TNF-α增強人臍帶間充質干細胞條件培養基的體外造血支持功能*
胡彩東, 韓之波, 楊舟鑫, 李麗娜, 羅偉峰, 及月茹, 王有為, 李揚秋, 韓忠朝,△
(1暨南大學第一臨床醫學院血液病研究所, 廣東 廣州 510632; 2中國醫學科學院血液學研究所泰達生命科學技術研究中心, 天津 300457; 3中國醫學科學院,北京協和醫學院血液學研究所,實驗血液學國家重點實驗室, 天津 300020)
TNF-α增強人臍帶間充質干細胞條件培養基的體外造血支持功能*
胡彩東1, 韓之波2,3, 楊舟鑫3, 李麗娜1, 羅偉峰1, 及月茹3, 王有為3, 李揚秋1, 韓忠朝1,2,3△
(1暨南大學第一臨床醫學院血液病研究所, 廣東 廣州 510632;2中國醫學科學院血液學研究所泰達生命科學技術研究中心, 天津 300457;3中國醫學科學院,北京協和醫學院血液學研究所,實驗血液學國家重點實驗室, 天津 300020)
目的研究腫瘤壞死因子α(tumor necrosis factor α, TNF-α)刺激后所得的臍帶間充質干細胞條件培養基對臍血CD34+細胞在半固體培養基中集落形成個數及種類的影響。方法將3~6代人臍帶來源間充質干細胞(human umbilical cord mesenchymal stem cells, hUC-MSCs)以2×106接種到75cm2培養瓶中,其中刺激組加入TNF-α(10 g/L),48 h后收集上清作為條件培養基。Real-time PCR檢測hUC-MSCs中各類造血因子mRNA的表達量。密度梯度離心法分離臍血單個核細胞,磁珠分選CD34+細胞,流式細胞術檢測細胞純度后分5組接種到6孔板內:TNF-α刺激hUC-MSC上清+不完全甲基纖維素培養基;hUC-MSC上清+不完全甲基纖維素培養基;TNF-α+DMEM/F12完全培養基+不完全甲基纖維素培養基;完全甲基纖維素培養基;DMEM/F12完全培養基+不完全甲基纖維素培養基。10 d后顯微鏡下計數各類集落形成單位(colony-forming unit, CFU)的數目,收集集落形成細胞,流式細胞術檢測其表型特征。結果(1)TNF-α刺激后hUC-MSCs中粒細胞集落刺激因子(granulocyte colony-stimulating factor, G-CSF)和白細胞介素6(interleukin-6, IL-6)mRNA表達上調。(2)兩種條件培養組均可見粒系CFU(granulocyte CFU, CFU-G)、巨噬系CFU(macrophage CFU, CFU-M)和粒巨噬系CFU(granulocyte-macrophage CFU, CFU-GM),但TNF-α刺激組CFU-G和CFU-M的數目約為未刺激組的1.5倍,CFU-GM約為未刺激組的2倍;陽性對照組中除粒系、巨噬系集落外還可見紅系集落;而DMEM/F12完全培養基加或不加TNF-α組10 d后均未見集落形成。(3)流式細胞術檢測TNF-α刺激組與未刺激組集落細胞表型CD14、CD45和CD11b,未見明顯差異。結論hUC-MSC上清作為條件培養基可在體外促進CD34+細胞分化增殖為髓系細胞,具有造血支持作用,TNF-α刺激后此作用增強。
間充質干細胞; 腫瘤壞死因子α; 造血支持
造血干/祖細胞的獲得及恢復一直被認為是各類血液腫瘤治療的關鍵,體外擴增造血干細胞后回輸到長期化療患者體內是主要治療手段,早期的實驗通過在長期培養體系中加入各種細胞因子如干細胞因子(stem cell factor, SCF)、白細胞介素(interleukin, IL)-3、IL-6、IL-1等來促進CD34+細胞自我更新及分化,為獲得理想的造血祖細胞構成,體系中各類因子的種類及劑量一直是研究的熱點。1989年Sutherland等[1]提取骨髓間充質干細胞作為滋養層細胞在體外行長期培養起始細胞(long-term culture-initiating cell, LTC-IC)實驗,成功培養出造血集落,骨髓來源間充質干細胞(bone marrow-derived mesenchymal stem cells, BM-MSCs)顯示出其獨立的造血支持能力。