以鄰菲咯啉衍生物及1,3-間苯二甲酸為配體的一維鏈狀Cd(Ⅱ)配位聚合物的合成及結(jié)構(gòu)
徐占林 賀 宇 馬 帥 伍錫榮
(1吉林師范大學化學學院,吉林師范大學環(huán)境友好材料制備與應用省部共建教育部重點實驗室,四平 136000)
(2馬來亞大學化學系,吉隆坡 50603,馬來西亞)
以鄰菲咯啉衍生物及1,3-間苯二甲酸為配體的一維鏈狀Cd(Ⅱ)配位聚合物的合成及結(jié)構(gòu)
徐占林*,1賀 宇1馬 帥1伍錫榮2
(1吉林師范大學化學學院,吉林師范大學環(huán)境友好材料制備與應用省部共建教育部重點實驗室,四平 136000)
(2馬來亞大學化學系,吉隆坡 50603,馬來西亞)
在水熱條件下,我們利用鄰菲咯啉衍生物配體(L=2-(3-fluorophenyl)-1H-imidazo[4,5-f][1,10]phenanthroline)和1,3-間苯二甲酸(1,3-H2BDC)反應得到了一維配位聚合物[Cd2(L)2(1,3-BDC)2]n,并對該化合物進行了元素分析、紅外和單晶X-射線表征。該化合物屬于三斜晶系,空間群P1,晶胞參數(shù)a=1.0808(4)nm,b=1.149 5(4)nm,c=1.924 8(7)nm,α=106.482(5)°,β=99.436(6)°,γ=93.093(6)°,V=2249.2(14)nm3,Z=4,C27H15CdFN4O4,Mr=590.83,Dc=1.745 g·cm-3,F(xiàn)(000)=1176,μ(Mo Kα)=1.024 mm-1,R=0.0445和wR=0.1117。該化合物為一維鏈狀結(jié)構(gòu),鏈與鏈之間又進一步地通過CH-π相互作用形成二維層狀超分子結(jié)構(gòu)。
配位聚合物;晶體結(jié)構(gòu);鄰菲咯啉衍生物;1,3-苯二甲酸
0 Introduction
Usually,coordination-bonded interactions for the construction of coordination polymers are the most effective force.Moreover,noncovalent interactions,such as π-π stacking interactions and hydrogen bonding interactions,which are described as supramolecular glues,are often used as structural directing tools in generating a number of novel supramolecular structures with promising properties[7-9].Therefore,versatile functional organic ligands(such as N-or O-containing ligands)which have strong coordination ability as well as providing the π-conjugated systems and hydrogen bond acceptors/donors are often employed in the construction of novel supramolecular framework[10].So far,N-containing ligands,such as 1,10-phenanthroline(phen),pyrazine,2,2′-bipyridine,4,4′-bipyridine and bis(imidazole)have been employed widely in the construction of coordination polymers[11-12].Among the N-containingligand,(2-(3-fluorophenyl)-1H-imidazo[4,5-f][1,10]phenanthroline(L)is an excellent N-donor chelating ligand for the construction of coordination polymers[13].In this work,we selected 1,3-benzenedicarboxylate(1,3-BDC)as an organic linker and L as a N-donor chelating ligand,generating a new one-dimensional coordination polymer,[Cd2(L)2(1,3-BDC)2]n(1).
1 Experimental
1.1 Generals
The L ligand was synthesized according to the reported method[13]and all other materials were analytical reagent grade and used as received without further purification.Elemental analysis was carried out with a Perkin-Elmer 240C analyzer;IR spectra were obtained on a Perkin-Elmer 2400LSⅡspectrometer.
1.2 Synthesis and crystal growth
A mixture ofCd(NO3)2·4H2O(0.5 mmol),1,3-H2BDC(0.5 mmol)and L(0.5 mmol)was dissolved in 8 mL distilled water.The pH value of the mixture was adjusted to between 5 and 6 by addition of triethylamine.The resultant solution was heated at 455 K in a Teflon-lined stainless steel autoclave for seven days.The reaction system was then slowly cooled to room temperature.Pale yellow crystals of 1 suitable for single crystal X-ray diffraction analysis were collected from the final reaction system by filtration,washed several times with distilled water and dried in air at ambient temperature.Yield:32%based on Cd(Ⅱ).IR(KBr,cm-1):1 621s,1 612m,1 580m,1 544m,1 460m,1 377w,1 340m,1 127w,865m,736w,629w.Anal.Calcd.for C20H20CdFN4O10(%):C,39.52;H,3.32;N,9.22.Found(%):C,39.72;H,3.25;N,9.30.
