carboxylate groups with μ1-η1∶η1-chelate coordination mode while the pyridyl group is free coordination,the third one coordinates to three Tb(Ⅲ)through the carboxylate groups with μ1-η1∶η1-chelate and μ2-η1∶η1bis-monodentate and the pyridyl group is also free coordination.Based on the special coordination of the Ni(Ⅱ)center,a(4,4)2D bilayer is formed by Tb(Ⅲ)-carboxylate groups and Ni-N and Ni-O connections as shown in Fig.2.Finally,the 2D networks are further linked together by O-H…O,O-H…N and N-H…O hydrogen bonding interactions to generate 3Dsupramolecular frameworks. The detailed data ofhydrogen bonds for 1 are shown in Table 3.
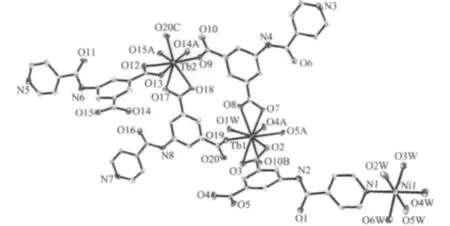
Fig.1 Coordination environment of Ni(Ⅱ)and Tb(Ⅲ)atoms in complex 1 with 30%thermal ellipsoids
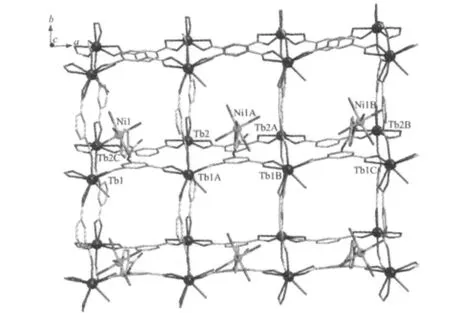
Fig.2 2D layer structure linked by INAIP2-ligands and Ni(Ⅱ)-Tb(Ⅲ)centers without the uncoordinated pyridyl groups
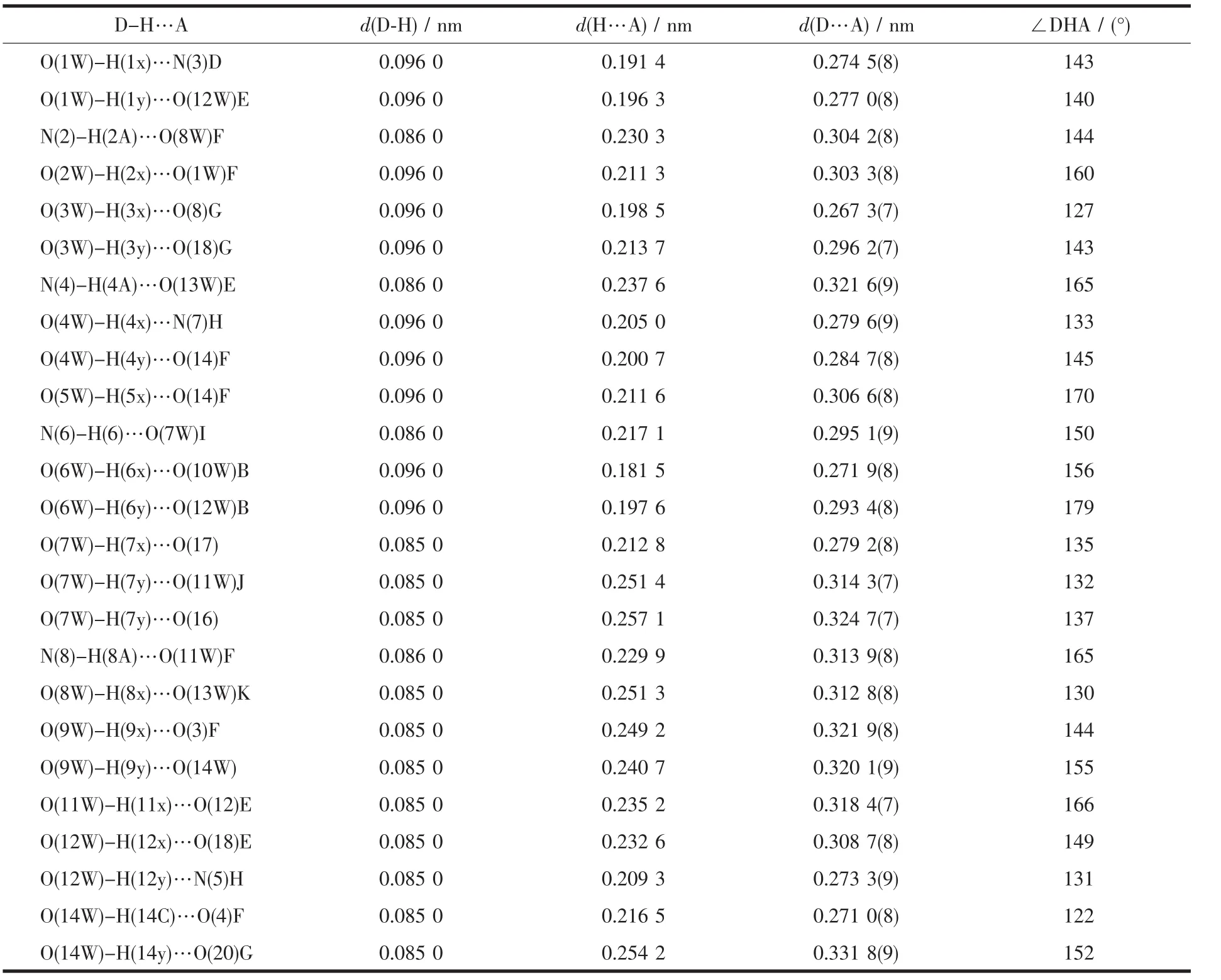
Table 3 Parameters of hydrogen bonds for the complex 1
2.2 IR,TG and photoluminescence property
The infrared spectra of the title complex has been recorded and some important data are shown in 1.2.In the IR spectrum of 1,the band at 3 422 cm-1,due to the ν(O-H)absorptions of water molecules.Strong