隨后的研究證實,間充質干細胞存在于骨髓組織中,通過分泌造血相關因子及細胞黏附作用等參與造血干細胞的自我增殖與分化,在造血系統微環境中發揮重要作用。2000年,Gerson等[2]將高劑量化療后晚期乳腺癌患者自身MSCs及外周血祖細胞回輸體內,發現在第8天患者可明顯提高中性粒細胞及血小板數,且未發生毒性反應。2002年,Kadereit等[3]在MSCs作為滋養層細胞的LTC-IC實驗中發現MSCs可促進CD34+細胞的擴增,同時通過調節BCL-2和P21降低造血干細胞的凋亡,而Noort等[4]提取胎肺MSCs與CD34+細胞同時移植入NOD/SCID小鼠體內,同樣證實了MSCs促進CD34+細胞自我更新與分化的作用。臍帶間充質干細胞由其易得性、易擴增及低免疫原性,其可利用性高于其它組織來源的間充質干細胞,而腫瘤壞死因子α(tumor necrosis factor-alpha,TNF-α)作為一類重要的炎癥因子,存在于各種病理生理反應中[5],其對hUC-MSCs的作用不容忽視。本研究通過TNF-α預先刺激hUC-MSCs,檢測其因子分泌的變化對造血干細胞(hematopoietic stem cells,HSCs)增殖分化的影響,為完善hUC-MSCs造血支持作用的研究提供實驗依據。
材 料 和 方 法
1主要試劑及儀器
DMEM/F12干粉培養基和胰酶購自Gibco;胎牛血清(fetal bovine serum,FBS)購自HyClone;人淋巴細胞分離液購自天津灝洋生物制品科技有限公司;TNF-α、甲基纖維素半固體培養基MethoCultTMH4230、H4435和人臍血CD34陽性分選試劑盒購自Stemcell;CD34-FITC (fluorescein isothiocyanate)、CD11b-PE (phycoerythrin)、CD19-PE、CD29-PE、CD54-PE、CD45-PE、CD73-PE、CD80-PE、CD86-PE、CD90-PE、CD105-PE、CD31-PE、CD117-PE、HLA-DR-PE和HLA-G-PE購自BD Biosciences;倒置相差顯微鏡 (Olympus);流式細胞儀(Becton Dickinson)。
2主要方法
2.1hUC-MSCs分離、培養、擴增和鑒定 臍帶和臍血標本取自天津市中心婦產醫院足月剖宮產新生兒,均經父母授權同意,母血檢測肝炎病毒、艾滋病病毒、巨細胞病毒、梅毒、衣原體等病原學均為陰性。臍帶用含青/鏈霉素的PBS充分沖洗去除殘留血液,剪碎至(1~2)mm×1 mm×1 mm大小,依次用0.1%膠原酶Ⅱ和0.125%的胰蛋白酶于37 ℃各消化30 min,10%的人AB血清終止消化。消化混合物經細胞篩過濾,收集濾液至離心管,DMEM/F12培養基洗滌2次。最后將細胞重懸于含10%FBS的DMEM/F12完全培養基,按1×106/cm2的密度將其接種于T-75培養瓶中,37 ℃、5%CO2、飽和濕度培養箱內培養。細胞貼壁生長至80%~90%融合時,胰酶消化傳代。收集80%~90%融合細胞,分別用FITC或PE標記的小鼠抗人單克隆抗體CD34、CD29、CD11b、CD19、CD45、CD54、CD73、CD80、CD86、CD90、CD105、CD31、CD117、HLA-G和HLA-DR孵育hUC-MSCs,以FITC-IgG和PE-IgG作為同型對照抗體,流式細胞術檢測。
2.2hUC-MSCs條件培養液收集 復蘇第3~5代hUC-MSCs,待細胞80%~90%融合時,胰酶消化細胞,臺盼藍計活細胞個數,取2×106個細胞,用10 mL含10%FBS的DMEM/F12培養基,刺激組加入TNF-α(10 μg/L),接種于75 cm2培養瓶中,置于37 ℃、5% CO2、飽和濕度的恒溫箱中,培養48 h時后收集hUC-MSCs培養上清,用0.22 μm濾膜過濾,分裝并凍存于-80 ℃備用。