1.3 X-ray structure determination
A single crystal with dimensions of 0.20 mm×0.15 mm×0.12 mm was selected and mounted on a Bruker Smart Apex CCD diffractometer equipped with a graphite-monochromatized Mo Kα(λ=0.071 073 nm)radiation by using an ω-2θ scanning method at a temperature of(20±2)℃.Out of the total 10 842 reflections collected in the 1.86°≤θ≤25.0°range,7 690 were independent with Rint=0.020 9,of which 6 106 were considered to be observed(I>2σ(I))and used in the succeeding refinement.The structure was solved by Direct Method with SHELXS-97 program[14]and refined with SHELXL 97[15]by full-matrix least-squares techniques on F2.All non-hydrogen atoms were refined anisotropically and hydrogen atoms isotropically.The flurophenyl unit is disordered over two sites in a 1∶1 ratio.The final R=0.044 5 and wR=0.111 7(w=1/[σ2(Fo2)+(0.054 6P)2+6.753 7P],where P=(Fo2+2Fc2)/3).S=1.062,(Δρ)max=0.979 e·nm-3,(Δρ)min=-0.598 e·nm-3and(Δ/σ)max=0.000.
CCDC:859146.
2 Results and discussion
2.1 Description of crystal structure
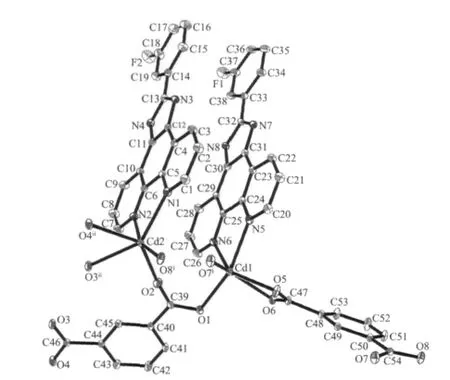
Fig.1 Coordination environments of Cd(Ⅱ)atoms in complex 1 with displacement ellipsoids at 30%probability level
The selected bond distances and angles are listed in Table 1.The asymmetric unit of 1 contains two crystallographically independent Cd(Ⅱ)atoms(Cd1 and Cd2),two kinds of L ligands,and two kinds of 1,3-BDC anions.As shown in Fig.1,each Cd(Ⅱ)atom is coordinated by two nitrogen atoms from one L ligand and four carboxylate oxygen atoms from three different 1,3-BDC anions,exhibiting a distorted CdN2O4octahedral coordination geometry.The Cd-O distances range from 0.2343(4)to 0.2848(5)nm.Notably,the two carboxylates of each 1,3-BDC show different coordination modes:one carboxylate chelates one Cd(Ⅱ)atom,while the other bridges two Cd(Ⅱ)atoms(Fig.1).The bridging carboxylate group of the1,4-bdcanion connects two Cd(Ⅱ)atoms to form a dimer with the Cd…Cd distance of 0.336 7(4)nm(Fig.2).The dinuclear units are bridged by the backbones of the 1,4-bdc ligands to form a chain structure(Fig.2).The ligands L are attached to both sides of the layers,allowing the formation of CH-π interactions(0.342 nm),and connect the adjacent chains to a two-dimensional supramolecular architecture(Fig.3).Obviously,the strong CH-π stacking interactions play an important role in stabilizing the supramolecular architecture of 1.Moreover,the N-H…Ohydrogen bonds(N(3)-H(3A)0.088 nm,H(3A)…O(6)iii0.205 nm,N(3)…O(6)iii0.289 2(6)nm,N(3)-H(3A)…O(6)iii=158.5°(symmetry code:iiix,y-1,z);N(8)-H(8A)0.088 nm,H(8A)…O(4)iv0.198 nm,N(8)…O(4)iv0.279 3(6)nm,N(8)-H(8A)…O(4)iv=152.5°(symmetry code:iv2-x,2-y,1-z))further stabilizes the structure of 1.Notably, when a similar phen derivativepyrazino [2,3-f][1,10]phenanthroline (L′) was usedto react with Cd 髤atoms in the presence of 1,4-naphthalenedicarboxylate (1,4-NDC), a structurallydifferentthree-dimensional α-polonium structure[Cd(1,4-NDC)(L′)]nwas reported[8].Clearly,the topological difference between 1 and the reported one is mainly attributed to the structural difference of the dicarboxylates.