absorptions at 1 619,1 542 cm-1[νas(COO-)]and 1 429,1 378 cm-1[νs(COO-)]are assigned to a carboxy-late[16].There is also a strong absorption at 1 665 cm-1,which clearly indicates the protonated carboxylate in complex 1.These IR results are coincident with the crystallographic structural analyses.
The results of thermogravimetric analyses(TGA)indicate that complex 1 looses its coordinated and noncoordinated water molecules in the temperature range of 30~215℃.The weight loss of 14.32%is consistent with the calculated one of 14.27%.After the loss of all the water molecules,the supramolecular framework is stable up to 310℃,followed by another weight loss at high temperature.
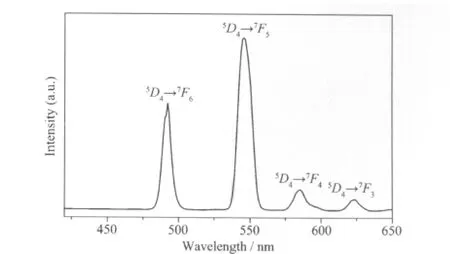
Fig.3 Solid-state emission spectrum of the title complex 1
Owing to the excellent luminescent property of Tb(Ⅲ)ions,the room temperature photoluminescence in the solid state of 1 was investigated.The emission spectrum of 1(Fig.3)upon excitation at 390 nm exhibits the characteristic transitions of5D4→7FJ(J=3~6)of Tb(Ⅲ),which the bands are at 489,544,587 and 624 nm can be attributed to the corresponding transitions[17-18].The peak at 544 nm is the strongest,ascribing to the5D4→7F5transition which gives intense green luminescence output for 1.