2.3Real-time PCR檢測hUC-MSCs各類造血相關因子基因表達 收集上述刺激組與未刺激組hUC-MSCs,提取RNA并逆轉為cDNA,行real-time PCR檢測粒細胞集落刺激因子(granulocyte colony-stimulating factor,G-CSF)、巨噬細胞集落刺激因子(macrophage colony-stimulating factor, M-CSF)、GM-CSF、干細胞因子(stem cell factor, SCF)、基質細胞衍生因子1(stromal-derived factor 1, SDF-1)和IL-6 mRNA表達量,反應條件為95 ℃ 2 min激活熱啟動酶,94 ℃ 15 s,60 ℃ 30 s,45個循環,60 ℃收集熒光信號。引物序列見表1。

表1 Real-time PCR引物序列
2.4分離純化臍血CD34+細胞 收集足月妊娠臍血標本,獲知情同意,病原學檢測后PBS按1∶2的比例稀釋,以2∶1的比例平鋪至人淋巴細胞分離液上,2 300 r/min離心20 min,抽取中間的白膜層(單個核細胞層),PBS溶液洗滌2次。用人臍血CD34陽性分選試劑盒分選CD34+細胞。部分分選細胞CD34-FITC抗體標記,流式細胞術檢測分選細胞純度,余細胞用DMEM/F12重懸,濃度1×107/L。
2.5造血祖細胞集落培養 按以下分組接種以上CD34+細胞至6孔板:TNF-α刺激hUC-MSC上清+不完全甲基纖維素培養基;hUC-MSC上清+不完全甲基纖維素培養基;TNF-α+DMEM/F12完全培養基+不完全甲基纖維素培養基;完全甲基纖維素培養基;DMEM/F12完全培養基+不完全甲基纖維素培養基。每組2個復孔,終體積2 mL,其中條件培養液與甲基纖維素比例為1∶4,CD34+細胞懸液與終培養基體積比例為1∶10,于37 ℃、5%CO2,飽和濕度孵箱中培養,觀察集落形成單位的形態和種類,第10天記錄≥50個細胞組成的集落形成單位數量。
2.6流式細胞術檢測分析 分別取第10天實驗組中形成集落形成單位的細胞孵育抗CD11b-PE、CD45-PE和CD14-PE鼠抗人抗體,流式細胞術分析,結果由CellQuest軟件處理,檢測集落形成單位細胞的免疫表型。
3統計學處理
數據用均數±標準差(mean±SD)表示,采用SPSS 13.0統計軟件進行兩樣本t檢驗,以P<0.05為差異有統計學意義。
結 果
1hUC-MSCs形態與表型特征
倒置顯微鏡觀察到hUC-MSCs在2~4 h內即貼壁生長,紡錘形,2~3 d細胞增長達高峰,呈旋渦狀排列。流式細胞術檢測發現,細胞表面標志CD11b、CD19、CD34、CD45、HLA-G、CD80、CD86、CD117、CD31和HLA-DR為陰性;CD29、CD54、CD73、CD90和CD105為陽性,可認為所分選細胞為臍帶間充質干細胞。
2Real-timePCR檢測造血相關因子的表達
Real-time PCR檢測TNF-α(10 μg/L)刺激48 h后臍帶間充質干細胞各類造血相關因子mRNA表達的變化,其中刺激后G-CSF和IL-6表達量上調,差異有統計學意義;GM-CSF雖無統計學意義,主要是因為不同個體間差異較大,導致方差較大,見圖1。

Figure 1. Quantitative analysis of G-CSF, M-CSF, GM-CSF, SCF, SDF-1 and IL-6 mRNA expression in control and TNF-α (10 μg/L)-stimulated hUC-MSCs. Mean±SD.n=3.*P<0.05,**P<0.01vscontrol group.
圖1TNF-α刺激組與未刺激組hUC-MSCs中G-CSF、M-CSF、GM-CSF、SCF、SDF-1和IL-6mRNA的相對表達量
3造血集落形成單位形態及集落數
流式細胞術檢測分選細胞中CD34+細胞比例大于95%,見圖2,符合后續實驗要求。
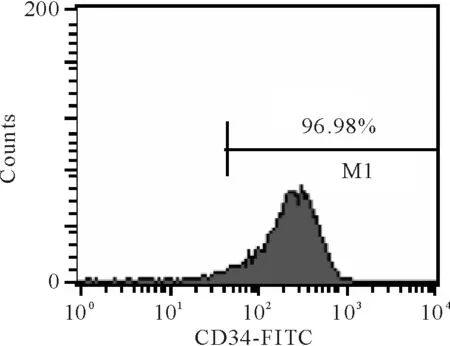
Figure 2. The frequency of the umbilical cord blood-derived CD34+cells after isolation assayed by flow cytometry.
