胰腺星狀細(xì)胞對(duì)胰腺癌AsPC-1細(xì)胞侵襲轉(zhuǎn)移的影響
高振軍 吳愷 王興鵬
·論著·
胰腺星狀細(xì)胞對(duì)胰腺癌AsPC-1細(xì)胞侵襲轉(zhuǎn)移的影響
高振軍 吳愷 王興鵬
目的探討胰腺星狀細(xì)胞(PSCs)對(duì)胰腺癌細(xì)胞侵襲轉(zhuǎn)移的影響及基質(zhì)細(xì)胞因子-1(SDF-1)在此過(guò)程中的作用。方法常規(guī)分離、培養(yǎng)PSCs,收集及濃縮PSCs條件培養(yǎng)液(PSC-CM),應(yīng)用不同濃度的PSC-CM、抗SDF-1抗體(anti-SDF-1)及兩者聯(lián)合應(yīng)用處理AsPC-1細(xì)胞,采用MTT法檢測(cè)AsPC-1細(xì)胞的增殖,Transwell小室檢測(cè)細(xì)胞遷移,體外侵襲實(shí)驗(yàn)觀察細(xì)胞侵襲能力。結(jié)果對(duì)照組及0.25、0.5、1 μg/μl PSC-CM組AsPC-1細(xì)胞增殖的吸光度值(A490)分別為0.437±0.041、0.472±0.048、0.553±0.057、0.690±0.051,PSC-CM呈劑量依賴性促進(jìn)細(xì)胞增殖,其中0.5、1 μg/μl PSC-CM組與對(duì)照組間以及0.5 μg/μl與1 μg/μl PSC-CM組間的差異具有統(tǒng)計(jì)學(xué)意義(P值均<0.05)。對(duì)照組、anti-SDF-1組、PSC-CM組及PSC-CM+anti-SDF-1組細(xì)胞增殖的A490值分別為0.407±0.028、0.416±0.030、0.629±0.048、0.481±0.049;穿膜細(xì)胞數(shù)分別為(35.3±7.1)、(34.8±5.6)、(140.9±12.7)、(56.5±5.9)個(gè);侵襲細(xì)胞數(shù)分別為(27.1±2.9)、(29.1±4.2)、(81.5±8.2)、(46.4±4.4)個(gè)。anti-SDF-1組與對(duì)照組間的差異無(wú)統(tǒng)計(jì)學(xué)意義,PSC-CM組細(xì)胞的增殖、遷移、侵襲能力較對(duì)照組顯著增強(qiáng)(P<0.05或P<0.01),PSC-CM+anti-SDF-1組細(xì)胞的增殖、遷移、侵襲能力較PSC-CM組顯著降低,但仍顯著高于對(duì)照組(P<0.05或P<0.01)。結(jié)論P(yáng)SC-CM可促進(jìn)AsPC-1的增殖、遷移及侵襲,其機(jī)制為部分通過(guò)SDF-1/CXCR4受體配體系統(tǒng)。
胰腺腫瘤; 星形細(xì)胞; 受體,CXCR4; 基質(zhì)細(xì)胞衍生因子-1
胰腺癌預(yù)后很差,早期即可局部或遠(yuǎn)處轉(zhuǎn)移,近年來(lái)發(fā)病率和病死率在國(guó)內(nèi)外均有明顯上升的趨勢(shì)。腫瘤進(jìn)展不僅取決于腫瘤細(xì)胞本身的生物學(xué)特性,而且與多種非腫瘤性基質(zhì)細(xì)胞構(gòu)成的微環(huán)境密切相關(guān),基質(zhì)與腫瘤細(xì)胞相互作用會(huì)進(jìn)一步促進(jìn)腫瘤細(xì)胞的生存、增殖和侵襲。在胰腺癌周圍產(chǎn)生基質(zhì)反應(yīng)的細(xì)胞是胰腺星狀細(xì)胞(pancreatic stellate cells,PSCs)[1-2],其數(shù)量有時(shí)甚至超過(guò)癌細(xì)胞[3]。基質(zhì)細(xì)胞衍生因子(stromal cell-derived factor 1,SDF-1)與它的特異性受體CXCR4作為最重要的受體配體復(fù)合體在胰腺癌的定向轉(zhuǎn)移中起重要作用[4]。研究發(fā)現(xiàn)[5-6],PSCs上清液可刺激腫瘤細(xì)胞增殖、侵襲及運(yùn)動(dòng),但具體機(jī)制尚不清楚。本研究旨在探討PSCs對(duì)胰腺癌細(xì)胞侵襲轉(zhuǎn)移的影響及SDF-1/CXCR4系統(tǒng)在此過(guò)程中的作用。