[1]Cheng J W,Zhang J,Zhang S T,et al.Angew.Chem.Int.Ed.,2006,45:73-77
[2]Liang Y C,Cao R,Su W P,et al.Angew.Chem.,Int.Ed.,2000,39:3304-3307
[3]Cai Y P,Zhou X X,Zhou Z Y,et al.Inorg.Chem.,2009,48:6341-6343
[4]Lin X M,Fang H C,Zhou Z Y,et al.CrystEngComm,2009,11:847-854
[5]Zhang M B,Zhang J,Zheng S T,et al.Angew.Chem.Int.Ed.,2005,44:1385-1388
[6]Gu X J,Xue D F.Inorg.Chem.,2006,45:9257-9261
[7]Shibasaki M,Yoshikawa N.Chem.Rev.,2002,102:2187-2210
[8]Zhao B,Cheng P,Chen X Y,et al.J.Am.Chem.Soc.,2004,126:3012-3013
[9]Sun Y Q,Zhang J,Yang G Y.Chem.Commun.,2006:4700-4702
[10]Zhao B,Chen X Y,Cheng P,et al.J.Am.Chem.Soc.,2004,126:15394-15395
[11]Lin X M,Ying Y,Chen L,et al.Inorg.Chem.Commun.,2009,12:316-320
[12]SAINT,Version 6.02a,Bruker AXS Inc.:Madison,W1,2002.
[13]Sheldrick G M.SADABS,Program for Bruker Area Detector Absorption Correction,University of G?ttingen,G?ttingen,Germany,1997.
[14]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of G?ttingen,G?ttingen,Germany,1997.
[15]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of G?ttingen,G?ttingen,Germany,1997.
[16]Cao T T,Ma Y,Zhou N,et al.J.Coord.Chem.,2010,63:988-995
[17]Li Z Y,Zhu G S,Guo X D,et al.Inorg.Chem.,2007,46:5174-5178
[18]Liu Z H,Qiu Y C,Li Y H,et al.Polyhedron,2008,27:3493-3499
Hydrothermal Synthesis,Crystal Structure and Luminescence of Ni-Tb Complex Based on 5-(Isonicotinamido)isophthalate
TAN Xiong-WenDENG Yi-FangZHANG Chun-Hua*KUANG Dai-ZhiCHEN Man-Sheng*
(Key Laboratory of Functional Organometallic Materials of Hengyang Normal University,College of Hunan Province,Department of chemistry and Materials Science,Hengyang,Hunan 421008,China)
A 3d-4f coordination polymer{[NiTb2(INAIP)4(H2O)6]·8H2O}n(1)was obtained by hydrothermal assembly of NiSO4·6H2O and Tb(NO3)3·6H2O with the H2INAIP ligand.Complex 1 crystallizes in triclinic,space group P1 with a=1.009 72(5)nm,b=1.083 43(5)nm,c=2.983 63(14)nm,V=3.209 3(3)nm3,Z=2,C56H60NiTb2N8O34,Mr=1765.67,Dc=1.827 g·cm-3,μ=2.575 mm-1,F(000)=1764,Rint=0.0315,R=0.0525,wR=0.1318.Single-crystal X-ray diffraction analysis reveals that each INAIP2-ligand uses its two carboxyl groups to connect two/three Tb(Ⅲ)centers,and the Ni(Ⅱ)only bonds to one pyridyl group and terminal water molecules,forming a two-dimensional(2D)bilayer.On the other hand,the 2D layers are further connected by hydrogen bonding interactions to give a three-dimensional(3D)supramolecular structure.The complex 1 at room temperature shows typical green photoluminescence of rare earth Tb3+ion.CCDC:853173.
3d-4f complex;crystal structure;luminescent property;hydrogen bond
O614.341;O614.81+3
A
1001-4861(2012)04-0856-05
2011-09-18。收修改稿日期:2011-10-19。
衡陽師范學院科學基金啟動項目(No.10B67),湖南省教育廳創新平臺開放基金(No.11K009)和衡陽市科技局(No.2011KJ24)資助項目。
*通訊聯系人。E-mail:cmsniu@163.com,zhangchunhua@163.com;會員登記號:S06N7223M1009。