圖2臍血分選CD34+細胞純度分析
倒置顯微鏡觀察實驗組、陽性對照組和陰性對照組集落形成情況。(1)陽性對照組與實驗組均有集落形成,但各集落形成單位的種類和數量不同;只加入TNF-α組及陰性對照組未見集落形成單位。(2)實驗組中可見粒系集落形成單位(granulocyte colony-forming unit,CFU-G)、巨噬系集落形成單位(macrophage colony-forming unit,CFU-M)和粒-巨噬集落形成單位(granulocyte-macrophage colony-forming unit,CFU-GM),未見紅系爆式集落形成單位 (erythroid burst-forming unit,BFU-E)、紅系集落形成單位(erythroid colony-forming unit,CFU-E)和粒-紅-巨噬-巨核集落形成單位(granulocyte-erythroid-macrophage-megakaryocyte colony-forming unit,CFU-GEMM),陽性對照各系集落形成單位均可見,見圖3。(3)計數刺激組與未刺激組各類集落數,差異有統計學意義,見圖4。由此可見,hUC-MSC條件培養基單獨作用能促進CD34+細胞的分化,即促進造血干細胞主要向除紅細胞以外的髓系細胞分化,加入TNF-α后該作用加強。

Figure 3. CFU-M(A),CFU-G(B),CFU-GM (C),BFU-E(D) and CFU-GEMM(E) after cultured for 10 d in conditioned culture medium and positive control(×40).
圖3條件培養基及陽性對照組培養第10天各類集落形態

Figure 4. The numbers of CFU formed by umbilical cord blood derived-CD34+cells in control and TNF-α stimulation group. Mean±SD.n=3.*P<0.05vscontrol.
圖4刺激組與未刺激組條件培養基中集落細胞數比較
4集落形成單位的細胞免疫表型檢測結果
收集第10天集落形成細胞,流式細胞術檢測CD11b-PE、CD45-PE和CD14-PE,刺激組與未刺激組相比無明顯差異,均高表達髓系標志CD45,見表2。
表2集落形成細胞表型分析
Table 2. Phenotypic characteristics of the colony-forming cells (%.Mean±SD.n=3)
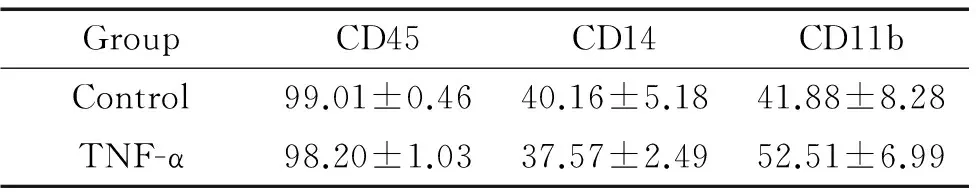
GroupCD45CD14CD11bControl99.01±0.4640.16±5.1841.88±8.28TNF-α98.20±1.0337.57±2.4952.51±6.99
討 論
骨髓造血微環境一般定義為基質干細胞、造血因子及細胞外基質,而其中主要的基質干細胞即BM-MSCs。BM-MSCs主要通過細胞-細胞接觸及分泌各類造血相關蛋白因子發揮其造血支持作用,其中包括維持HSCs的穩定性,促進其自我更新及多向分化[6-7]。本實驗主要收集TNF-α刺激48 h后hUC-MSC培養上清,體外檢測其對CD34+細胞集落形成的影響,從分泌因子的角度考慮其造血支持能力。
一般認為TNF-α單獨作用于造血集落是一種造血負調控因子,在體外實驗中從1~100 U/mL的濃度,TNF-α對CFU-E、CFU-G、CFU-M、CFU-GM和CFU-GEMM表現出不同的抑制作用[8-10],然而動物實驗中,直接注射TNF-α到正常及白血病小鼠體內,小鼠骨髓及脾臟CFU-M、CFU-GM及BFU-E表現為促進,僅CFU-E表現為抑制[11]。可見TNF-α對造血干/祖細胞的影響不能單獨定論,其作用方向與其作用環境相關。G-CSF、M-CSF、GM-CSF、SCF、SDF-1和IL-6均為公認的造血相關因子,且在過去的研究中證實MSCs主要通過分泌造血相關因子,結合其基質成分調節造血功能[12-13]。本實驗real-time PCR 結果顯示hUC-MSCs本身表達上述各類因子,經TNF-α刺激48 h后G-CSF、M-CSF、GM-CSF和IL-6表達量上調或具有上調趨勢,這與集落形成中對照組與刺激組均形成集落且刺激組CFU-GM較未刺激組大約兩倍的結果相符。由此可見,hUC-MSCs單獨具有促進CD34+細胞分化為造血祖細胞形成造血集落的功能,而TNF-α刺激后通過上調其造血因子的表達加強此作用,且此加強作用遠遠超過了TNF-α本身對上述集落形成的抑制作用。此外,TNF-α刺激hUC-MSCs后,其表達SCF下調,我們認為這是間充質干細胞置身于類似炎癥微環境中,表現出與各類血細胞類似的分化成熟趨勢,但并未改變其基本性狀,相反有可能激發了其它因子的分泌。同時刺激前后hUC-MSCs均未檢測到促紅細胞生成素(erythropoietin, EPO),這可能與MSCs本身特性有關,在以往實驗中BM-MSCs同樣未檢測到EPO表達,有報道顯示BM-MSCs與CD34+共培養體系集落形成實驗中可形成BFU-E,但其數量相對于粒系集落則少之又少。且Roodman等[9]在體外實驗中證實TNF-α刺激15 min后即表現出對CFU-E和BFU-E形成的抑制效果,同時不同個體紅系集落對TNF-α的敏感性差異達上百倍。本實驗最后收集記錄形成在流式細胞術中CD45的近乎全陽性表達的結果與上述RT-PCR結果是相符的。因此我們認為本實驗中無紅系集落形成可能與hUC-MSCs本身支持紅系分化較弱[14]及TNF-α對CFU-E的抑制作用有關[15]。綜合來看,我們可以認為TNF-α在體內實驗中出現與體外實驗相反結果可以用其在體內與MSCs相互作用來解釋,即與本實驗結果相符。