材料與方法
一、PSCs的分離、培養(yǎng)
取胰腺癌根治術(shù)中的新鮮癌旁組織,在無(wú)菌環(huán)境、無(wú)血清培養(yǎng)液中剪成0.5 mm3大小的組織塊。把組織塊貼放在培養(yǎng)皿中,間隔0.5~1.0 cm,加入含20%胎牛血清(FCS)的DMEM/F12培養(yǎng)液,培養(yǎng)24 h后更換培養(yǎng)液,以后每2~3 d更換培養(yǎng)液一次。培養(yǎng)約3~5 d,組織塊周圍有星狀細(xì)胞爬出,并形成克隆。小心去除組織塊后用含乙二胺四乙酸(EDTA)的0.25%胰蛋白酶消化細(xì)胞,洗滌后置培養(yǎng)瓶中培養(yǎng)。待細(xì)胞貼壁生長(zhǎng)至70%~80%融合時(shí)按1∶3比例傳代。收集對(duì)數(shù)生長(zhǎng)期PSCs,棄去培養(yǎng)液,用PBS沖洗2遍,每個(gè)培養(yǎng)瓶加無(wú)血清DMEM/F12培養(yǎng)液(serum-free medium, SFM)5 ml培養(yǎng)48 h,收集培養(yǎng)液,過(guò)濾去除細(xì)胞碎片,用YM3超濾離心管濃縮,混合。該濃縮的培養(yǎng)液取名為PSCs條件培養(yǎng)液(PSCs conditioned media, PSC-CM),應(yīng)用Bio-Rad法檢測(cè)蛋白濃度后置-80°C保存?zhèn)溆谩?/p>
二、人胰腺癌AsPC-1細(xì)胞增殖的檢測(cè)
人胰腺癌細(xì)胞株AsPC-1購(gòu)自中科院細(xì)胞庫(kù),常規(guī)培養(yǎng)、傳代。取對(duì)數(shù)生長(zhǎng)期AsPC-1細(xì)胞制成單細(xì)胞懸液,按每孔5×103個(gè)細(xì)胞(每孔100 μl)接種于96孔板,貼壁生長(zhǎng)后換SFM培養(yǎng)過(guò)夜。實(shí)驗(yàn)分5組,分別加入含0.25、0.5、1 μg/μl PSC-CM的SFM和含10%小牛血清(NCS)的培養(yǎng)液,以只加SFM作為對(duì)照組。另取上述細(xì)胞,分為4組,分別加入含1 μg/ml SDF-1中和抗體(SDF-1 neutralizing antibody, anti-SDF-1)、1 μg/μl 的PSC-CM、1 μg/μl的PSC-CM+1 μg/ml anti-SDF-1的SFM,以只加SFM為對(duì)照組。每組均設(shè)5個(gè)平行孔,繼續(xù)培養(yǎng)24 h后每孔加入濃度為5 mg/ml的MTT20 μl培養(yǎng)4 h,吸棄上清液,每孔加入150 μl DMSO振蕩10 min,用酶標(biāo)儀測(cè)定各孔在490 nm波長(zhǎng)處的吸光度(A490)值。
三、細(xì)胞遷移實(shí)驗(yàn)