Table 1 Selected bond distances(nm)and angles(°)fro the title complex
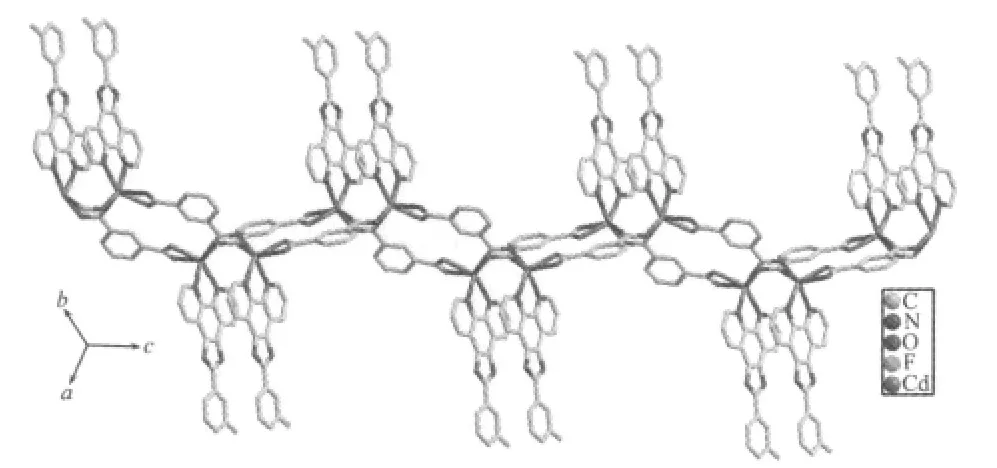
Fig.2 View of the chain structure of complex 1
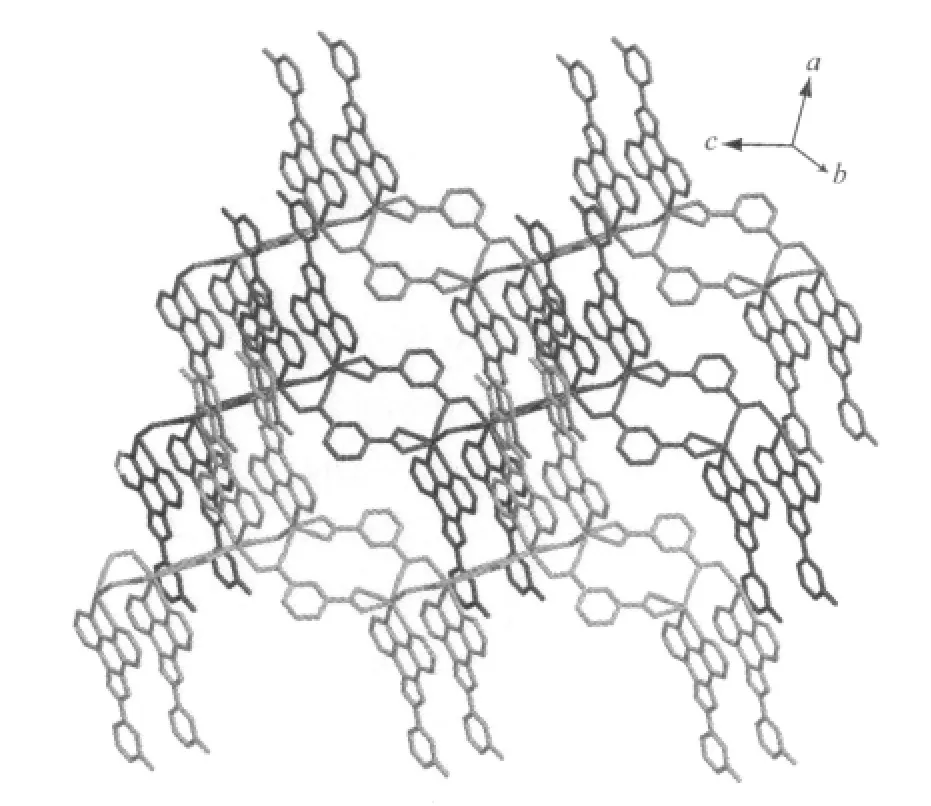
Fig.3 View of the 2D supramolecular layer architecture of 1 constructed through interchain CH-π interactions
2.2 IR analysis
The infrared spectrum of the compound 1 has a strong peak at about 1 621 cm-1corresponding to the stretching vibration of imino C=N bonds of the L ligand.Asymmetric and symmetric stretching of the carboxylate group of the 1,3-BDC appear at 1 612,1 580 cm-1(ν(OCO)assym)and 1 340,1 377 cm-1(ν(OCO)sym),respectively.The absence of characteristic bands at about 1 700 cm-1in the compound 1 indicates the complete deprotonation of 1,3-BDC ligand upon reaction with Cd(Ⅱ)atoms.