鑒于本實驗僅收集培養上清而無hUC-MSCs與CD34+細胞共培養,即無細胞間直接接觸作用,其中hUC-MSCs對造血干細胞的作用僅限其分泌的因子,與經典的LTC-IC實驗結果可能有出入,但足以說明hUC-MSCs間接對造血干/祖細胞的作用,即hUC-MSCs能通過分泌造血相關因子促進造血集落形成,TNF-α通過上調hUC-MSCs的G-CSF、M-CSF、GM-CSF和IL-6表達量加強其造血支持作用,而深入、更全面的TNF-α對hUC-MSCs造血支持能力的影響有待于體內試驗的開展。
[1] Sutherland HJ, Eaves CJ, Eaves AC, et al. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesisinvitro[J]. Blood,1989,74(5):1563-1570.
[3] Kadereit S, Deeds LS, Haynesworth SE, et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34+/CD38-early progenitors cultured over human MSCs as a feeder layer[J]. Stem Cells,2002,20(6):573-582.
[4] Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34+cells in NOD/SCID mice[J]. Exp Hematol,2002,30(8):870-878.
[5] Satomi N, Haranaka K, Kunii O. Research on the production site of tumor necrosis factor (TNF)[J]. Jpn J Exp Med,1981,51(6):317-322.
[6] Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells[J]. Science,1999,284(5411):143-147.
[7] Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche[J]. Nature,2010,466(7308):829-834.
[8] Broxmeyer HE, Williams DE, Lu L, et al. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma[J]. J Immunol,1986,136(12):4487-4495.
[9] Roodman GD, Bird A, Hutzler D, et al. Tumor necrosis factor-alpha and hematopoietic progenitors: effects of tumor necrosis factor on the growth of erythroid progenitors CFU-E and BFU-E and the hematopoietic cell lines K562, HL60, and HEL cells[J]. Exp Hematol,1987,15(9):928-935.
[10] Beran M, O’Brien S, Gutterman JU, et al. Tumor necrosis factor and human hematopoiesis: I. Kinetics and diversity of human bone marrow cell response to recombinant tumor necrosis factor alpha in short-term suspension culturesinvitro[J]. Hematol Pathol,1988,2(1):31-42.
[11] Johnson CS, Chang MJ, Furmanski P.Invivohematopoietic effects of tumor necrosis factor-alpha in normal and erythroleukemic mice: characterization and therapeutic applications[J]. Blood,1988,72(6):1875-1883.