收集對(duì)數(shù)生長(zhǎng)期AsPC-1細(xì)胞,加入SFM制成2×105/ml的單細(xì)胞懸液。取Transwell小室,在膜的底面鋪100 μg/ml的纖連蛋白(Fn,Sigma-Aldrich公司)50 μl,上室均加入200 μl的細(xì)胞懸液,下室分別加入500 μl含1 μg/ml anti-SDF-1、1 μg/μl PSC-CM、1 μg/μl PSC-CM+1 μg/ml的SFM,以單加500 μl SFM作為對(duì)照組。每組5個(gè)Transwell小室,常規(guī)培養(yǎng)12 h,加0.1%結(jié)晶紫染色20 min,置高倍顯微鏡下隨機(jī)取10個(gè)視野,計(jì)數(shù)穿膜細(xì)胞數(shù),取均值。實(shí)驗(yàn)重復(fù)3次。
四、AsPC-1細(xì)胞體外侵襲實(shí)驗(yàn)
收集對(duì)數(shù)生長(zhǎng)期AsPC-1細(xì)胞,加入SFM制成1×106/ml的單細(xì)胞懸液。應(yīng)用體外侵襲試劑盒ECM550(Chemicon公司)檢測(cè)細(xì)胞體外侵襲能力。上室均加入300 μl的細(xì)胞懸液,下室分別加入500 μl含1 μg/ml anti-SDF-1、 1 μg/μl PSC-CM、 1 μg/μl PSC-CM+1 μg/ml anti-SDF-1的2% FCS培養(yǎng)液,以單加500 μl 2% FCS的培養(yǎng)液為對(duì)照組。每組5個(gè)小室,嚴(yán)格按試劑盒說(shuō)明書(shū)操作,孵育48 h后隨機(jī)計(jì)數(shù)10個(gè)高倍鏡視野中浸潤(rùn)細(xì)胞的數(shù)量,取均值。實(shí)驗(yàn)重復(fù)3次。
五、統(tǒng)計(jì)學(xué)處理
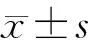
結(jié) 果
一、AsPC-1細(xì)胞增殖的變化
對(duì)照組,0.25、0.5、1 μg/μl PSC-CM組及10% NCS組AsPC-1細(xì)胞增殖的A490值分別為0.437±0.041、0.472±0.048、0.553±0.057、0.690±0.051及0.591±0.056(F=27.116,P<0.05),PSC-CM呈劑量依賴性促進(jìn)細(xì)胞增殖,其中0.5、1 μg/μl PSC-CM組與對(duì)照組間以及0.5μg/μl與1 μg/μl PSC-CM組間的差異具有統(tǒng)計(jì)學(xué)意義(P值均<0.05)。10% NCS組的細(xì)胞增殖亦較對(duì)照組顯著增加(P<0.05)。
對(duì)照組、anti-SDF-1組、PSC-CM組及PSC-CM+anti-SDF-1組細(xì)胞增殖的A490值分別為0.407±0.028、0.416±0.030、0.629±0.048、0.481±0.049,anti-SDF-1組與對(duì)照組的差異無(wú)統(tǒng)計(jì)學(xué)意義,PSC-CM組細(xì)胞增殖較對(duì)照組顯著增加(P<0.05),而PSC-CM+anti-SDF-1組細(xì)胞增殖較PSC-CM組顯著下降,但仍高于對(duì)照組(P值均<0.05)。
二、AsPC-1細(xì)胞穿膜細(xì)胞數(shù)的變化
對(duì)照組、anti-SDF-1組、PSC-CM組及PSC-CM+anti-SDF-1組的穿膜細(xì)胞數(shù)分別為(35.3±7.1)、(34.8±5.6)、(140.9±12.7)、(56.5±5.9)個(gè),anti-SDF-1組與對(duì)照組間的差異無(wú)統(tǒng)計(jì)學(xué)意義,PSC-CM組的穿膜細(xì)胞數(shù)較對(duì)照組顯著增多(P<0.01),而PSC-CM+anti-SDF-1組的穿膜細(xì)胞數(shù)較PSC-CM組顯著減少,但仍高于對(duì)照組(P值均<0.01)。