2.3 Thermal analysis
Thermogravimetric analysis was carried out for compound 1 in order to characterize the compound more fully in terms of thermal stability.The experiment was performed under N2atmosphere with a heating rate of 10℃·min-1in temperatures ranging from room temperature to 800℃.As shown in Fig.4,the anhydrous compound 1 is thermally stable up to around 395℃.The first weight loss corresponds to the release of 1,3-BDC ligand in the temperature range of 395~450℃(obsd.25.9%,calcd.27.3%).The second weight loss from 450 to 630℃can be attributed to the decomposition of L ligand(obsd.49.2%,calcd.51.7%).This result is in good accordance with the composition of the complex.
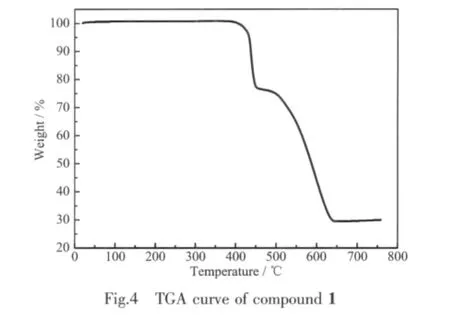
Fig.4 TGA curve of compound 1
2.4 Luminescent property
The luminescent properties of compound 1,free ligands L and 1,3-H2BDC have been studied at room temperature(Fig.5).The photoluminescent spectra of L and 1,3-H2BDC show the emissions maxima at 545(λex=325 nm)and 385 nm(λex=325 nm),respectively.These emissions may be assigned to π*-n or π*-π transitions of the intraligands.Compound 1 shows a maximum emission at 403 nm(λex=325 nm).In comparison with the 1,3-H2BDC ligand,the emission maximum of compound 1 has slightly changed and show a little red shift.Therefore,the origin of the emission for compound 1 might be attributed to the intraligand transition of 1,3-H2BDC anion[12].
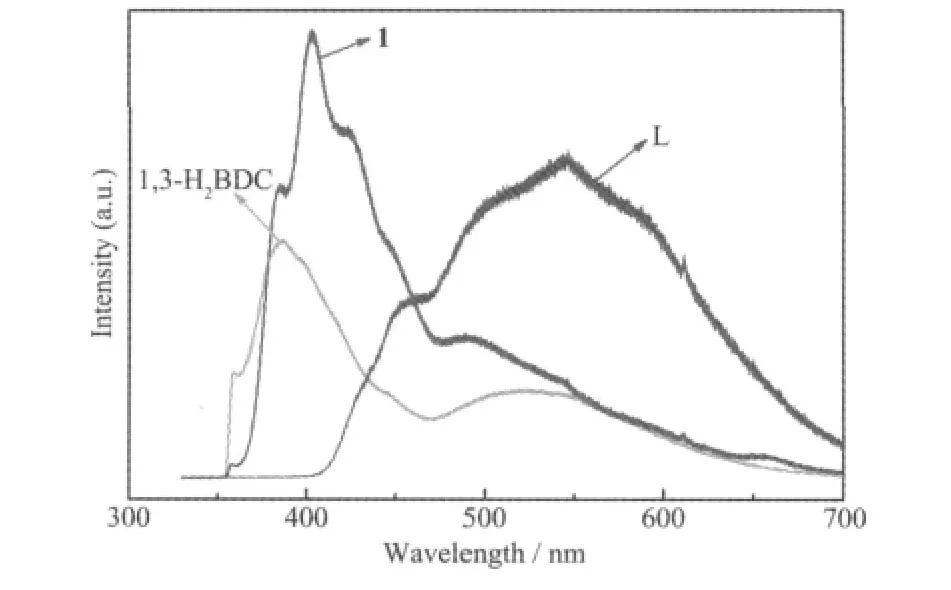
Fig.5 Emission spectra of compound 1,free ligands L and 1,3-H2BDC
[1]Carlucci L,Ciani G,Proserpio D M.Coord.Chem.Rev.,2003,246:247-289
[2]Batten S R,Robson R.Angew.Chem.Int.Ed.,1998,37:1460-1494
[3]Yang J,Ma J F,Batten S R,et al.Chem.Commun.,2008:2233-2235
[4]Dinolfo P H,Hupp J T.Chem.Mater.,2001,13:3113-3125
[5]Blake A J,Champness N R,Hubberstey P,et al.Coord.Chem.Rev.,1999,183:117-138
[6]Qiao Q,Zhao Y J,Tang T D.Acta Cryst.,2008,C64:m336-m338
[7]Kong Z G,Ma X Y,Xu Z L.Z.Naturforsch.,2010,65b:1173-1176
[8]Qiao Q,Wu G Q,Tang T D,et al.Acta Crystallogr.,2009,C65:m146-m148
[9]HU Bin(胡斌),QU Zhi-Rong(瞿志榮).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(2):283-285
[10]Wang X Y,Wang J J,Ng S W.Acta Cryst.,2008,C64:m401-m404
[11]Chen X M,Liu G F.Chem.Eur.J.,2002,18:4811-4817
[12]Yang J,Li G D,Cao J J,et al.Chem.Eur.J.,2007,13:3248-3261
[13]Yang J,Ma J F,Liu Y Y,et al.Cryst.Growth Des.,2009,9:1894-1911
[14]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of G?ttingen,Germany,1997.