[12] Sze SK, De Kleijn DP, Lai RC, et al. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells[J]. Mol Cell Proteomics,2007,6(10):1680-1689.
[13] 毛文哲, 許 超, 李揚秋, 等. 長期培養人臍帶間充質干細胞PCNA、IL-6、IL-11和galectin-3的表達[J]. 中國病理生理雜志,2012,28(6):1051-1056.
[14] 莫世靜, 童秀珍, 鐘 茜, 等. 骨髓間充質干細胞通過上調EPO表達減輕缺氧損傷引起的PC12細胞凋亡[J]. 中國病理生理雜志,2013,29(1):62-69.
[15] Chen HW, Chen HY, Wang LT, et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines[J]. J Immunol,2013,190(10):5065-5077.
TNF-αenhanceshematopoiesis-supportiveeffectofconditionedculturemediumfromhumanumbilicalcord-derivedMSCsinvitro
HU Cai-dong1, HAN Zhi-bo2,3, YANG Zhou-xin3, LI Li-na1, LUO Wei-feng1, JI Yue-ru3, WANG You-wei3, LI Yang-qiu1, HAN Zhong-chao1, 2, 3
(1InstituteofHematology,theFirstClinicalMedicalCollege,JinanUniversity,Guangzhou510632,China;2TEDALifeScienceandTechnologyResearchCenter,InstituteofHematology,ChineseAcademyofMedicalSciences,Tianjin300457,China;3StateKeyLaboratoryofExperimentalHematology,InstituteofHematology,ChineseAcademyofMedicalSciencesandPekingUnionMedicalCollege,Tianjin300020,China.E-mail:hanzhongchao@hotmail.com)
AIM: To study the influence of tumor necrosis factor-alpha (TNF-α)-stimulated conditioned culture medium from human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) on the colony-forming ability of umbilical cord blood CD34+cells in semisolid medium.METHODSThe hUC-MSCs were cultured in 75-cm2culture flasks at a concentration of 2×106cells per flask, with or without TNF-α (10 μg/L), and their culture supernatants were harvested as the conditioned culture medium 48 h later. The hUC-MSCs were collected and their RNA was extracted. Real-time PCR was performed to detect the mRNA expression of hematopoietic factors. Umbilical cord blood mononuclear cells were isolated by Ficoll-Paque density gradient centrifugation, and then CD34+cells were isolated using Human Cord Blood CD34 Positive Selection Kit. The CD34+cells were divided into the following five groups: TNF-α group (TNF-α-stimulated hUC-MSC culture supernatant added into incomplete methylcellulose medium), control group (unstimulated hUC-MSC culture supernatant added into incomplete methylcellulose medium), positive group (complete methylcellulose medium with recombinant human cytokines), TNF-α+DMEM/F12 group (TNF-α and DMEM/F12 medium with 10% FBS added into incomplete methycellulose medium) and DMEM/F12 group (DMEM/F12 medium with 10% FBS added into incomplete methycellulose medium). Ten days later, the number of the colony-forming units (CFU) was counted, and the cells were collected to detect the surface markers by flow cytometry.RESULTS(1) TNF-α stimulation significantly up-regulated the mRNA expression of granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6) in hUC-MSCs. (2) Granulocyte CFU (CFU-G), macrophage CFU (CFU-M) and granulocyte-macrophage CFU (CFU-GM) were observed in both TNF-α and control groups. The numbers of CFU-G and CFU-M in TNF-α group were 1.5 times as large as those in control group, and the number of CFU-GM in TNF-α group was even twice as large as that in control group. Granulocyte-erythroid-macrophage-megakaryocyte CFU (CFU-GEMM) and erythroid burst-forming units (BFU-E) were only observed in positive group, and no CFU was observed in TNF-α+DMEM/F12 and DMEM/F12 groups. (3) Flow cytometry showed no differences of CD14, CD45 and CD11b expression on the colony-forming cells between TNF-α and control groups.CONCLUSIONTNF-α can enhance the hematopoiesis-supportive effect of conditioned culture medium from hUC-MSCsinvitro.
Mesenchymal stem cells; Tumor necrosis factor-alpha; Hematopoiesis
R363
A
1000- 4718(2013)09- 1679- 06
2013- 05- 10
2013- 07- 15
國家重大科學計劃“973計劃”(No.2011CB964800)
△通訊作者 Tel:022-23909172;E-mail:hanzhongchao@hotmail.com
10.3969/j.issn.1000- 4718.2013.09.025