三、AsPC-1細(xì)胞體外侵襲能力的變化
對(duì)照組、anti-SDF-1組、PSC-CM組及PSC-CM+anti-SDF-1組的侵襲細(xì)胞數(shù)分別為(27.1±2.9)、(29.1±4.2)、(81.5±8.2)、(46.4±4.4)個(gè),anti-SDF-1組與對(duì)照組間的差異無(wú)統(tǒng)計(jì)學(xué)意義,PSC-CM組的侵襲細(xì)胞數(shù)較對(duì)照組均顯著增多(P<0.01),PSC-CM+anti-SDF-1組的侵襲細(xì)胞數(shù)較PSC-CM組顯著減少,但仍高于對(duì)照組(P值均<0.01)。
討 論
腫瘤局部微環(huán)境在腫瘤的進(jìn)展中起重要作用[7-8]。腫瘤微環(huán)境中存在著復(fù)雜的趨化因子及其受體系統(tǒng),其中,起首要作用的是SDF-1。SDF-1多以自分泌或旁分泌形式刺激腫瘤細(xì)胞及腫瘤間質(zhì)細(xì)胞,使腫瘤細(xì)胞存活和生長(zhǎng)。近來(lái)研究提示,間質(zhì)細(xì)胞是SDF-1的重要來(lái)源,腫瘤相關(guān)性成纖維細(xì)胞(carcinoma associated fibroblasts, CAFs)能分泌SDF-1,通過(guò)與CXCR4作用來(lái)直接刺激腫瘤生長(zhǎng),并能介導(dǎo)內(nèi)皮前體細(xì)胞進(jìn)入腫瘤而促進(jìn)腫瘤血管生成,進(jìn)一步促進(jìn)腫瘤的增殖及侵襲、轉(zhuǎn)移[9]。SDF-1是分泌型蛋白質(zhì),間質(zhì)成纖維細(xì)胞產(chǎn)生后釋放到細(xì)胞間隙,在局部微環(huán)境中通過(guò)旁分泌方式直接刺激造血或非造血性正常或惡性細(xì)胞的遷移[10-11]。胰腺癌中有大量間質(zhì)組織,胰腺癌-間質(zhì)之間的相互作用在腫瘤進(jìn)展中也起重要作用[2]。
本研究結(jié)果顯示,應(yīng)用PSCs培養(yǎng)上清處理AsPC-1細(xì)胞后可促進(jìn)其增殖,與文獻(xiàn)報(bào)道[6,12]相似。本研究結(jié)果還顯示,應(yīng)用PSCs培養(yǎng)上清處理AsPC-1細(xì)胞后可促進(jìn)AsPC-1細(xì)胞遷移、侵襲,而應(yīng)用抗SDF-1抗體后,PSCs培養(yǎng)上清對(duì)AsPC-1細(xì)胞增殖、遷移、侵襲的促進(jìn)作用被抑制,提示PSC-CM可能通過(guò)PSCs分泌的SDF-1促進(jìn)胰腺癌細(xì)胞增殖、遷移及侵襲,表明SDF-1/CXCR4軸通過(guò)旁分泌調(diào)控機(jī)制在PSCs與胰腺癌細(xì)胞的相互作用中扮演重要角色。本研究結(jié)果還顯示,盡管抗SDF-1抗體可明顯抑制PSC-CM所引起的促AsPC-1細(xì)胞增殖、遷移及侵襲作用,但處理后的AsPC-1細(xì)胞的增殖、遷移、侵襲能力仍顯著高于對(duì)照組,提示在PSC-CM中尚存在其他一些可溶性因子具有促進(jìn)胰腺癌細(xì)胞增殖、遷移及侵襲的作用,有待于進(jìn)一步深入研究。
[1] Bachem MG, Schünemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology, 2005,128: 907-921.
[2] Ohuchida K, Mizumoto K, Murakami M, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res, 2004, 64: 3215-3222.