[15]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of G?ttingen,Germany,1997.
Synthesis and Crystal Structure of a One-Dimensional Cd(Ⅱ)Coordination Polymer Based on 1,10-Phenanthroline Derivative and 1,3-Benzenedicarboxylic Acid
XU Zhan-Lin*,1HE Yu1MA Shuai1NG Seik Weng2
(1Department of Chemistry,Jilin Normal University,Key Laboratory of Preparation and Applications of Environmental Friendly Materials(Jilin Normal University),Ministry of Education,Siping,Jilin 136000,China)
(2Department of Chemistry,University of Malaya,Kuala Lumpur 50603,Malaysia)
A new 1D chain coordination polymer,[Cd2(L)2(1,3-BDC)2]n1(L=2-(3-fluorophenyl)-1H-imidazo[4,5-f][1,10]phenanthroline and 1,3-H2BDC=1,3-benzenedicarboxylic acid)has been hydrothermally synthesized and characterized by elemental analysis,IR and single-crystal X-ray diffraction.It crystallizes in triclinic,space group P1 with a=1.080 8(4)nm,b=1.149 5(4)nm,c=1.924 8(7)nm,α=106.482(5)°,β=99.436(6)°,γ=93.093(6)°,V=2.249 2(14)nm3,Z=4,C27H15CdFN4O4,Mr=590.83,Dc=1.745 g·cm-3,F(000)=1 176,μ(Mo Kα)=1.024 mm-1,R=0.0445 and wR=0.1117.In compound 1,the 1,3-BDC ligands linked the adjacent Cd(Ⅱ)atoms to generate a onedimensional chain structure.The CH-π interactions between L and 1,3-BDC extended the adjacent chains into a two-dimensional supramolecular layer.The N-H…O hydrogen bond further stabilizes the structure of 1.CCDC:859146.
coordination polymer;crystal structure;1,10-phenanthroline derivative;1,3-benzenedicarboxylic acid
considerable interest in coordination chemistry and material science due to their intriguing structural diversities and potential applications in functional materials,nanotechnology,and biological recognition[1-3].Up to now, lots of coordination polymers withinteresting structures and topologies have been investigated and reported[4].However,their controllable syntheses are still a great challenge because many factors play important roles in their self-assemblies,such as the chemical structures of the ligands,the metal,the anions,reaction temperature and pH value[5].In this regard,the selection of ligand is a vital subject in the construction of the coordination polymers[6].
O614.24+2
A
1001-4861(2012)04-0851-05
2011-09-12。收修改稿日期:2011-11-27。
四平市研究基金(No.2009011)和吉林師范大學研究生創(chuàng)新基金(No.2011004)資助項目。
*通訊聯(lián)系人。E-mail:xuzljl@yahoo.com.cn
Metal-organic coordination polymers have