[3] Lee CS, Montebello J, Georgiou T, et al. Distribution of type IV collagen in pancreatic adenocarcinoma and chronic pancreatitis. Int J Exp Pathol, 1994, 75: 79-83.
[4] Wang Z, Ma Q. Beta-catenin is a promising key factor in the SDF-1/CXCR4 axis on metastasis of pancreatic cancer. Med Hypotheses, 2007, 69: 816-820.
[5] Bachem MG, Zhou S, Buck K,et al. Pancreatic stellate cells-role in pancreas cancer. Langenbecks Arch Surg, 2008, 393: 891-900.
[6] Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res, 2008, 68: 918-926.
[7] Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature, 2001, 411: 375-379.
[8] Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer, 2006, 6: 392-401.
[9] Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell, 2005, 121: 335-348.
[10] Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood, 2006, 107: 1761-1767.
[11] Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett, 2006, 238: 30-41.
[12] Bachem MG, Zhou S, Buck K, et al. Pancreatic stellate cells-role in pancreas cancer. Langenbecks Arch Surg, 2008, 393:891-900.
EffectofpancreaticstellatecellsoninvasionandmetastasisofhumanpancreaticcancercelllineAsPC-1
GAOZhen-jun,WUKai,WANGXing-peng.
DepartmentofGastroenterology,ZhongshanHospitalQingpuBranch,FudanUniversity,Shanghai201700,China
Correspondingauthor:WANGXing-peng,Email:xpwcn@public7.sta.net.cn
ObjectiveTo investigate the effect of pancreatic stellate cells on invasion and metastasis of human pancreatic cancer cell AsPC-1, and to determine the role of SDF-1 in this process.MethodsPSCs were routinely isolated and cultured, and PSCs conditioned media(PSC-CM) was collected and concentrated. Different concentrations of PSC-CM, anti SDF-1 and their combination were used to treat AsPC-1 cells, and MTT assay was applied to detect the proliferation of pancreatic cancer cells. Transwell chamber migration assay was employed to detect the migration of AsPC-1 cells. In vitro invasion assay was used to determine the invasion of AsPC-1 cells.ResultsA490values of AsPC-1 cell in control group and 0.25, 0.5, 1 μg/μl PSC-CM group were 0.437±0.041, 0.472±0.048, 0.553±0.057, 0.690±0.051, and PSC-CM promoted cell proliferation in a dose dependant manner. The difference between 0.5, 1 μg/μl PSC-CM group and control group, and between 1 μg/μl and 0.5 μg/μl PSC-CM group was statistically significant (P<0.05).A490values of control group, anti SDF-1 group, PSC-CM group and PSC-CM+anti SDF-1 group were 0.407±0.028, 0.416±0.030, 0.629±0.048, 0.481±0.049. The numbers of penetrating cells were 35.3±7.1, 34.8±5.6, 140.9±12.7, 56.5±5.9, and the numbers of invasive cells were 27.1±2.9, 29.1±4.2,81.5±8.2, 46.4±4.4. The difference between anti SDF-1 group and control group was not statistically significant. The proliferation, migration and invasion of pancreatic cancer cells in PSC-CM group was significantly higher than those of control group (P<0.05 orP<0.01). The proliferation, migration and invasion of pancreatic cancer cells in PSC-CM+anti SDF-1 group was significantly lower than those of PSC-CM group, but they were significantly higher than those of control group (P<0.01).ConclusionsPSCs can promote proliferation, migration and invasion of pancreatic cancer cells AsPC-1, and the mechanism may be partly due to SDF-1/CXCR4 receptor ligand axis.
Pancreatic neoplasm; Astrocytes; Receptors, CXCR4; Stromal cell-derived factor 1
2013-04-24)
(本文編輯:屠振興)
10.3760/cma.j.issn.1674-1935.2013.04.009
201700 上海,復(fù)旦大學(xué)附屬中山醫(yī)院青浦分院消化科(高振軍);上海交通大學(xué)附屬第一人民醫(yī)院消化科(吳愷、王興鵬)
王興鵬,Email: xpwcn@public7.sta. net. cn

